Titanium enolates have found widespread use in organic synthesis as a result of their unique reactivity and ease of preparation from inexpensive and nontoxic titanium reagents under mild conditions that are compatible with a range of functional groups.[1] The most prevalent application of titanium enolates is for stereoselective aldol reactions.[2] Several examples of the alkylation of titanium enolates with strongly electrophilic reagents have also been reported.[3] Recently, we described a different mode of alkylation of titanium enolates in which they serve as efficient radical acceptors for haloalkyl radicals generated by a ruthenium-catalyzed redox process.[4] It has been postulated that the enolate serves as an electroactive ligand for the titanium center,[5] whereby it facilitates the radical addition process and participates in the ruthenium catalytic cycle. The utility of this method in the synthesis of a range of chloroleucine-derived natural products has been illustrated.[4,6] One intriguing aspect of the reaction that emerged during our investigations is the possibility of the catalytic generation and alkylation of the titanium enolate species. Herein, we describe the development of a haloalkylation process that is catalytic in both ruthenium and titanium and thus constitutes the first example of a catalytic enolate alkylation involving titanium enolates.
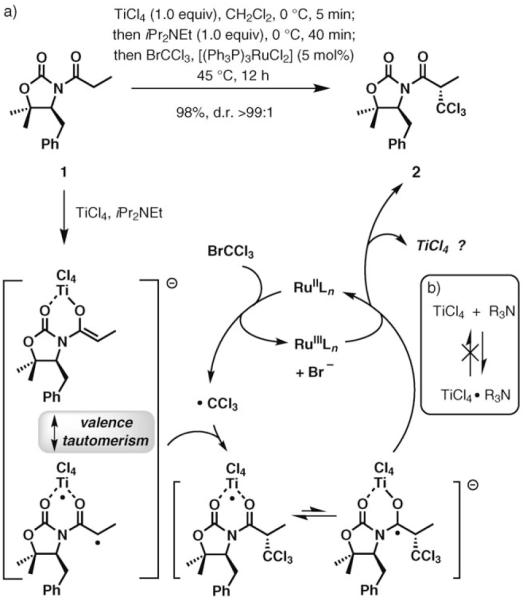
During an examination of the putative mechanism of the trichloromethylation process outlined in Scheme 1,[4,5] we directed our attention toward the function of titanium tetrachloride. This mechanism suggests that TiCl4 should be regenerated at the completion of the reaction, which creates potential for the development of a catalytic process. According to the seminal studies by Evans et al., initial complexation of the substrate and titanium tetrachloride is prerequisite to the addition of the amine base and the ensuing enolization.[3a] If the order of the reagent addition is reversed, enolization is precluded by the apparently irreversible formation of an unreactive TiCl4–amine adduct. Thus, the potential interception of the regenerated TiCl4 by the amine base would be a major challenge in the catalytic recovery of the reagent.
Scheme 1.
Radical trichloromethylation based on valence tautomerism in titanium enolates: a) an initial overview of the mechanism; b) potential sequestering of TiCl4 by the amine.
Preliminary experimentation revealed that indeed the haloalkylation reaction could reach completion with a substoichiometric amount of titanium tetrachloride under the original reaction conditions with diisopropylethylamine (Hünig base). On the other hand, the conversion decreased precipitously when less than 0.3 equivalents of TiCl4 were used (Table 1, entries 1–4). We hypothesized that the decline in conversion at around 0.3–0.4 equivalents of TiCl4 could be attributed to one of the following factors: 1) the possible formation of higher-order titanium enolates with a 2:1 or 3:1 ligand-to-metal ratio, in which case no catalytic turnover of TiCl4 would occur, or 2) inefficient catalytic recovery of the titanium reagent and, possibly, a strong inhibitory effect by the amine.
Table 1.
Influence of the amine on the course of the catalytic trichloromethylation reaction.
| Entry | TiCl4 [mol%] | Amine (equiv) | Conversion [%][a] |
|---|---|---|---|
| 1 | 40 | iPr2NEt (1.1) | >95 |
| 2 | 30 | iPr2NEt (1.1) | 75 |
| 3 | 20 | iPr2NEt (1.1) | 50 |
| 4 | 10 | iPr2NEt (1.1) | 14 |
| 5 | 30 | iPr2NEt (3.0) | 47 |
| 6 | 30 | iPr2NEt (4.5) | 28 |
| 7 | 30 | Et3N (1.1) | 76 |
| 8 | 30 | Et3N (3.0) | 100 |
| 9 | 10 | Et3N (3.0) | 75 |
| 10[b] | 10 | Et3N (3.0) | 74 |
| 11 | 10 | Et3N (4.5) | 76 |
| 12 | 30 | PMP (1.1) | 100 |
| 13 | 10 | PMP (1.1) | 100 |
Conversion was determined by 1H NMR spectroscopy of the crude mixture of products.
The reaction time was 48 h. PMP=1,2,2,6,6-pentamethylpiperidine.
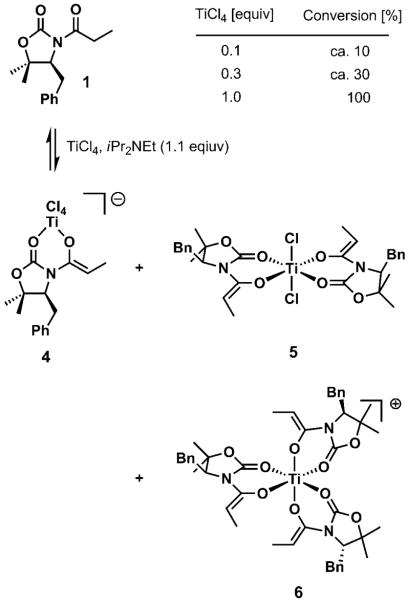
Although it is well-known that titanium forms hexa- or octacoordinated complexes,[7] higher-order titanium enolates such as those depicted in Scheme 2 have not been documented. We monitored the enolization reaction with the Hünig base (1.1 equiv) and varying amounts of titanium tetrachloride by NMR spectroscopy and found no conclusive support for the existence of higher-order titanium enolates; however, it was evident that the degree of enolization diminished proportionally with decreasing substoichiometric amounts of TiCl4. Thus, whereas complete enolization was observed with 1.0 equivalent of TiCl4, the conversion was approximately 10% with 0.1 equivalents of TiCl4 and about 30% with 0.3 equivalents of TiCl4. Notably, it could be clearly observed that more than one distinct enolate species was generated, and the relative ratios changed substantially as the amount of the titanium reagent was varied. Although the nature of the additional species is presently unclear,[8] we concluded that full conversion in the trichloromethylation reaction with a substoichiometric amount of TiCl4 could not be rationalized through the implication of higher-order titanium enolates.
Scheme 2.
Hypothetical formation of higher-order enolates during the enolization of 1 with substoichiometric TiCl4. Bn =benzyl.
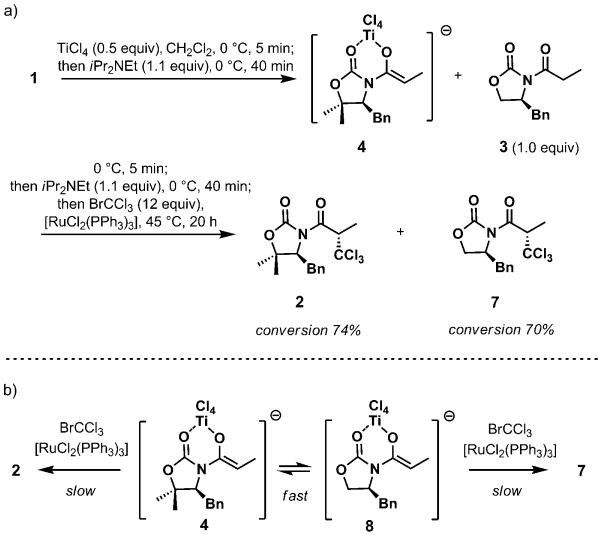
The next series of experiments was designed to probe the catalytic recovery of titanium tetrachloride. Specifically, we aimed to establish whether titanium tetrachloride could be efficiently transferred between different N-acyl oxazolidinone species once enolization was completed. The cross-over experiment in Scheme 3 was particularly illuminating and revealed a number of notable factors. When the enolization of 1 was complete (TiCl4 (0.5 equiv), Hünig base (1.1 equiv), 0°C, 45 min), the N-acyl oxazolidinone 3 (1.0 equiv) was added along with additional Hünig base (1.1 equiv), and, after 40 min at 0°C, the ruthenium-catalyzed radical trichloromethylation was initiated under the standard conditions. With the ultimate loading of titanium tetrachloride at 0.25 equivalents, products 2 and 7 resulting from the trichloromethylation of both substrates were produced in nearly equimolar amounts, which provided conclusive support for a rapid transfer of TiCl4 between different N-acyl oxazolidinone species in the presence of diisopropylethylamine. This experiment corroborates the feasibility of the catalytic turnover of TiCl4 and suggests inhibition by the amine as a reason for its poor efficiency. It can be hypothesized that a fast equilibrium is established between titanium enolates 4 and 8 (Scheme 3b). This process is accompanied by a slow radical addition, and the distribution of products 2 and 7 is determined by the Curtin–Hammett principle.
Scheme 3.
a) Cross-over experiment; b) product distribution is in accord with the Curtin–Hammett principle.
To ascertain the influence of the amine on the efficiency of the catalytic turnover of titanium tetrachloride and to probe further the equilibrium in Scheme 1b, we carried out the trichloromethylation reaction with various amounts of the Hünig base as well as less-hindered triethylamine and more-hindered 1,2,2,6,6-pentamethylpiperidine (PMP; Table 1). An increase in the concentration of the Hünig base consistently resulted in lower conversion (Table 1, entries 5 and 6), which supports the proposed inhibitory effect of the amine.
Remarkably, both less-hindered triethylamine and more-hindered PMP proved to be superior to the Hünig base for the catalytic turnover of TiCl4. Complete conversion was observed with 30 mol% of TiCl4 with 3 equivalents of Et3N, whereas conversion was 76% with 1.1 equivalents of the amine (Table 1, entries 7 and 8). The same conversion (ca. 75%) was observed with 10 mol% of TiCl4 and Et3N (3 equiv). Interestingly, no inhibitory effect was observed for triethylamine. With PMP (1.1 equiv), full conversion was observed with either 10 or 30 mol% of TiCl4 (Table 1, entries 12 and 13). As with Et3N, no inhibition of the trichloromethylation reaction was observed in the presence of excess PMP. Thus, the inhibitory effect appears to be confined to the Hünig base.
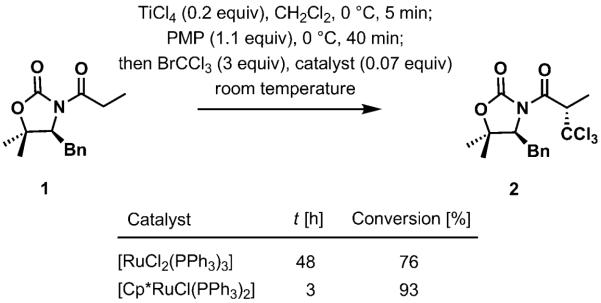
We compared the original catalyst, [RuCl2(PPh3)3], with a more active catalyst, [Cp*RuCl(PPh3)2][9], to ascertain whether the rate of trichloromethylation was limited by low catalytic turnover frequency of TiCl4 or other factors associated with the radical process (Scheme 4). When the reaction was carried out at room temperature in the presence of TiCl4 (20 mol%) and PMP (1.1 equiv), a substantial acceleration of the process was observed with [Cp*RuCl(PPh3)2] as the redox catalyst. This observation indicates that radical generation is the rate-limiting step, and both the turnover frequency of TiCl4 and the addition of ·CCl3 to the titanium enolate are relatively fast (Scheme 3b).
Scheme 4.
Comparison between [RuCl2(PPh3)3] and [Cp*RuCl(PPh3)2] as the redox catalyst for radical generation. Cp* =1,2,3,4,5-pentamethylcyclopentadienyl anion.
In the next series of experiments, the order of reagent addition during the formation of the titanium enolate was changed to examine the irreversibility of the formation of TiCl4–amine complexes under the trichloromethylation reaction conditions. The seminal studies by Evans et al. emphasized the importance of the initial TiCl4–substrate complexation; no enolate formation was observed if diisopropylethylamine or triethylamine were allowed to react with uncomplexed titanium tetrachloride first.[3a] Therefore, we were surprised to discover substantial conversion in experiments in which TiCl4 and the amine were mixed prior to the addition of the substrate (Table 2). Although the conversion was lower than that observed under the original enolization conditions, these results indicate notable, albeit rather inefficient, reversibility in the formation of TiCl4–amine adducts.
Table 2.
Influence of the inverse addition of the amines on conversion.
| Entry | Amine | T [°C] | t [h] | Conversion [%] |
|---|---|---|---|---|
| 1 | iPr2NEt | 20 | 12 | 41 |
| 2 | iPr2NEt | 45 | 12 | 45 |
| 3 | iPr2NEt | 50 | 24 | 57 |
| 4 | PMP | 20 | 12 | 57 |
| 5 | PMP | 45 | 12 | 63 |
| 6 | Et3N | 45 | 12 | 35 |
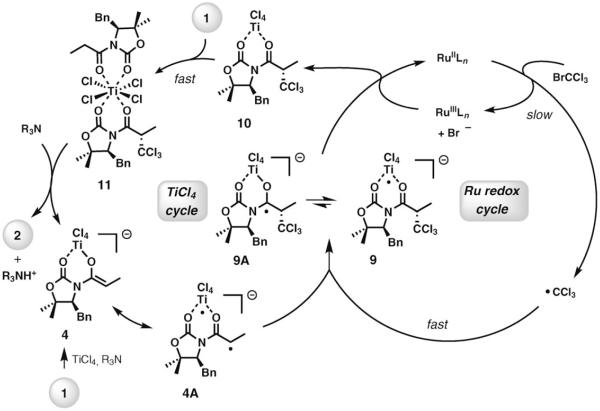
On the basis of the experimental results of this study, we propose the catalytic cycle depicted in Scheme 5 for the dual Ru–Ti catalysis in the direct trichloroalkylation of N-acyl oxazolidinones. An initial complexation of TiCl4 and substrate 1, followed by addition of the amine, produces enolate 4 along with its biradical valence tautomer 4A.[5,10] A slow, rate-limiting electron transfer from the RuII catalyst to BrCCl3 generates ·CCl3, which adds to the electron-rich titanium enolate to directly afford 9, rather than the carbon-centered radical 9A, along with the RuIII counterpart.[11] Intermediate 9, which is essentially the final product 2 complexed to a TiIII center, regenerates the RuII catalyst, possibly by another single-electron-transfer event, to give the TiIV chelate 10. At this stage, TiCl4 is transferred directly and at a relatively high rate to another molecule of the substrate, potentially via an octacoordinated species, such as 11. Alternatively, TiCl4 can be intercepted by excess amine, which accounts for the inhibitory effect of the amine. It is highly unlikely that uncoordinated TiCl4 is present at any time during the course of the reaction.[12]
Scheme 5.
Proposed mechanism for Ti–Ru catalysis in the radical haloalkylation of titanium enolates.
In summary, a direct radical trichloromethylation reaction that is catalytic in both the titanium and ruthenium reagents has been developed as a result of a deeper mechanistic investigation of the process. This reaction constitutes the first alkylation of titanium enolates that is catalytic in titanium and provides a foundation for expanding the scope of catalytic radical alkylation reactions of titanium enolates. Important findings include: 1) the discovery that the complexation of trialkyl amines to TiCl4 appears to be reversible, especially at elevated temperatures, although the decomplexation is of limited efficiency; and 2) the observation that the turnover frequency for the catalytic transfer of TiCl4 is relatively high in comparison with the rate of radical generation, even with the more reactive ruthenium catalyst [Cp*RuCl(PPh3)2].
Supplementary Material
Footnotes
We thank Eli Lilly and Amgen for unrestricted support of this research. This research was supported by the National Institute of General Medical Sciences, National Institutes of Health (NIGMS, R01 GM077379), and the University of California Cancer Research Coordinating Committee.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201101364.
References
- [1].Marek I. Titanium and Zirconium in Organic Synthesis. Wiley-VCH; Weinheim: 2002. [Google Scholar]
- [2].Mahrwald R. Modern Aldol Reactions. Vol. 1. Wiley-VCH; Weinheim: 2004. pp. 63–125. [Google Scholar]
- [3].a) Evans DA, Urpi F, Somers TC, Clark JS, Bilodeau MT. J. Am. Chem. Soc. 1990;112:8215–8216. [Google Scholar]; b) Evans DA, Dow RL, Shih TL, Takacs JM, Zahler R. J. Am. Chem. Soc. 1990;112:5290–5313. [Google Scholar]; c) Hintermann T, Seebach D. Helv. Chim. Acta. 1998;81:2093–2126. [Google Scholar]; d) Gessier F, Schaeffer L, Kimmerlin T, Fl gel O, Seebach D. Helv. Chim. Acta. 2005;88:2235–2250. [Google Scholar]; e) Chen C-C, Chen S-T, Chuang T-H, Fang J-M. J. Chem. Soc. Perkin Trans. 1. 1994:2217–2221. [Google Scholar]; f) Barnett CJ, Wilson TM, Evans DA, Somers TC. Tetrahedron Lett. 1997;38:735–738. [Google Scholar]
- [4].Beaumont S, Ilardi EA, Monroe LR, Zakarian A. J. Am. Chem. Soc. 2010;132:1482–1483. doi: 10.1021/ja910154f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) de I, Moreira PR, Bofill JM, Anglada JM, Solsona JG, Nebot J, Romea P, Urp F. J. Am. Chem. Soc. 2008;130:3242–3243. doi: 10.1021/ja076625f. [DOI] [PubMed] [Google Scholar]; b) Evangelio E, Ruiz-Molina D. Eur. J. Inorg. Chem. 2005:2957–2971. [Google Scholar]; c) Ouchi M, Terashima T, Sawamoto M. Chem. Rev. 2009;109:4963–5050. doi: 10.1021/cr900234b. [DOI] [PubMed] [Google Scholar]
- [6].Gu Z, Zakarian A. Angew. Chem. 2010;122:9896–9899. Angew. Chem. Int. Ed. 2010;49:9702–9705. doi: 10.1002/anie.201005354. see also: Li Y, Cao Y, Gu J, Wang W, Wang H, Zheng T, Sun Z. Eur. J. Org. Chem. 2011:676–679.
- [7].a) Reetz MT. Organotitanium Reagents in Organic Synthesis. Springer; Berlin: 1986. [Google Scholar]; b) Dell Amico DB, Calderazzo F, Ianelli S, Labella L, Marchetti F, Pelizzi G. J. Chem. Soc. Dalton Trans. 2000:4339–4342. [Google Scholar]
- [8].Besides the expected titanium enolate 4, an additional species with substantially broadened peaks in the 1H NMR spectrum was detected. These peaks became sharper at higher temperatures. See the Supporting Information for more details.
- [9].a) Simal F, Wlodarczak L, Demonceau A, Noels AF. Tetrahedron Lett. 2000;41:6071–6074. [Google Scholar]; b) Simal F, Wlodarczak L, Demonceau A, Noels AF. Eur. J. Org. Chem. 2001:2689–2695. [Google Scholar]; c) Severin K. Curr. Org. Chem. 2006;10:217–224. [Google Scholar]
- [10].The original computational study by de P. R. Moreira et al.[5a] shows no energy barrier connecting species such as 4 and 4A and suggests that 4 and 4A should be viewed as resonance forms rather than distinct equilibrating intermediates.
- [11].Fischer H. Chem. Rev. 2001;101:3581–3610. doi: 10.1021/cr990124y. [DOI] [PubMed] [Google Scholar]
- [12].For a recent Highlight, see: Amatov T, Jahn U. Angew. Chem. 2011;123:4636–4638. Angew. Chem. Int. Ed. 2011;50:4542–4544. doi: 10.1002/anie.201007672.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.