Abstract
Plasmacytoid dendritic cells (pDC) are rarely present in normal skin but have been shown to infiltrate lesions of infections or autoimmune disorders. Here, we report that several DC subsets including CD123+ BDCA-2/CD303+ pDC accumulate in the dermis in indurations induced by the tuberculin skin test (TST), used to screen immune sensitization by Mycobacterium tuberculosis. Although the purified protein derivate (PPD) used in the TST did not itself induce pDC recruitment or IFNα production, the positive skin reactions showed high expression of the IFNα inducible protein MxA. In contrast, the local immune response to PPD was associated with substantial cell death and high expression of the cationic antimicrobial peptide LL37, which together can provide a means for pDC activation and IFNα production. In vitro, pDC showed low uptake of PPD compared to CD11c+ and BDCA-3/CD141+ myeloid DC subsets. Furthermore, supernatants from pDC activated with LL37-DNA complexes reduced the high PPD uptake in myeloid DC as well as decreased their capacity to activate T cell proliferation. Infiltrating pDC in the TST reaction site may thus have a regulatory effect upon the antigen processing and presentation functions of surrounding potent myeloid DC subsets to limit potentially detrimental and excessive immune stimulation.
Keywords: dendritic cells, plasmacytoid dendritic cells, skin, tuberculin skin test, LL37, delayed hypersensitivity reaction, PPD
Introduction
Tuberculin skin tests (TST) are performed by intradermal injection of purified protein derivate (PPD), produced and sterilized from multiple strains of Mycobacterium tuberculosis (MTB) (Magnusson and Bentzon, 1958), and are clinically used to evaluate immune sensitization by MTB or the BCG (Bacillus Calmette-Guérin) vaccine. In sensitized individuals a delayed type hypersensitivity (DTH) reaction is induced at the injection site. The sequence of events leading to the local immune reaction associated with a positive TST is incompletely defined but likely initiated by PPD uptake of dendritic cells (DC) in the skin for subsequent antigen presentation and stimulation of MTB-specific memory T cells (Poulter et al., 1982; Scheynius et al., 1982). DC are known to orchestrate immune reactions occurring at the interface of innate and adaptive immunity (Banchereau et al., 2000). There are several distinct subsets of DC described based on lineage origin and anatomical location. DC resident in the skin are almost exclusively of the myeloid lineage. Recently, a distinctly different subset, plasmacytoid dendritic cells (pDC), normally absent in skin, were shown to migrate to inflamed skin lesions of autoimmune reactions such as psoriasis (Albanesi et al., 2010; Nestle et al., 2005) and lupus erythematosus (Farkas et al., 2001; Meller et al., 2005), as well as to virally induced lesions of herpes simplex (Donaghy et al., 2009; Peng et al., 2009) and varicella (Gerlini et al., 2006; Huch et al., 2010). pDC are thought to play a central role in the response to viruses through their capacity to produce high amounts of IFNα, which is primarily induced by microbial nucleic acids signalling via Toll-Like Receptor (TLR) 7 and 9 (Gilliet et al., 2008). In addition, pDC can produce IFNα in response to human (self) DNA or RNA if complexed to the antimicrobial peptide LL37 (Ganguly et al., 2009; Lande et al., 2007). This complex formation enables endocytosis of self-DNA and RNA and subsequent TLR signalling in the endosome. Thus, pDC may also regulate immune responses via IFNα in the absence of pathogen exposure.
In this study, we investigated the presence and relative roles of distinct DC subsets in the skin in the TST reaction. In biopsies from healthy individuals displaying a positive TST, we found a large infiltration of several DC subsets expressing markers associated with myeloid DC (mDC) and pDC. This was accompanied by upregulation of LL37 and an increased expression of multiple markers for cell death suggesting the release of self nucleic acids. No infiltration of cells was found after PPD injection in individuals with a negative TST, nor did PPD exposure activate DC in vitro, suggesting that it is the milieu in the positive TST reaction that leads to recruitment of DC. Supernatants from LL37/DNA-activated pDC in vitro reduced the otherwise high uptake of PPD by mDC, induced their maturation as well as altered the activation of T cell proliferation and cytokine production profile. Infiltrating pDC may therefore exhibit a regulatory function to control the level of antigen-presentation and immune activation in the TST reaction.
Results
DC infiltrate the TST site
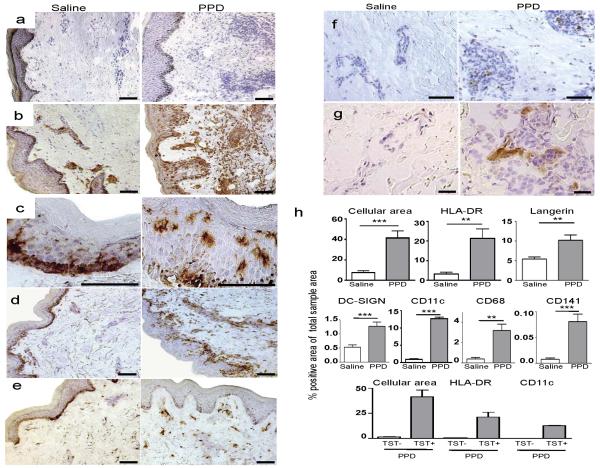
Cryosections of skin punch biopsies taken at 48 hours after injection of PPD, or saline, in healthy donors with a positive TST reaction were analyzed by immunohistochemistry and quantitative image analysis. Inflammation was observed in all positive TST sites as evidenced by a significant increase in cellularity quantified by the area of hematoxylin-stained nuclei in the tissue (Figure 1a, h, p=0.0008, n=7). The efficiency of DC to present PPD antigen to CD4+ and CD8+ T cells, which are known to infiltrate the positive TST reaction (Platt et al., 1983; Poulter et al., 1982; Scheynius et al., 1982), likely depends on both recruitment of DC to the skin and their antigen processing capacity. To evaluate the degree of DC influx to the TST site, we first assessed the level of MHC class II (HLA-DR) expression. The percentage of HLA-DR+ area out of the total area of the dermis was found to be significantly higher in the TST reaction than in the donor-matched saline injected control sites (Figure 1b, h, p=0.0019, n=9). In the controls, the majority of HLA-DR+ cells were typically present in clusters in the dermis. In contrast, the HLA-DR+ cells in the TST reaction were scattered throughout both the dermis and epidermis, though predominantly in the pericapillary areas. To further characterize these cells, a series of well-defined markers were used. The C-type lectin receptor (CLR) Langerin, specific for Langerhans cells, was constitutively present in the epidermis (Valladeau et al., 2003), and significantly upregulated in the TST reaction (Figure 1c, h, p=0.0061, n=8). DC-SIGN was constitutively expressed in dermis, and significantly increased in the TST reaction (Figure 1d, h, p=0.0006, n=8). Furthermore, CD11c (Figure 1e, h, p≤0.0001, n=8) and CD68 (Figure 1f, h, p=0.0013, n=10), expressed by DC of the myeloid lineage as well as macrophages were almost exclusively present in the dermis and dramatically increased in the TST sites as compared to controls. CD141 (BDCA-3), recently reported to define a distinct myeloid DC subset (Jongbloed et al., 2010), was absent or expressed at very low levels in the controls but was detected in all TST biopsies (Figure 1g, h, p=0.0009, n=10). In accordance with that PPD is designed to not induce immune-activation in individuals lacking pre-existing immunity, there was no infiltration after injection of individuals displaying a negative TST as evidenced by the absence of increased cellularity or expression of HLA-DR and CD11c (Figure 1h). From these results, we concluded that multiple DC of the myeloid lineage infiltrated the positive TST indurations.
Figure 1. PPD-injected sites show increased cellularity and infiltration of DC.
Cryosections of donor-matched skin punch biopsies of the TST site were taken 48 hours post-injection. Representative images are shown of saline injected versus PPD injected sites from TST+ donors. Increased cellularity (a) as measured by cell nuclei counterstained blue with hematoxylin, and increased expression (brown) of HLA-DR (b), Langerin (c), DC-SIGN (d), CD11c (e), CD68 (f) and CD141/BDCA-3 (g) at the TST site. All scale bars = 25μm. Note that the dark basal membrane represents melanin-rich cells and not positive staining. (h) Bar graphs show mean ± SEM. Data collected from image analysis of dermis only, except for Langerin where epidermis only was analyzed.
pDC are recruited to the positive TST site
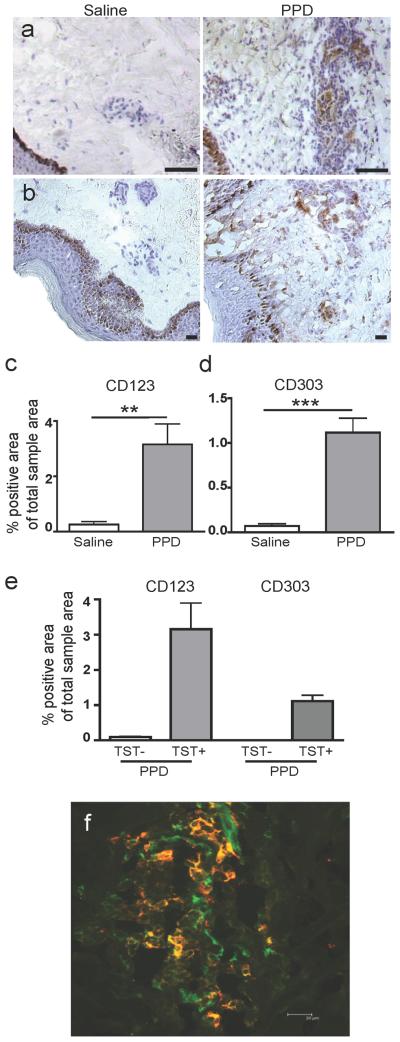
As mentioned, pDC do not usually reside in the skin. This was confirmed by rare or absent CD123 expression in the saline controls. In contrast, CD123+ pDC were present in all positive TST biopsies (p=0.0043, n=10, Figure 2a, c). The expression of CD123 was confined to the dermis and mainly found in clusters where distinct individual cells with high CD123 expression were discernible. In addition, there was occasionally low intensity CD123 staining in streak formations, presumably representing endothelial cells as described (Hirbod et al., 2009). Another pDC marker, CD303 (BDCA-2), was also significantly upregulated in the TST sites (p≤0.0001, n=9, Figure 2b, d), and was highly co-expressed with CD123 (Figure 2f). However, CD303 was less prevalent consistent with its documented downregulation on activated pDC (Figdor et al., 2002). No increase in CD123 or CD303 expression was detected, indicating an absence of pDC recruitment, in biopsies from individuals with a negative TST (Figure 2e).
Figure 2. Recruitment of CD123+CD303+ pDC to the TST site.
pDCs were detected by expression of both CD123 (a and c) and CD303 (BDCA-2) (b and d), and were significantly increased at the PPD injection site of TST+ individuals. The expression was restricted to the dermis. Scale bars = 25μm. All graphs show mean ± SEM. (e) pDC were not recruited to the site of PPD injection in TST-individuals. (f) Representative immunofluorescent co-staining (which appear yellow) of CD123 (green) and CD303 (red) to confirm the presence of pDC at the TST site.
High expression of the IFNα inducible protein MxA and cell death markers in the TST reaction
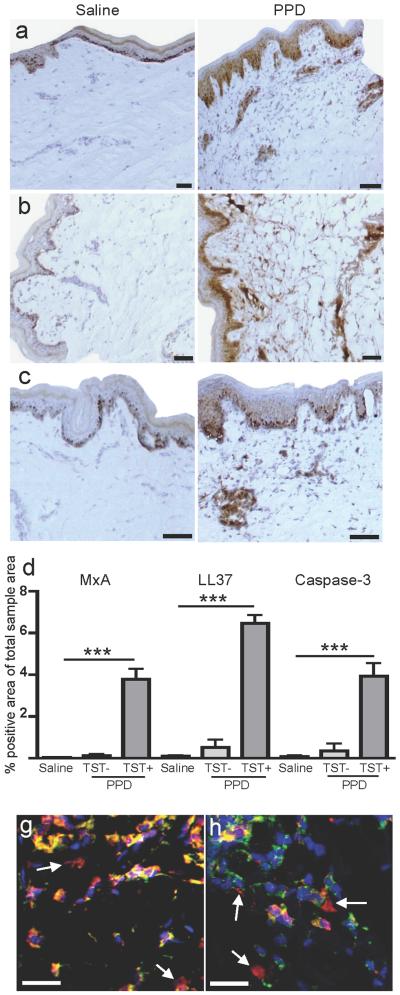
Despite the infiltration of pDC in response to the positive TST, IFNα was not detected (data not shown). In contrast, staining of MxA, a GTPase specifically induced by IFNα/β exposure (Fah et al., 1995; Simon et al., 1991), was readily detected after PPD injection whereas it was undetectable in the saline controls (Figure 3a, d, p≤0.0001, n=9). Since the presence of MxA strongly implies production of IFNα/β, the time of biopsy collection or production levels may explain the lack of IFNα detection. MxA was present in the dermis, in close proximity to CD123+ CD303+ pDC, but also in the epidermis. It is plausible that pDC-produced IFNα/β can diffuse through the tissue inducing upregulation of MxA. Alternatively, cells in the epidermis distinct from pDC may have produced IFNα. IFNα production by pDC in psoriatic lesions, a non-pathogen-containing yet inflammatory milieu, was recently demonstrated to be induced by self-DNA or RNA from dying cells in complex with the antimicrobial peptide LL37 (Ganguly et al., 2009; Lande et al., 2007). Analyses of the expression levels of LL37 at the TST sites showed a strong upregulation in dermis, compared to a low constitutive expression in the controls (Figure 3b, d, p≤0.0001, n=10). With regard to cell death, the levels of active Caspase-3, primarily indicating apoptosis (Duan et al., 2003), were highly upregulated in the positive TST site (Figure 3c-d, p=0.0002, n=9). PPD injection per se induced no or a very small increase in MxA, LL37 or Caspase-3 expression, as shown in donors with negative TST reactions (Figure 3d). High intracellular as well as extracellular expression of high-mobility group protein B 1 (HMGB-1) (Figure 3e) and lactate dehydrogenase (LDH) (Figure 3f) in the TST sites indicated leakage of these proteins due to loss of cell membrane integrity associated with necrosis in particular (Duan et al., 2003; Morishima et al., 2010; Scaffidi et al., 2002; Yun et al., 2008). This cell leakage implies a simultaneous release of self nucleic acids from cells undergoing necrosis or secondary apoptosis in the TST reaction, which could thus be available for complex formation with the high levels of LL37.
Figure 3. Presence of potential stimuli for IFNα production at the TST site.
(a, d) High expression of the IFNα inducible protein MxA in the TST site but not the saline site was detected. (b, d) High levels of LL37 were present in the dermis of the TST sites. In addition, Caspase-3, associated with cell death, was also increased (c-d). These increases were not seen in TST-individuals. Representative images of (e) HMGB-1 (red) and (f) LD (red) together with HLA-ABC (green) and DAPI (blue). Arrows denote extracellularly located HMGB-1 and LD as a possible consequence of leakage out of necrotic cells. Scale bars = 25μm. All graphs show mean ± SEM.
Differential capacity for PPD uptake in DC subsets
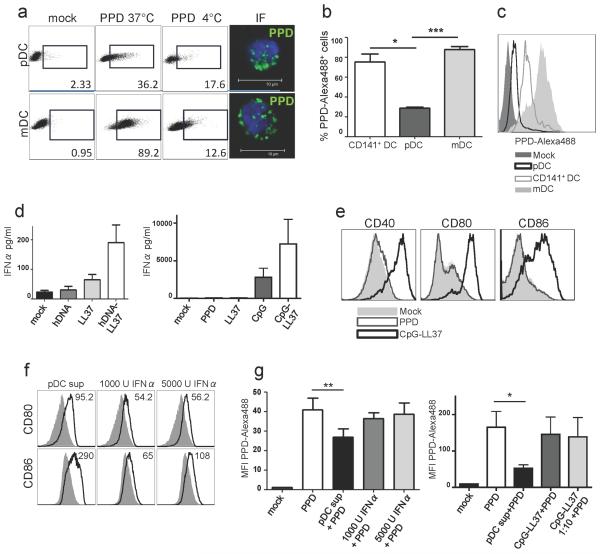
To investigate DC functions pertaining to uptake and processing of PPD for antigen presentation and whether a milieu of LL37-DNA complexes influenced this, we performed experiments in vitro using isolated DC subsets. pDC are generally considered to be inefficient at antigen presentation compared to other DC subsets, which may partially relate to lesser capacity to internalize antigens (Villadangos and Young, 2008). To this end, CD123+ pDC and CD11c+ mDC were pulsed with fluorescently-labeled PPD for two hours. The internalization of PPD was assessed by comparing PPD binding at 4°C with active uptake at 37°C by flow cytometry as well as by verifying intracellular punctuate signals by microscopy (Figure 4a). pDC showed significantly lower uptake of PPD compared to both conventional mDC (p=0.0008) and cross-presenting CD141+ DC (Figure 4b-c, p=0.02, n=3).
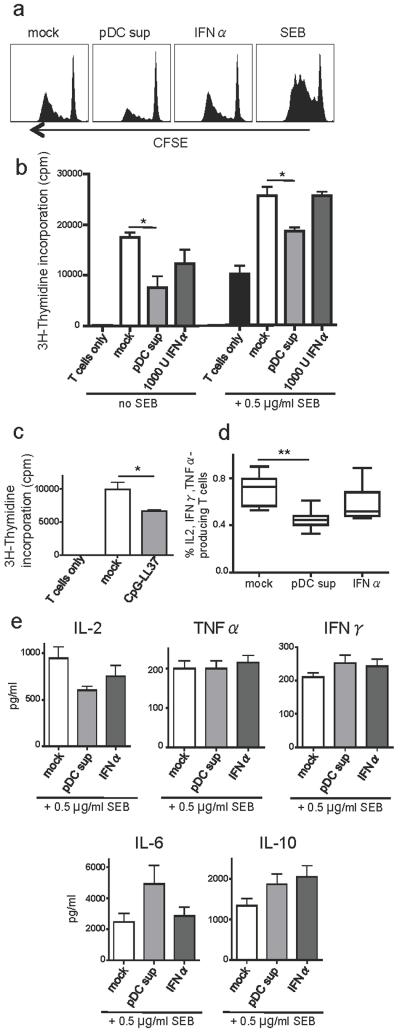
Figure 4. LL37/DNA-activated pDC affect mDC phenotype and function.
(a) Gates indicate percentages of cells with binding (4°C) and/or uptake (37°C) of PPD-Alexa488. Single plane confocal images show intracellular PPD-Alexa488 in DC pulsed at 37°C. (b) Bar graph shows mean ± SEM of mean fluorescence intensity values (MFI) of PPD-Alexa488 uptake, n=3 (c) Representative histogram of the PPD uptake. (d) IFNα production of pDC exposed to complexes of LL37 and human or CpG DNA. (e) Phenotypic maturation of pDC exposed to LL37-DNA. (f) Phenotypic maturation in mDC exposed overnight to supernatants from stimulated pDC. Solid grey lines represent unexposed mDC. Numbers indicate MFI. (g) mDC pulsed with PPD-Alexa488 for 2 hours after overnight stimulation.
LL37-DNA stimulated pDC can regulate mDC and T cell function
In line with earlier reports (Ganguly et al., 2009; Lande et al., 2007), we found that IFNα secretion by pDC exposed to complexes of LL37 and human DNA was enhanced compared to the low levels found in response to either LL37 or DNA alone (Figure 4d). This was even more pronounced using complexes of LL37 and synthetic CpG ODN. LL37-DNA complexes also induced phenotypic maturation in pDC as evidenced by upregulation of CD40, CD80 and CD86 (Figure 4e). Exposure of pDC to PPD alone did not induce IFNα or maturation. mDC did not show any detectable IFNα or maturation in response to either PPD or LL37-DNA (data not shown). In contrast, mDC matured when exposed to supernatants from LL37-DNA exposed pDC (Figure 4f). Further, the high uptake of PPD by mDC was significantly reduced when cultured in the presence of pDC supernatants (Figure 4g). This reduced capacity to internalize PPD was unlikely due to IFNα alone since the addition of recombinant IFNα at similar levels as in the pDC supernatants did not reproduce this effect. Importantly, LL37-DNA complexes alone did not reduce PPD uptake in mDC (data not shown). The decreased PPD uptake by mDCs caused by pDC supernatants is unlikely to exclusively relate to the functional transformation associated with maturation since the effect was observed as early as after two hours of incubation (data not shown). Finally, mDC exposed to pDC supernatants showed significantly reduced capacity to stimulate allogeneic CD4+ T cell proliferation as evidenced by both CFSE dilution (Figure 5a, representative of 7 donors) and thymidine incorporation assays (Figure 5b, n=4, p=0.02). Again, IFNα alone did not have the same effect. LL37/DNA-exposed pDC also directly suppressed T cells proliferation found in pDC:T cell co-cultures (Figure 5c, p=0.035, n=4). In addition to reduced proliferation, T cells co-cultured with mDC exposed to pDC supernatant showed altered production of effector cytokines IL-2, TNFα and IFNγ as measured by intracellular cytokine staining (Figure 5d, p=0.0036, n=8). Further separate assessment showed that the production of IL-2 was particularly reduced while the levels of TNFα and IFNγ remained unchanged (Figure 5e). This concurred with elevated production of IL-6 and IL-10. Thus, pDC activated by LL37/DNA complexes in the TST site may acquire a function to regulate immune activation by directly or indirectly skewing cytokine production and controlling proliferation of T cells.
Figure 5. LL37/DNA-activated pDC suppress the T cell stimulatory capacity of mDC.
T cell proliferation as measured by (a) CFSE dilution and (b) thymidine-incorporation in allogeneic CD4+ T cells after 5 days of co-culture with mDC exposed overnight to indicated stimuli. (c) T cell proliferation after 5 days of co-culture of T cells and pDC exposed to indicated stimuli. (d) Frequencies of cytokine (IL-2, TNFα, IFNγ) producing CD4+ T cells measured by intracellular staining after co-culture for 16 hours with mDC exposed overnight to the indicated stimuli and (e) levels of IL-2, IL-6, IL-10, TNFα and IFNγ in supernatants from mDC-T cell co-cultures pulsed with SEB.
Discussion
pDC have emerged as a highly specialized DC subset, that has been shown to possess poorer antigen uptake and presentation capacity compared to other DC subsets, but instead equipped with a high capacity to produce IFNα/β (Cella et al., 1999). Thus, their role in controlling viral infections has been particularly explored. However, pDC have also shown to exert other immuno-regulatory properties not involving pathogen responses (Swiecki and Colonna, 2010). Indeed, pDC were initially described for their role in maintaining tolerance. High levels of pDC were shown to represent a good prognostic factor for discontinuation of immunosuppressive treatment after liver and stem cell transplantation and may hence be important to prevent transplant rejection or graft versus host disease, respectively (Mazariegos et al., 2003; Rajasekar et al., 2008). To this end, pDC were shown to mediate oral tolerance to food antigens by suppressing CD4+ and CD8+ T cell-specific DTH responses (Goubier et al., 2008). The TST reaction examined in this study also represents a DTH. A role for DC in the development of positive TST reactions was early implied (Beck, 1991; Sarrazin et al., 2009; Scheynius et al., 1982). Our data indicate that there is a rapid and rather robust infiltration of several DC subsets including pDC in the positive TST reaction. As this was not found in negative TST, the inflammation induced locally by infiltrating, presumably PPD-specific, T cells likely regulates the pDC recruitment. Although pDC are not normally located in the skin, they are recruited to allergic contact hypersensitivity reactions (Bangert et al., 2003) as well as the autoimmune reactions associated with lupus erythematosus (Farkas et al., 2001; Meller et al., 2005) and psoriasis (Albanesi et al., 2010; Nestle et al., 2005). In the latter, infiltrated pDC were shown to produce IFNα. The classical stimuli that induce high levels of IFNα in pDC are nucleic acids derived from microbes activating cells via intracellular TLR signaling (Akira et al., 2006; Stacey et al., 2003). However, recent reports show that pDC can react to human RNA or DNA if they are presented as complexes with (the antimicrobial peptide) LL37 (Ganguly et al., 2009; Lande et al., 2007). These studies prompted our further investigation of whether LL37 was upregulated at the TST sites and if this occurred simultaneously with cell death leading to release of nucleic acid material. Based on our data showing high levels of LL37 and signs of necrotic tissue, we speculate that LL37-DNA complexes may also be available and activate pDC that infiltrate the TST sites. In this regard, pDCs could also be activated by complexes formed by self-DNA and the high levels of HMGB-1 at the TST site (Tian et al., 2007). Due to the quite distinct differences in the characteristics of DC subsets, they are likely to exhibit different and complementary functions to generate, enhance or suppress immune responses. pDC showed significantly lower capacity to take up PPD than the mDC subsets. This included the distinct CD141+ mDC subset with superior cross-presenting capacity (Bachem et al., 2010; Jongbloed et al., 2010; Villadangos and Shortman, 2010). Infiltration of CD141+ DC in response to antigen delivery and inflammation in the skin has not previously been described. Since pDC showed much lower uptake of PPD, their primary contribution in the TST reaction may not pertain to antigen processing and presentation to T cells. Instead, they may have an important bystander effect of conditioning surrounding mDC subsets. This bystander effect could consist of a suppressor function to restrain otherwise potentially harmful, excessive immune activation. While pDC have been identified to mediate immune responses in skin inflammation in psoriasis (Lande et al., 2007) and wound healing (Gregorio et al., 2010), they may also function to regulate the magnitude of the responses. On this note, pDC have shown different immune-stimulatory capacities at different differentiation/activation stages (Bjorck et al., 2011; Hadeiba et al., 2008; Schwab et al., 2010). The nature of the antigen likley also play a role in shaping pDC function.
We found that supernatants from pDC stimulated by LL37-DNA-complexes reduced the high capacity of mDC to take up PPD. This reduction may in part be mediated by IFNα though it was evident in our experiments that this it was not the sole factor. Anti-IFNα/β antibodies have been shown to block maturation of mDC (Ganguly et al., 2009) and thus IFNβ could potentially also mediate this effect. Here, mDC cultured in the presence of pDC supernatants showed reduced capacity to induce effector cytokine production and proliferation of allogeneic CD4+ T cells. Type I IFN produced by pDC have been shown to limit T cell proliferation (Chi et al., 2006), but again the inhibition we observed was not evident with exposure to recombinant IFNα alone suggesting that there are alternative suppressive mediators released from the pDC. Taken together, our data suggest that mDC subsets would take up more PPD delivered at the site of injection than pDC, and consequently are most likely to perform the majority of the antigen presentation to activate antigen-specific memory T cells. pDC could still contribute to these functions, but as mDC are by far the most frequent DC subsets at the site, even after PPD injection, the uptake and presentation by pDC may be negligible. Instead, pDC activated by components from the local inflammation and tissue-destruction may partake in controlling the magnitude of immune responses by directly exerting a regulatory effect on T cell activation or by influencing the functions of mDCs. In our in vitro studies we found that LL37/DNA activated pDC can suppress T cell proliferation and skew the cytokine production. To this end, pDC have under other culture conditions shown to restrain T cell activation by inducing T regulatory cells (Moseman et al., 2004; Varani et al., 2007). However, these interactions remain to be shown in the skin in vivo. Also, the kinetics by which the different DC subsets and T cells are recruited after PPD injection could influence the regulation of T cell activation but are still to be determined. In conclusion, our data further elucidate the emerging multi-facetted roles that pDCs play in shaping immune responses. Understanding the different roles of the specific DC subsets infiltrating the skin is critical for defining the responses elicited to pathogens targeting the skin as well as for the development of new therapies, vaccines, and delivery strategies.
Materials and Methods
Collection of human skin punch biopsies
Written informed consent was obtained from all study subjects. The Institutional Review Boards of Ethics of the Karolinska Institutet and the University of Cape Town approved this study. Study subjects were recruited in Khayelitsha Township, Cape Town, South Africa. Exclusion criteria are earlier described (Rangaka et al., 2007) and included previously diagnosed TB, HIV infection, and/or presentation of one of several symptoms e.g. cough, chest pain or fever. TST was performed according to international standards and considered positive at ≥10 mm. The study subjects who displayed a positive TST reaction showed mean 18.6±2.3 mm. Punch biopsies were taken as described (Sarrazin et al., 2009) from the PPD injection site and a saline injected site on the opposite arm at 48 hours and snap frozen.
Immunohistochemical staining and quantitative image analysis
Cryosections (8 μm) of skin biopsies were stained as described (Lore et al., 2002) with anti-HLA-DR, CD11c, CD123, CD8, active Caspase-3 (BD Biosciences, San José, CA), CD68 (Dako, Glostrup, Denmark), CD303 (BDCA-2), DC-SIGN, Langerin (R&D Systems, Minneapolis, MN), LL37 (Innovagen, Lund, Sweden), CD141 (BDCA-3) (Miltenyi Biotech, Auburn, CA), MxA (Prof. Haller and Kochs, University of Freiburg, Germany), followed by secondary biotinylated antibodies (Abs) i.e. anti-mouse, anti-goat (Dako) or anti-rabbit (Vector Laboratories, Burlingame, CA). The secondary Abs were detected with the peroxidase-based Vectastain Elite ABC kit (Vector Laboratories), and the reaction developed by diaminobenzidine tetrahydrochloride (DAB) peroxidase substrate kit (Vector Labo-ratories). Cell nuclei were counterstained with Mayer’s Haematoxylin (Histolab Products, Gothenburg, Sweden). The sections were analyzed by a Leica DMR-X microscope (Leica Microsystems GmbH, Wetzlar, Germany) coupled to computerized image analysis (Leica Qwin 5501W, Leica Imaging Systems) as described (Bjork et al., 1996). The epidermis was excluded from the analysis of all markers except for Langerin where the dermis was excluded. Analyses were confirmed by at least two separate investigators.
Fluorescent co-staining
The stainings were performed as above with modifications. Following the incubation with the secondary biotinylated Abs, streptavidin (SA)-conjugated Alexa488 or Alexa647 (Molecular Probes, Eugene, OR) were added or, alternatively, Alexa594 conjugated secondary Abs were used. In addition to the cell specific Abs mentioned above, anti-HLA-ABC (Dako), Lactate Dehydrogenase (Abcam, Cambridge, UK), HMGB-1 (BD) and Alexa594-labeled anti-goat (Molecular Probes) were used. Sections were mounted using SlowFade® Gold antifade regent with DAPI (Invitrogen) for nuclear staining.
Isolation of blood pDC and mDC
Primary DC were purified from blood as described (Adams et al., 2009; Douagi et al., 2009). In short, pDC and mDC were isolated from elutriated monocytes by anti-BDCA-4 and anti-CD1c microbeads (Miltenyi) and AutoMACS separation (Miltenyi). pDC and mDC were cultured in RPMI1640 with 10% fetal calf serum (Sigma-Aldrich, Schelldorf, Germany) supplemented with IL-3 (1 ng/ml; R&D Systems) and GM-CSF (2 ng/ml; PeproTech, Rocky Hill, NJ), respectively.
Stimulation of pDC with LL-37-DNA complexes
Complexes were formed by co-incubation of either 10 μg/ml human DNA (Biochain Institute Inc., Hayward, CA) or 5 μg/ml CpG ODN (class B 10103, Coley Pharmaceutical Group GmbH, Düsseldorf, Germany) with 10-50 μg/ml LL37 (Innovagen, Lund, Sweden) for 30 min at RT. pDC were exposed to the complexes at 1×106/ml for 16 h at 37°C. Cell-free supernatants were harvested and analyzed. Supernatants of pDC stimulated by CpG-LL37 complexes were used for culture experiments with mDC.
Analysis of cytokine production and maturation of DC
IFNα was measured in supernatants from pDC by ELISA (VeriKine™, PBL Interferon Source, NJ). For analysis of phenotypic maturation, isolated DC were stained with combinations of Abs, including CD1c, CD11c, CD123, CD80, CD83, CD86 as described (Lore et al., 2005). The cells were collected on a FACSCalibur flow cytometer (Becton Dickinson) and data was analyzed using FlowJo software (Treestar Inc., San Carlos, CA). Luminex assays (Human Cytokine 10-Plex Panel, Invitrogen), for analysis of cytokines in supernatants from DC and DC:T cell cultures, were performed according to the manufacturers instructions and analyzed by Luminex 200™ system (Invitrogen).
Evaluation of PPD uptake capacity
PPD (Statens Serum Institut, Copenhagen, Denmark) was labelled by Alexa488 protein labelling kit (Molecular Probes). Isolated pDC and mDC were pulsed with 4 μg/ml PPD-Alexa488 for 2 h at 37°C or 4°C and analyzed by flow cytometry. Alternatively, 2×106 cells of monocyte-enriched populations (Rosette Separation, StemCell technologies, Grenoble, France) were exposed to PPD-Alexa488 for 2 h and cells expressing CD1c, CD123 or CD141 were analyzed. For confocal microscopy imaging, sorted pDC and mDC were transferred to adhesion slides (BioRad Lab, Munich, Germany) as described (Bond et al., 2009). The slides were mounted with Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories) for nuclear staining.
Functional assays of pDC effects on mDC
IFNα at 1000-5000 U/ml or supernatants from pDC at 1:1 were added to cultures of mDC. mDC were incubated for 24 h, washed, exposed to PPD-Alexa488 for 2 h and analyzed for PPD uptake and CD80/CD86 expression. For analysis of T cell stimulatory capacity, pDC or mDCs were co-cultured with unlabeled or CFSE-labeled, purified allogeneic CD4+ T cells at a ratio of 1:10 for 16 h for detection of IL-2, TNFα and IFNγ expression by intracellular staining and FACS analysis or for 5 days for assessment of proliferation as described (Lore et al., 2005). Alternatively, proliferation was measured by thymidine incorporation as described (Gujer et al., 2011).
Statistical analyses
Data was analyzed by Student’s t test, with GraphPad Prism software (San Diego, CA), and considered significant at *p≤0.05, **p≤0.01 and ***p≤0.001.
Acknowledgements
The authors thank Sayma Rahman, Linda Johansson and Gunilla Karlsson-Hedestam (Karolinska Institutet) for valuable advice. KL is supported by grants from Vetenskapsrådet, the Swedish International Development Agency (Sida), the Swedish Society of Medicine, and Swedish Physicians Against AIDS Foundation. RJW and MXR are supported by the Wellcome Trust and the Medical Research Council (UK), and the H.W. and J. Hector Foundation for logistic and shipping costs.
Nonstandard abbreviations
- pDC
plasmacytoid dendritic cell
- mDC
myeloid dendritic cell
- PPD
purified protein derivate
- TST
tuberculin skin test
- hDNA
human DNA
- HMGB-1
high mobility group B 1
- LDH
lactate dehydrogenase
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Adams WC, Bond E, Havenga MJ, Holterman L, Goudsmit J, Karlsson Hedestam GB, et al. Adenovirus serotype 5 infects human dendritic cells via a coxsackievirus-adenovirus receptor-independent receptor pathway mediated by lactoferrin and DC-SIGN. J Gen Virol. 2009;90:1600–10. doi: 10.1099/vir.0.008342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Albanesi C, Scarponi C, Bosisio D, Sozzani S, Girolomoni G. Immune functions and recruitment of plasmacytoid dendritic cells in psoriasis. Autoimmunity. 2010;43:215–9. doi: 10.3109/08916930903510906. [DOI] [PubMed] [Google Scholar]
- Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–81. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Bangert C, Friedl J, Stary G, Stingl G, Kopp T. Immunopathologic features of allergic contact dermatitis in humans: participation of plasmacytoid dendritic cells in the pathogenesis of the disease? J Invest Dermatol. 2003;121:1409–18. doi: 10.1111/j.1523-1747.2003.12623.x. [DOI] [PubMed] [Google Scholar]
- Beck JS. Skin changes in the tuberculin test. Tubercle. 1991;72:81–7. doi: 10.1016/0041-3879(91)90033-o. [DOI] [PubMed] [Google Scholar]
- Bjorck P, Leong HX, Engleman EG. Plasmacytoid dendritic cell dichotomy: identification of IFN-alpha producing cells as a phenotypically and functionally distinct subset. J Immunol. 2011;186:1477–85. doi: 10.4049/jimmunol.1000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork L, Fehniger TE, Andersson U, Andersson J. Computerized assessment of production of multiple human cytokines at the single-cell level using image analysis. J Leukoc Biol. 1996;59:287–95. doi: 10.1002/jlb.59.2.287. [DOI] [PubMed] [Google Scholar]
- Bond E, Adams WC, Smed-Sorensen A, Sandgren KJ, Perbeck L, Hofmann A, et al. Techniques for time-efficient isolation of human skin dendritic cell subsets and assessment of their antigen uptake capacity. J Immunol Methods. 2009;348:42–56. doi: 10.1016/j.jim.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Chi B, Dickensheets HL, Spann KM, Alston MA, Luongo C, Dumoutier L, et al. Alpha and lambda interferon together mediate suppression of CD4 T cells induced by respiratory syncytial virus. J Virol. 2006;80:5032–40. doi: 10.1128/JVI.80.10.5032-5040.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaghy H, Bosnjak L, Harman AN, Marsden V, Tyring SK, Meng TC, et al. Role for plasmacytoid dendritic cells in the immune control of recurrent human herpes simplex virus infection. J Virol. 2009;83:1952–61. doi: 10.1128/JVI.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douagi I, Gujer C, Sundling C, Adams WC, Smed-Sorensen A, Seder RA, et al. Human B cell responses to TLR ligands are differentially modulated by myeloid and plasmacytoid dendritic cells. J Immunol. 2009;182:1991–2001. doi: 10.4049/jimmunol.0802257. [DOI] [PubMed] [Google Scholar]
- Duan WR, Garner DS, Williams SD, Funckes-Shippy CL, Spath IS, Blomme EA. Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J Pathol. 2003;199:221–8. doi: 10.1002/path.1289. [DOI] [PubMed] [Google Scholar]
- Fah J, Pavlovic J, Burg G. Expression of MxA protein in inflammatory dermatoses. J Histochem Cytochem. 1995;43:47–52. doi: 10.1177/43.1.7822763. [DOI] [PubMed] [Google Scholar]
- Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon-alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol. 2001;159:237–43. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206:1983–94. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlini G, Mariotti G, Bianchi B, Pimpinelli N. Massive recruitment of type I interferon producing plasmacytoid dendritic cells in varicella skin lesions. J Invest Dermatol. 2006;126:507–9. doi: 10.1038/sj.jid.5700052. [DOI] [PubMed] [Google Scholar]
- Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–75. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorio J, Meller S, Conrad C, Di Nardo A, Homey B, Lauerma A, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921–30. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujer C, Sandgren KJ, Douagi I, Adams WC, Sundling C, Smed-Sorensen A, et al. IFN-{alpha} produced by human plasmacytoid dendritic cells enhances T cell-dependent naive B cell differentiation. J Leukoc Biol. 2011 doi: 10.1189/jlb.0810460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253–60. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirbod T, Kaldensjo T, Lopalco L, Klareskog E, Andersson S, Uberti-Foppa C, et al. Abundant and superficial expression of C-type lectin receptors in ectocervix of women at risk of HIV infection. J Acquir Immune Defic Syndr. 2009;51:239–47. doi: 10.1097/QAI.0b013e3181a74f89. [DOI] [PubMed] [Google Scholar]
- Huch JH, Cunningham AL, Arvin AM, Nasr N, Santegoets SJ, Slobedman E, et al. Impact of varicella-zoster virus on dendritic cell subsets in human skin during natural infection. J Virol. 2010;84:4060–72. doi: 10.1128/JVI.01450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–60. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- Lore K, Smed-Sorensen A, Vasudevan J, Mascola JR, Koup RA. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J Exp Med. 2005;201:2023–33. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lore K, Sonnerborg A, Brostrom C, Goh LE, Perrin L, McDade H, et al. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS. 2002;16:683–92. doi: 10.1097/00002030-200203290-00003. [DOI] [PubMed] [Google Scholar]
- Magnusson M, Bentzon MW. Preparation of purified tuberculin RT 23. Bull World Health Organ. 1958;19:829–43. [PMC free article] [PubMed] [Google Scholar]
- Mazariegos GV, Zahorchak AF, Reyes J, Ostrowski L, Flynn B, Zeevi A, et al. Dendritic cell subset ratio in peripheral blood correlates with successful withdrawal of immunosuppression in liver transplant patients. Am J Transplant. 2003;3:689–96. doi: 10.1034/j.1600-6143.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- Meller S, Winterberg F, Gilliet M, Muller A, Lauceviciute I, Rieker J, et al. Ultraviolet radiation-induced injury, chemokines, and leukocyte recruitment: An amplification cycle triggering cutaneous lupus erythematosus. Arthritis Rheum. 2005;52:1504–16. doi: 10.1002/art.21034. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Kawashima H, Takekuma K, Hoshika A. Changes in serum lactate dehydrogenase activity in children with atopic dermatitis. Pediatr Int. 2010;52:171–4. doi: 10.1111/j.1442-200X.2009.02908.x. [DOI] [PubMed] [Google Scholar]
- Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–42. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–43. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Zhu J, Klock A, Phasouk K, Huang ML, Koelle DM, et al. Evasion of the mucosal innate immune system by herpes simplex virus type 2. J Virol. 2009;83:12559–68. doi: 10.1128/JVI.00939-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt JL, Grant BW, Eddy AA, Michael AF. Immune cell populations in cutaneous delayed-type hypersensitivity. J Exp Med. 1983;158:1227–42. doi: 10.1084/jem.158.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter LW, Seymour GJ, Duke O, Janossy G, Panayi G. Immunohistological analysis of delayed-type hypersensitivity in man. Cell Immunol. 1982;74:358–69. doi: 10.1016/0008-8749(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Rajasekar R, Mathews V, Lakshmi KM, Sellathamby S, George B, Viswabandya A, et al. Plasmacytoid dendritic cell count on day 28 in HLA-matched related allogeneic peripheral blood stem cell transplant predicts the incidence of acute and chronic GVHD. Biol Blood Marrow Transplant. 2008;14:344–50. doi: 10.1016/j.bbmt.2007.12.494. [DOI] [PubMed] [Google Scholar]
- Rangaka MX, Wilkinson KA, Seldon R, Van Cutsem G, Meintjes GA, Morroni C, et al. Effect of HIV-1 infection on T-Cell-based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med. 2007;175:514–20. doi: 10.1164/rccm.200610-1439OC. [DOI] [PubMed] [Google Scholar]
- Sarrazin H, Wilkinson KA, Andersson J, Rangaka MX, Radler L, van Veen K, et al. Association between tuberculin skin test reactivity, the memory CD4 cell subset, and circulating FoxP3-expressing cells in HIV-infected persons. J Infect Dis. 2009;199:702–10. doi: 10.1086/596735. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Scheynius A, Klareskog L, Forsum U. In situ identification of T lymphocyte subsets and HLA-DR expressing cells in the human skin tuberculin reaction. Clin Exp Immunol. 1982;49:325–30. [PMC free article] [PubMed] [Google Scholar]
- Schwab N, Zozulya AL, Kieseier BC, Toyka KV, Wiendl H. An imbalance of two functionally and phenotypically different subsets of plasmacytoid dendritic cells characterizes the dysfunctional immune regulation in multiple sclerosis. J Immunol. 2010;184:5368–74. doi: 10.4049/jimmunol.0903662. [DOI] [PubMed] [Google Scholar]
- Simon A, Fah J, Haller O, Staeheli P. Interferon-regulated Mx genes are not responsive to interleukin-1, tumor necrosis factor, and other cytokines. J Virol. 1991;65:968–71. doi: 10.1128/jvi.65.2.968-971.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey KJ, Young GR, Clark F, Sester DP, Roberts TL, Naik S, et al. The molecular basis for the lack of immunostimulatory activity of vertebrate DNA. J Immunol. 2003;170:3614–20. doi: 10.4049/jimmunol.170.7.3614. [DOI] [PubMed] [Google Scholar]
- Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–62. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- Valladeau J, Dezutter-Dambuyant C, Saeland S. Langerin/CD207 sheds light on formation of birbeck granules and their possible function in Langerhans cells. Immunol Res. 2003;28:93–107. doi: 10.1385/IR:28:2:93. [DOI] [PubMed] [Google Scholar]
- Varani S, Cederarv M, Feld S, Tammik C, Frascaroli G, Landini MP, et al. Human cytomegalovirus differentially controls B cell and T cell responses through effects on plasmacytoid dendritic cells. J Immunol. 2007;179:7767–76. doi: 10.4049/jimmunol.179.11.7767. [DOI] [PubMed] [Google Scholar]
- Villadangos JA, Shortman K. Found in translation: the human equivalent of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1131–4. doi: 10.1084/jem.20100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–61. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Yun SJ, Choi MS, Piao MS, Lee JB, Kim SJ, Won YH, et al. Serum lactate dehydrogenase is a novel marker for the evaluation of disease severity in the early stage of toxic epidermal necrolysis. Dermatology. 2008;217:254–9. doi: 10.1159/000148255. [DOI] [PubMed] [Google Scholar]