Abstract
Purpose
Claudication is a typical symptom of peripheral arterial disease (PAD) and lumbar spinal stenosis (LSS). Differential diagnosis of PAD and LSS is often difficult due to the subjective natures of symptoms and atypical signs. The authors aimed to determine the usefulness of ankle-brachial index (ABI) measurement for the differential diagnosis of PAD and LSS when the etiology of claudication is uncertain.
Methods
Forty-two consecutive patients who had been referred by spine surgeons to a lower extremity vascular surgeon for atypical claudication were retrospectively analyzed. Atypical claudication was defined as claudication not caused by PAD, as determined by clinical manifestations, or by LSS, as determined by MR imaging. A final diagnosis of PAD was established by CT angiography (CTA) and of LSS by excluding PAD. Diagnostic validity of ABI for PAD in atypical presentation was assessed.
Results
Sixty-two legs of 42 atypical claudication patients were analyzed. Mean patient age was 65.8 ± 8.2 years (38–85) and 29 (69.0%) had diabetes mellitus. Mean ABI was 0.73 ± 0.14 (0.53–0.94) in the PAD group and 0.92 ± 0.18 (0.52–1.10) in the LSS group (P < 0.001). Of the 33 legs with a low ABI (ABI < 0.9), 29 legs were diagnosed as true positives for PAD by CTA and 4 were false positives, and of the 29 legs with a high ABI, 5 were false negatives and 24 were true negatives. The sensitivity and specificity of ABI for the diagnosis of PAD in patients with atypical claudication were 85.3 and 85.7%, respectively, and its positive and negative predictive values were 87.9 and 82.8%.
Conclusions
ABI is a recommended screening test for the differential diagnosis of lower leg claudication when clinical symptoms are atypical.
Keywords: Claudication, Peripheral arterial disease, Lumbar spinal stenosis, Ankle-brachial index, Validity
Introduction
The term “Claudication” was coined by Charcot who first ascribed this symptom to peripheral arterial disease (PAD) of the lower extremity in 1858 [1]. The word derives from the Latin word claudicare, which means to be lame, or to limp [2]. However, rather than limping, the typical symptom of claudication is being unable to walk due to intermittent cramping pain in the lower legs which is precipitated by exercise and relieved by rest. Furthermore, lumbar spinal stenosis (LSS) produces similar symptoms [1, 3, 4].
When the majority of claudication patients make an initial visit for leg pain, a number of clinical features enable orthopaedic surgeons to differentiate the vascular and neurogenic etiologies [5–7]. Vascular claudication typically occurs after activity or ambulation for a distance due to a vascular insufficiency caused by an imbalance between muscular oxygen demand and supply [7]. In such cases, resting from activity even in a standing position may help relieve symptoms. On the other hand, neurogenic claudication is associated with activity and position, because narrowing of the spinal canal and neural foramen is aggravated by standing and relieved by sitting and flexion [8]. Therefore, neurogenic claudication may be relieved by sitting down or leaning over.
Nevertheless, the differential diagnosis of vascular and neurogenic etiologies is often difficult due to variable subjective symptoms and atypical signs [1, 7, 9]. In particular, the diagnostic validities and reliabilities of clinical manifestations remain unclear. Furthermore, patients with a degenerative condition can be affected by PAD and LSS concomitantly [6, 10, 11].
Ankle-brachial index (ABI) is a measurement of the blood pressure in the lower leg compared to the blood pressure in the arm. ABI remains a gold standard for the diagnosis of lower-extremity PAD in an outpatient department setting, because it is simple, non-invasive, inexpensive, objective, reliable, and valid [12–15]. However, the usefulness of ABI measurement in case of atypical claudication has not been previously reported. The aim of this study was to determine the diagnostic validity of ABI for the differential diagnosis of PAD and LSS when the etiology of claudication is uncertain.
Materials and methods
This study involved a retrospective analysis of 42 consecutive patients that were referred by spine surgeons to a lower extremity vascular surgeon for atypical claudication at an outpatient department of orthopaedic surgery in a tertiary hospital.
Atypical claudication was defined as claudication not caused by PAD, as determined by clinical manifestations, or by LSS, as determined by MR imaging. Included patients had a walking difficulty for more than 5 min due to an intermittent cramping pain in the lower legs without a stenotic lesion by MR (magnetic resonance) imaging at the central canal and neural foramen of lumbar spine (from L1-2 to L5-S1 levels), that is, the smallest cross-sectional area of dural sac was >100 mm2 and epidural fat in the intervertebral foramen was not obliterated. The cross-sectional areas of dural sacs were calculated in T2-weighed axial MR images using a picture archiving and communication system (PiViewerSTAR; INFINITT, Seoul) equipped with an integrated digital area measurement facility (Fig. 1). Epidural fat obliteration in the intervertebral foramen was evaluated in T1-weighed sagittal images using the criteria described by Wildermuth et al. [16] (Table 1). All measurements were performed by three orthopaedic surgeons three times.
Fig. 1.
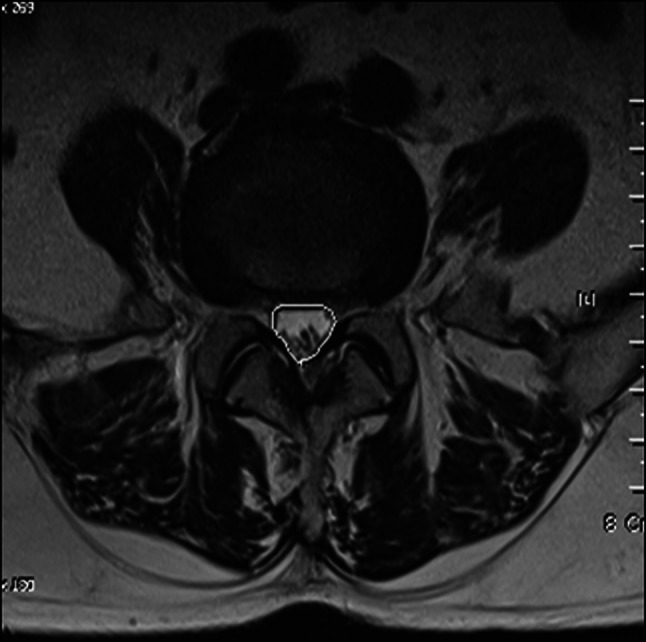
Measurement of a cross-sectional area of dural sac at L5-S1 level. A region of interest was drawn with a graphic cursor around the cross-sectional area of the dural sac and the area was calculated
Table 1.
Wildermuth’s MR grading system for lumbar foraminal stenosis
| Grade | |
|---|---|
| 0 | Normal foramina [normal dorsolateral border of the intervertebral disk and normal form at the foraminal epidural fat (oval or inverted pear shape)] |
| 1 | Slight foraminal stenosis and deformity of the epidural fat, with the remaining fat still completely surrounding the exiting nerve root |
| 2 | Marked foraminal stenosis, with epidural fat only partially surrounding the nerve root |
| 3 | Advanced stenosis with obliteration of the epidural fat |
Patients with typical symptoms and signs of PAD, that is, a decreased pedal pulse, a previous diagnosis of PAD, and a history of significant trauma in legs were excluded.
Patients’ information on gender, age, weight, height, diabetes mellitus (DM), hypertension, current smoking status, and described symptoms including provocation and palliative factors were obtained from medical records. Our institutional review board approved the present study.
Evaluation of PAD using ABI and angiography
Both ABI and computed tomographic angiography (CTA) were performed on each patient. ABI was measured by a trained technician using a Nicolet VasoGuard® (Nicolet Vascular Inc. Madison, WI) using photoplethysmography (PPG). The patients were sent to a separate room for the ABI measurements under optimal conditions. The room temperature was maintained at 23–25°C, and at least 10 min was allowed for thermal acclimatization and relaxation in the supine position. Ambient lighting in the room was turned off during measurements. Photoplethysmographic sensors were attached to the tips of the greater toes, and cuffs were placed on the patient’s arms and lower calves (just above the ankles). Systolic blood pressures from bilateral upper and lower extremities were measured simultaneously, and bilateral great toe pulses were captured to compute ABI. For individual legs, ABI was considered abnormal when it was <0.9.
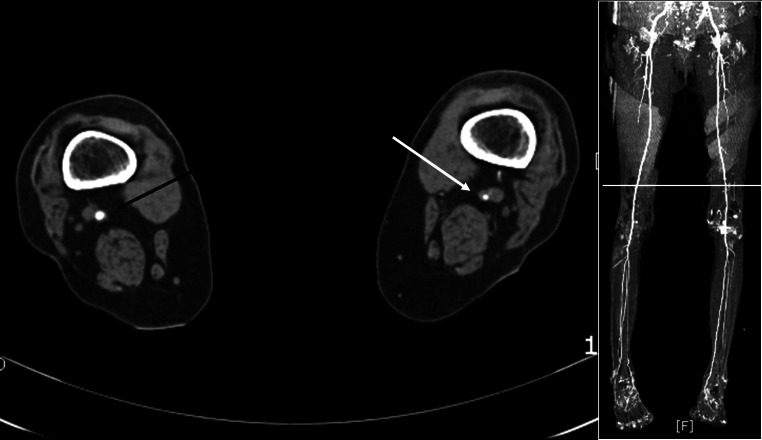
All patients then, underwent CTA (Brilliance CT 64®, Phillips Medical Systems, Cleveland, OH). CTA images were given to a vascular radiologist who interpreted images without any clinical information. PAD was diagnosed when >50% narrowing of arterial lumen was observed in any lower extremity segment. Degrees of stenosis were recorded as mild (50–74%), moderate (75–94%), or severe (more than 95%), and locations of stenosis were recorded as iliac, femoral/popliteal, and below trifurcation (Fig. 2). Mean time between ABI determination and CTA was 2.9 ± 1.7 (0–5) days. A diagnosis of PAD was established for the individual leg. LSS was diagnosed by excluding PAD.
Fig. 2.
An illustration of moderate PAD at femoral/popliteal location. Compared with the unaffected side (black arrow), >75% narrowing of arterial lumen (white arrow) was observed at the distal femoral artery
Statistical analysis
Descriptive statistics are summarized as frequencies and percentages for categorical variables, and as means and standard deviations for continuous variables. The student’s t-test and the chi-square test were used to compare group demographic and measured variables. The reliabilities of dural sac measurement and of epidural fat obliteration in the neural foramen were determined using intraclass correlation coefficients (ICCs) and kappa values, respectively. Kappa values were interpreted as follows; moderate (0.41 ≤ κ < 0.60), substantial (0.60 ≤ κ < 0.80), and almost perfect (0.80 ≤ κ ≤ 1.00) [17].
Statistical analysis was carried out using SPSS version 12.0 (SPSS Inc, Chicago, IL, USA), and P values of ≤0.05 were considered significant.
Results
Demographics of the PAD and LSS patients
Of the 42 patients (62 symptomatic legs) enrolled, a final diagnosis of PAD was established for 22 patients (52.4%), and the remaining 20 patients were diagnosed as having LSS. Table 2 presents details of these two groups. Groups demographic variables (gender, age, BMI, DM, hypertension, and smoking) were similar, as were claudication characteristics (bilaterality, relieving factor, and back pain).
Table 2.
Demographics of PAD and LSS groups
| PAD (N = 22) | LSS (N = 20) | P | |
|---|---|---|---|
| Male gender | 13 (59.1) | 9 (45.0) | 0.273 |
| Age (range) | 66.4 ± 10.4 (38–85) | 64.1 ± 6.6 (41–83) | 0.089 |
| Body mass index (m/kg2) (range) | 24.4 ± 3.5 (14.9–27.9) | 23.6 ± 2.8 (21.5–27.3) | 0.297 |
| Diabetes mellitus | 16 (72.7) | 13 (65.0) | 0.239 |
| Hypertension | 13 (59.1) | 11 (55.0) | 0.894 |
| Smoking | 10 (45.5) | 6 (30.0) | 0.137 |
| Claudication | |||
| Bilateral | 12 (54.5) | 8 (40.0) | 0.264 |
| Relieved by lumbar flexion | 11 (50) | 8 (40.0) | 0.367 |
| With back pain | 8 (36.4) | 7 (35.0) | 0.591 |
Unless otherwise noted, data are numbers of subjects and percentages in parentheses
MR, ABI, and CTA for individual legs (Table 3)
Table 3.
MR, ABI, and CTA of individual legs
| Legs of PAD (N = 34) | Legs of LSS (N = 28) | P | |
|---|---|---|---|
| MR images | |||
| Dural sac area(mm2) (range) | 146.7 ± 28.3 (102.3–188.2) | 125.5 ± 26.1 (100.89–160.49) | 0.003 |
| Foraminal stenosis | 0.429 | ||
| Normal | 18 (52.9) | 12 (42.9) | |
| Slight decreased | 16 (47.1) | 16 (57.1) | |
| ABI (range) | 0.73 ± 0.14 (0.53–0.94) | 0.92 ± 0.18 (0.52–1.10) | <0.001 |
| Angiography | |||
| Severity | |||
| Mild | 29 | – | |
| Moderate | 5 | – | |
| Severe | 0 | ||
| Location | |||
| Iliac | 3 | – | |
| Femoral/popliteal | 15 | – | |
| Below trifurcation | 16 | – | |
Unless otherwise noted, data are numbers of subjects and percentages in parentheses
Of the 84 individual legs of the 42 patients, 62 legs were symptomatic. Final diagnoses for symptomatic legs were; PAD for 34 (54.8%) legs and LSS for the other 28 legs.
The mean smallest cross-sectional area of the dural sac was 146.7 ± 28.3 mm2 (102.3–188.2) in PAD, and 125.5 ± 26.1 mm2 (100.89–160.49) in LSS (P = 0.003). The epidural fat obliteration in the neural foramen was evaluated as normal for 18 legs (52.9%) and as slight for 16 legs (47.1%) in the PAD group, and as normal for 12 (42.9%) and slight for 16 (57.1%) in the LSS group (P = 0.429).
Mean ABIs were 0.73 ± 0.14 (0.53–0.94) for PAD legs and 0.92 ± 0.18 (0.52–1.10) for LSS legs, and these values were significantly different (P < 0.001).
Of the 34 PAD legs, 29 and 5 legs were regarded as mild and moderate, respectively. PAD locations were at the iliac level for 3 legs, the femoral/popliteal level for 15 legs, and below trifurcate for 16 legs.
Diagnostic validity of ABI for PAD
Of the 33 legs with a low ABI (ABI < 0.9), 29 legs were diagnosed as true positives for PAD by CTA and 4 were false positives, and of the 29 legs with a high ABI, 5 were false negative, and 24 were true negative. Therefore, the sensitivity and specificity of ABI for a diagnosis of PAD in case of atypical claudication were 85.3 and 85.7%, respectively, and the positive and negative predictive values of ABI were 87.9 and 82.8%, respectively.
Interobserver and intraobserver reliability of the measurement of LSS (Table 4)
Table 4.
Interobserver and intraoberver reliability of MR imaging
| Intrarater correlation coefficient | Kappa | |||
|---|---|---|---|---|
| Interobserver | Intraobserver | Interobserver | Intraobserver | |
| Foraminal height | 0.75–0.81 | 0.79–0.88 | ||
| Superior foraminal width | 0.69–0.74 | 0.71–0.77 | ||
| Middle foraminal width | 0.63–0.69 | 0.69–0.75 | ||
| Sagittal cross-sectional area | 0.61–0.72 | 0.69–0.74 | ||
| Epidural fat obliteration | 0.85–0.89 | 0.86–0.91 | ||
For smallest cross-sectional areas of dural sacs, interobserver and intraobserver ICCs were 0.61–0.81 and 0.69–0.87, respectively, indicating fair to good agreement, and for epidural fat obliteration in the neural foramen, the interobserver and intraobserver kappa values were 0.85–0.89 and 0.86–0.91, respectively, indicating excellent agreement.
Discussion
De Jerine [18] discovered that claudication can have a spinal etiology in 1911, and later, Verbiest [4] detailed the clinical symptoms of neurogenic claudication as opposed to vascular claudication. As LSS and PAD are usually associated with degenerative conditions, atypical presentations and concomitant affections are not rare [6, 10, 11]. However, although a number of clinical features have been reported to differentiate vascular and neurogenic claudication [5–7], achieving a differential diagnosis in a clinical situation is often difficult.
Currently, no diagnostic guideline is available for differentiating PAD and LSS when claudication is atypical. Plain radiography of the lumbar spine is frequently performed initially to examine spinal lesions, and if degenerative findings are positive, a prejudiced impression of LSS might result. Then, additional imaging test including CT, myelography, or MR are considered to identify more details of spinal pathologic structures. If these additional imaging studies produce positive findings for LSS, the impression of LSS might be confirmed and the possibility of PAD discarded. On the other hand, if results are ambiguous for LSS, results are taken to indicate PAD. These processes represent common flaws during the differential diagnosis of atypical claudication, because imaging studies are not specific for LSS [19]. Although electrodiagnostic testing is highly specific for the diagnosis of LSS [20], its clinical utilization is limited due to the high cost and invasiveness.
Reduced pulses at the foot and leg are a pathologic finding of vascular claudication, but the reliability and validity of pulse palpation are low [21–23]. Alternatively, ABI can be useful either as a screening tool for the primary prevention of PAD or as a tool for monitoring the therapeutic efficacy in secondary prevention. Furthermore, ABI has prognostic value in terms of the prediction of limb survival, wound healing, and the risk factors of the survival of coronary artery disease and stroke [24, 25]. In addition, ABI (threshold of 0.9) has been reported to have a sensitivity of 79–95% and a specificity of 96–100% for the diagnosis of PAD [14].
Few reports have addressed the usefulness of ABI for the differential diagnosis of claudication by PAD and LSS. In the present study, the sensitivity and specificity of ABI for a diagnosis of PAD in atypical claudication were 85.3 and 85.7%, respectively, and its positive and negative predictive values were 87.9 and 82.8%, respectively. Based on our findings, we recommended that ABI be used in claudication patients when spinal images raise suspicion of LSS. Moreover, in view of its simplicity, we suggest that ABIs be determined at initial diagnostic work-ups.
This study has several limitations. The retrospective design introduces a degree of uncertainty due to missing and erroneous data in medical records, and a lack of clinical information. Another limitation was that the study subjects had a mean age of 65.8 ± 8.2 years and 69.0% had DM. And only mild cases of LSS as determined by MR imaging were included. Furthermore, other psychosocial and physiologic factors that affected patients’ symptoms might have affected results, for example, depressive mood, osteoarthritis, and neuropathic pain. On the other hand, it can be inferred that atypical caludication is prevalent among older patients with DM.
Clinically, patients that present with severe degenerative changes of both lumbar spine and vascular structures would be more challenge to orthopaedic surgeons in terms of proper management (Fig. 3). Further studies on the epidemiology and natural course of claudication are required.
Fig. 3.
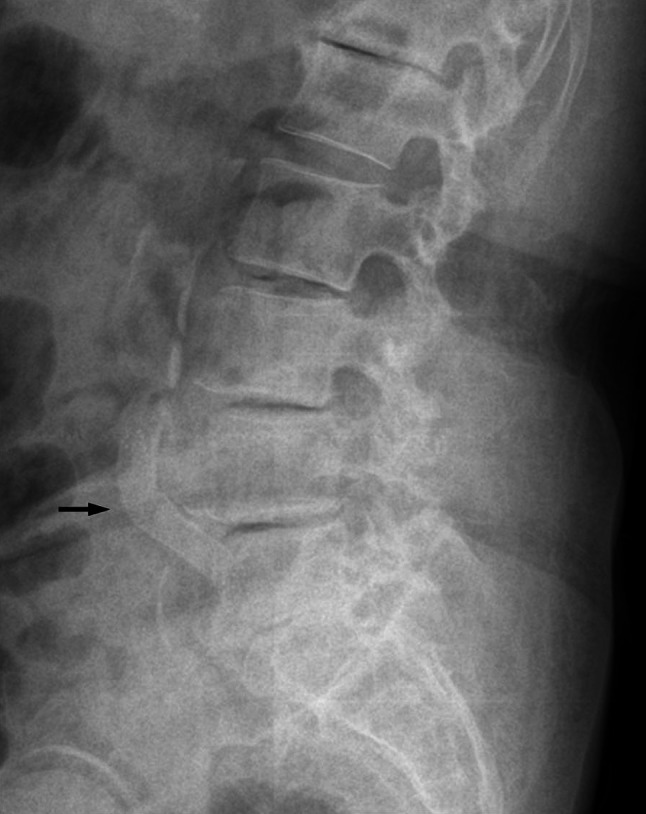
An illustration of severe degenerative changes of both lumbar spine and vascular structures. The black arrow indicates a calcification of the abdominal aorta
Conclusion
In the present study, the diagnostic sensitivity and specificity of ABI for differential diagnosis of PAD in atypical claudication were found to be 85.3 and 85.7%, respectively. Demographic factors of old age and DM were found to be associated with atypical caludication. We recommend that ABI be used in claudication patients as an initial diagnostic work-up when spinal imaging findings are suspicious for LSS.
Conflict of interest
None.
References
- 1.Maher M, Hehir DJ, Neary P, Hinchion J, O’Donnell JA. Spinal claudication versus arterial claudication. Ir J Med Sci. 1996;165:118–120. doi: 10.1007/BF02943798. [DOI] [PubMed] [Google Scholar]
- 2.Pearce JM. (Neurogenic) Claudication. Eur Neurol. 2005;54:118–119. doi: 10.1159/000088648. [DOI] [PubMed] [Google Scholar]
- 3.Warren R. Editorial: two kinds of intermittent claudication. Arch Surg. 1976;111:739. doi: 10.1001/archsurg.1976.01360250015001. [DOI] [PubMed] [Google Scholar]
- 4.Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br. 1954;36-B:230–237. doi: 10.1302/0301-620X.36B2.230. [DOI] [PubMed] [Google Scholar]
- 5.Hawkes CH, Roberts GM. Neurogenic and vascular claudication. J Neurol Sci. 1978;38:337–345. doi: 10.1016/0022-510X(78)90140-5. [DOI] [PubMed] [Google Scholar]
- 6.De Villiers JC. Combined neurogenic and vascular claudication. S Afr Med J. 1980;57:650–654. [PubMed] [Google Scholar]
- 7.Stanton PE, Jr, Rosenthal D, Clark M, Vo N, Lamis P. Differentiation of vascular and neurogenic claudication. Am Surg. 1987;53:71–76. [PubMed] [Google Scholar]
- 8.Nowicki BH, Yu S, Reinartz J, Pintar F, Yoganandan N, Haughton VM. Effect of axial loading on neural foramina and nerve roots in the lumbar spine. Radiology. 1990;176:433–437. doi: 10.1148/radiology.176.2.2367657. [DOI] [PubMed] [Google Scholar]
- 9.Okoro T, Qureshi A, Sell B, Sell P. The accuracy of assessment of walking distance in the elective spinal outpatients setting. Eur Spine J. 2010;19:279–282. doi: 10.1007/s00586-009-1152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson JE, Barrington TW, Ameli M. Combined vascular and neurogenic claudication. Spine (Phila Pa 1976) 1982;7:150–158. doi: 10.1097/00007632-198203000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Heidrich H, Hermann GM. Concomitant neurological and orthopaedic diseases in the presence of peripheral arterial disease: a prospective study. Vasa. 2006;35:101–105. doi: 10.1024/0301-1526.35.2.101. [DOI] [PubMed] [Google Scholar]
- 12.Yao ST, Hobbs JT, Irvine WT. Ankle systolic pressure measurements in arterial disease affecting the lower extremities. Br J Surg. 1969;56:676–679. doi: 10.1002/bjs.1800560910. [DOI] [PubMed] [Google Scholar]
- 13.Greenland P, Abrams J, Aurigemma GP, Bond MG, Clark LT, Criqui MH, Crouse JR, 3rd, Friedman L, Fuster V, Herrington DM, Kuller LH, Ridker PM, Roberts WC, Stanford W, Stone N, Swan HJ, Taubert KA, Wexler L. Prevention conference V: beyond secondary prevention: identifying the high-risk patient for primary prevention: noninvasive tests of atherosclerotic burden: Writing Group III. Circulation. 2000;101:E16–E22. doi: 10.1161/01.cir.101.1.e16. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 15.de Graaff JC, Ubbink DT, Legemate DA, de Haan RJ, Jacobs MJ. Interobserver and intraobserver reproducibility of peripheral blood and oxygen pressure measurements in the assessment of lower extremity arterial disease. J Vasc Surg. 2001;33:1033–1040. doi: 10.1067/mva.2001.108011. [DOI] [PubMed] [Google Scholar]
- 16.Wildermuth S, Zanetti M, Duewell S, Schmid MR, Romanowski B, Benini A, Boni T, Hodler J (1998) Lumbar spine: quantitative and qualitative assessment of positional (upright flexion and extension) MR imaging and myelography. Radiology 207:391–398 [DOI] [PubMed]
- 17.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 18.Joffe R, Appleby A, Arjona V. “Intermittent ischaemia” of the cauda equina due to stenosis of the lumbar canal. J Neurol Neurosurg Psychiatry. 1966;29:315–318. doi: 10.1136/jnnp.29.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:1178–1184. [PubMed] [Google Scholar]
- 20.Haig AJ, Tong HC, Yamakawa KS, Quint DJ, Hoff JT, Chiodo A, Miner JA, Choksi VR, Geisser ME. The sensitivity and specificity of electrodiagnostic testing for the clinical syndrome of lumbar spinal stenosis. Spine (Phila Pa 1976) 2005;30:2667–2676. doi: 10.1097/01.brs.0000188400.11490.5f. [DOI] [PubMed] [Google Scholar]
- 21.Brearley S, Shearman CP, Simms MH. Peripheral pulse palpation: an unreliable physical sign. Ann R Coll Surg Engl. 1992;74:169–171. [PMC free article] [PubMed] [Google Scholar]
- 22.Khan NA, Rahim SA, Anand SS, Simel DL, Panju A. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536–546. doi: 10.1001/jama.295.5.536. [DOI] [PubMed] [Google Scholar]
- 23.Imagama S, Matsuyama Y, Sakai Y, Ito Z, Wakao N, Deguchi M, Hachiya Y, Osawa Y, Yoshihara H, Kamiya M, Kanemura T, Kato F, Yukawa Y, Yoshida T, Harada A, Kawakami N, Suzuki K, Matsubara Y, Goto M, Sato K, Ito S, Maruyama K, Yanase M, Ishida Y, Kuno N, Hasegawa T, Ishiguro N. An arterial pulse examination is not sufficient for diagnosis of peripheral arterial disease in lumbar spinal canal stenosis: a prospective multicenter study. Spine (Phila Pa 1976) 2011;36:1204–1210. doi: 10.1097/BRS.0b013e3181ebd86f. [DOI] [PubMed] [Google Scholar]
- 24.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Lee AJ, Price JF, d’Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doobay AV, Anand SS. Sensitivity and specificity of the ankle-brachial index to predict future cardiovascular outcomes: a systematic review. Arterioscler Thromb Vasc Biol. 2005;25:1463–1469. doi: 10.1161/01.ATV.0000168911.78624.b7. [DOI] [PubMed] [Google Scholar]