Abstract
Purpose
Lateral lumbar interbody fusion (LLIF) is a minimally invasive technique that has gained growing interest in recent years. We performed a retrospective review of the medical records and operative reports of patients undergoing LLIF between March 2006 and December 2009. We seek to identify the incidence and nature of neurological deficits following LLIF.
Methods
New occurring sensory and motor deficits were recorded at 6 and 12 weeks as well as 6- and 12 months of follow-up. Motor deficits were grouped according to the muscle weakness and severity and sensory deficits to the dermatomal zone. New events were correlated to the patient demographics, pre-operative diagnosis, operative levels, and duration of surgery. At each post-operative time-point patients were queried regarding the presence of leg pain.
Results
A total of 235 patients (139 F; 96 M) with a total of 444 levels fused were included. Average age was 61.5 and mean BMI 28.3. At 12 months’ follow-up, the prevalence of sensory deficits was 1.6%, psoas mechanical deficit was 1.6% and lumbar plexus related deficits 2.9%. Although there was no significant correlation between the surgical level L4–5 and an increased psoas mechanical flexion or lumbar plexus related motor deficit, a trend was observed. Independent risk factors for both psoas mechanical hip flexion deficit and lumbar plexus related motor deficit was duration of surgery.
Conclusion
LLIF is a valuable tool for achieving fusion through a minimally invasive approach with little risk to neurovascular structures.
Keywords: Lateral transpsoatic interbody fusion, Lateral lumbar interbody fusion, Lumbar plexus injury, Anterior thigh pain, Neurologic deficit, Motor deficit
Introduction
Lateral lumbar interbody fusion (LLIF) is a relatively new fusion technique with potentially broad applications used to achieve anterior column fusion, deformity correction, and indirect decompression of the lumbar spine. It is applied for a wide range of degenerative spinal disorders such as degenerative disc disease, spondylolisthesis, scoliosis, as well as adjacent segment degeneration. In addition to fusion, the technique has demonstrated efficacy in selective deformity correction and indirect central and foraminal decompression [1, 2]. It was shown that this procedure is associated with less tissue trauma and postoperative pain, shorter hospital stay and faster return to activities of daily living when compared with traditional anterior or posterior lumber interbody fusion techniques [3].
In LLIF the operative level is reached through a lateral retroperitoneal trans-psoas approach. The psoas muscle is bluntly dissected followed by docking of a minimally invasive, self-lighted retractor system on the disc space. The lumbar plexus runs loosely within the substance of the psoas muscle. Additionally, the ilioinguinal, iliohypogastric, and lateral femoral cutaneous nerves lie on or near the psoas muscle in the retroperitoneal space where they travel obliquely, inferiorly, and anteriorly to reach the iliac crest and the abdominal wall.
Recent advances in neuromonitoring techniques that allow for improved geographic “mapping” of the lumbar plexus within the psoas muscle have been developed to improve the safety of this approach [4]. Despite this, due to the anatomic proximity of these structures to the operative zone within the intervertebral disc and due to the need to dilate or expand the retractor system to allow access to the disc space, these neural structures are at risk during the procedure. Anatomical studies have shown that the majority of the plexus travels within the posterior part of the psoas major muscle and migrates in a ventral direction as it travels caudally [5]. Further, it was shown that the average distance between the nerves to the lateral mid-point of the disc decreases from cranial to caudal levels [6]. Therefore, it has been theorized that the risk of iatrogenic neurologic injury varies at each level. Several authors defined safe-zones for each level, concluding that particular L4–5 is at greater risk [7, 8]. Furthermore, the approach for LLIF is also anecdotally associated with postoperative groin/thigh pain.
With increasing numbers of LLIF being performed each year, expected complications and their predictors and potential mitigating factors need to be better understood. There continues to be uncertainly surrounding the absolute risk posed by this procedure as well as both patient and technique mitigating factors. To our knowledge, no study to date has attempted to correlate patient-, surgeon-, and technique-related factors to postoperative sensory and motor deficits as well as groin/thigh pain. Last, no study has attempted to examine the natural history of these deficits over time in the post operative period.
The objective of this study was to identify the incidence of sensory and motor deficits as well as groin/thigh pain and their natural history over time, as well as elucidate mitigating patient, surgeon, and technique factors. A retrospective review of a group of consecutive LLIF patients’ complete medical records with 1-year follow-up was performed. We hypothesized that patients undergoing LLIF at the L4–5 level have a higher risk of transient and permanent nerve damage. We predicted an increasing risk of sensory and motor deficit postoperatively with an increase in the number of levels approached and operative time.
Materials and methods
Under institutional review board approval we retrospectively reviewed medical records of 235 patients, who underwent minimally invasive LLIF between March 2006 and December 2009 at a single institution by four attending surgeons. All patients underwent minimally invasive LLIF surgery either by utilizing extreme lateral interbody fusion (XLIF) (XLIF-Nuvasive, Inc., San Diego, CA) technique or the COUGAR-System (COUGAR-Depuy Spine Inc., Raynham, MA). All patients included for this study had their pre-operative consultation and at 6, 12 weeks, 6- and 12 months’ follow-up visit at the same institution. Ipsilateral sensory and motor deficits (at the same side as the surgical approach) were recorded at the follow-up time points. Patients who had their follow-up appointment within the first 6 weeks post-operatively by another institution were excluded from this study.
All patients were evaluated pre-operatively with standard radiographs, magnetic resonance imaging and computed tomography scans. The indications were neurogenic claudation due to central or foraminal spinal stenosis and axial back pain due to degenerative scoliosis, spondylolisthesis, and/or junctional disc degeneration. In every case, intra-operative electromyography probes and active-run electromyography were used to prevent injury to neural structures.
Per institutional protocol, the presence of anterior thigh or groin pain as well as complete neurologic examination including tactile detection, two-point discrimination was performed by the treating surgeon and recorded at each post operative visit. The presence of pain at the anterior thigh or groin region was only recorded if the patient, subjectively, commented on a new occurring event since the surgical procedure. The motor strength testing was scored using the manual muscle test scale (MMTS) for each muscle group. For the statistical analysis, the motor deficits were grouped into psoas mechanical flexion deficit (any weakness in hip flexion) and lumbar plexus related deficits to differentiate between psoas muscle dissection deficits and lumbar plexus deficits. The severity of motor deficit within each subgroup was divided into three groups: mild (4/5 in one or more muscle groups within the same lower extremity), moderate (3/5 in one or more muscles of the same lower extremity), and severe (≤2/5 in one or more muscles of the same lower extremity). Additionally, operative records were reviewed for surgical technique details, side of approach, levels of surgery, number of levels, and duration of surgery.
Descriptive analyses of patients and characteristics were reported using means and standard deviations for continuous variables and frequencies and percentages for discrete variables. Inferential statistics were evaluated using independent samples t tests or one-way analysis of variance (ANOVA) for continuous variables and Chi-square or Fischer’s exact test for categorical variables. Bonferroni correction was used for any multiple comparisons of continuous data and odds ratios (OR) and 95% confidence intervals (95% CI) calculated to assess the magnitude of the association for categorical. Statistical significance for the inferential analysis was set at α = 0.05 following the initial analyses; multivariate logistic regression models were created to evaluate the independent associations of each potential explanatory variable to predict the likelihood of the independent variable having an impact on sensory and motor deficits and/or groin/thigh pain at the 6-week follow-up. Additionally, another multivariate logistic regression analysis at 6 weeks’ follow-up was performed to assess the contributing factors to psoas mechanical flexion deficit and lumbar plexus related motor deficits. Severity grade in this study was dichotomized into mild versus moderate or severe, with the mild group serving as the reference group.
For all regression models, variables with a univariate significance level of 0.25 or less, or those variables that were thought to be clinically relevant were eligible for inclusion in the model. Because of the explanatory nature of the analyses, 0.15 was chosen as the threshold for retention in the final model; however, statistical significance was still set α = 0.05 and those variables that achieved statistical significance were deemed significant risk factors for the dependent outcome of the model. For all regression models, adjusted odds ratios and their 95% confidence intervals were reported. Data analysis was conducted using SPSS version 14.0 (SPSS Inc., Chicago, IL).
Results
A total of 282 patients underwent lateral lumbar interbody fusion at 527 levels between March 2006 and December 2009 and 235 patients met our inclusion criteria. The study population included 139 females and 96 males with an average age of 61.49 (range 30–88) years and BMI of 28.25 kg/m2 (range 17.4–46.0). A total of 131 patients had previous lumbar surgery. The operations were performed by five different surgeons. Four surgeons performed the LLIF through a mini-open approach and one surgeon used a single-incision percutaneous approach. An XLIF cage and minimally invasive self-lighted retractor system was used 210 times (in 57 cases a standalone procedure without posterior fixation at the same time). The COUGAR cage and minimally invasive self-lighted retractor system was used 25 times (in 9 cases a standalone procedure without posterior fixation at the same time).
Two-hundred and thirty-five medical records and operative reports were reviewed to find a correlation between surgical level and postoperative neurological deficit following LLIF. Pre-operative diagnosis were lumbar spondylosis with neurogenic claudication due to central or foraminal spinal stenosis, axial back pain due to degenerative scoliosis, spondylolisthesis, and adjacent segment disc degeneration.
With an average of 1.89 levels per patient, a total of 444 levels were fused (Th12-L1: n = 2, L1–L2: n = 39, L2–L3: n = 106, L3–L4: n = 164, L4–L5: n = 133). Three different types of graft material were used, including autograft alone from the iliac crest (ICBG) (n = 59) as well as allograft alone (n = 5) and a combination of bone morphogenetic protein with ICBG or allograft (n = 180). In the analysis, the different bone marrow substitutes did not influence the sensory or motor deficits at any given time point and was therefore not included in the regression analysis.
Sensory deficits and anterior groin/thigh pain
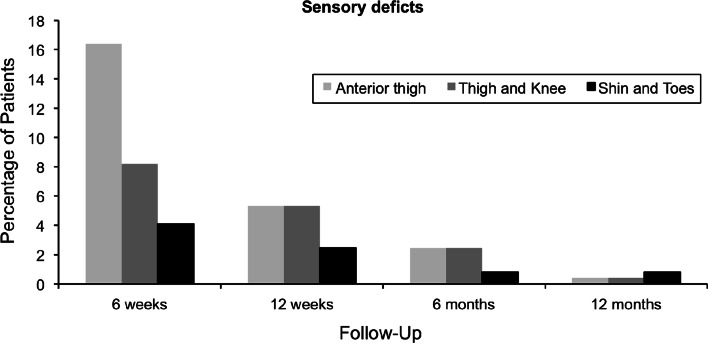
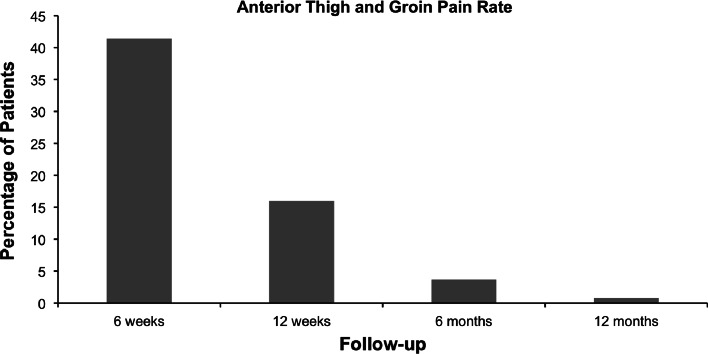
We found sensory deficits at 6 weeks of 28.7% (n = 70), 13.1% at 12 weeks (n = 32), 5.7% at 6 months (n = 14), and 1.6% at 12 months (n = 4). The detailed analysis of the sensory deficits is depicted in Fig. 1. Not only did the total number of sensory deficits decrease over time, but a reduction of deficit in each nerve zone was also observed. The rate of anterior groin/thigh pain per 100 patients was 41% (n = 101) at 6 weeks, 16% (n = 39) at 12 weeks, 3.7% (n = 9) at 6 months, and 0.8% (n = 2) at 12 months (Fig. 2).
Fig. 1.
This figure illustrates the occurrence of sensory deficits in each distribution zone at different time points
Fig. 2.
This figure shows the percentage of anterior thigh pain at different time points
Psoas mechanical flexion deficits
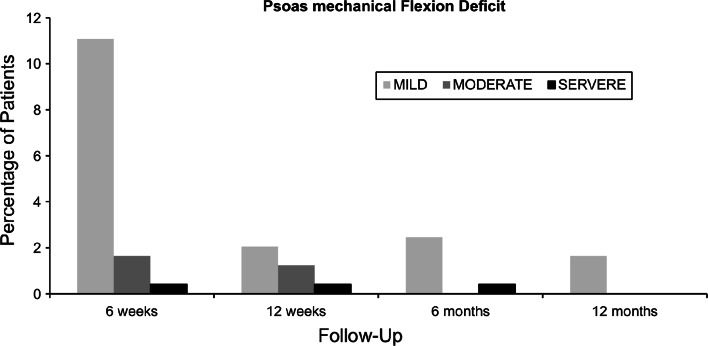
Psoas mechanical flexion deficits were observed in 13.1% (n = 32) at 6 weeks, 3.7% (n = 9) at 12 weeks, 2.9% (n = 7) at 6 months, and 1.6% (n = 4) at 12 months. The detailed analysis of the psoas mechanical flexion deficits is shown in Fig. 3. The total number and severity of psoas mechanical flexion deficits decreases over time. At 12 months’ follow-up there were 4 mild deficits (1.6%) observed, compared with 32 (13.1%) at 6 weeks.
Fig. 3.
Depicted are the psoas mechanical flexion deficits of different severities at each time point
Lumbar plexus related motor deficits
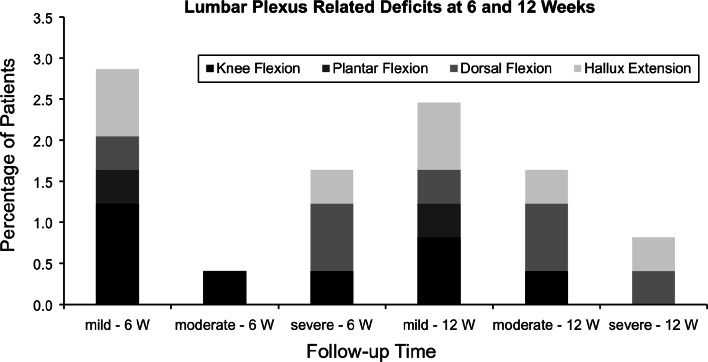
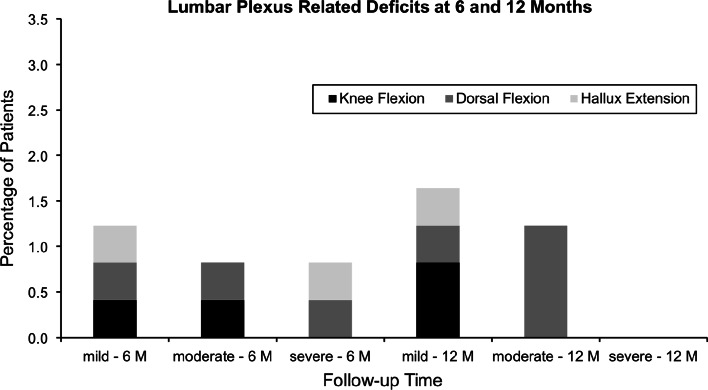
Lumbar plexus related motor deficits were observed in 4.9% (n = 12) at 6 weeks, 4.9% (n = 12) at 12 weeks, 2.9% (n = 7) at 6 months, and 2.9% (n = 7) at 12 months. The detailed analysis of the lumbar plexus related motor deficits is shown in Figs. 4 and 5. As with the psoas mechanical flexion deficits, the total number and severity of lumbar plexus related motor deficits decreases over time.
Fig. 4.
Depicted are percentages of lumbar plexus related deficits at 6- and 12 weeks’ follow-up according to each joint movement of the lower extremity (W weeks)
Fig. 5.
Depicted are percentages of lumbar plexus related deficits at 6- and 12 months’ follow-up according to each joint movement of the lower extremity (M months)
Multivariate regression analysis
The multivariate logistic regression models were created to evaluate the independent associations of each potential explanatory variable to predict the likelihood of the dependent variable, presence of a psoas mechanical flexion deficit, and lumbar plexus related motor deficits at the 6 weeks’ follow-up time point. Multivariate logistic regression analysis is for psoas mechanical flexion deficits are illustrated in Table 1 and lumbar plexus related motor deficits in Table 2. Female sex and duration of surgery were independent risk factors for psoas mechanical flexion deficits. Duration of surgery was the only independent risk factor for lumbar plexus related motor deficits. However, a trend towards multiple level fusion, previous lumbar surgeries and involvement of L4–L5 was observed. For both, psoas mechanical flexion deficits and lumbar plexus related motor deficits increased odd-ratios were observed for an involvement of L4–L5, however not statistically significant.
Table 1.
Multivariate regression analysis of patients with psoas mechanical flexion deficit
| Adjusted odds ratio | 95% CI lower | 95% CI upper | p value | |
|---|---|---|---|---|
| Female sex | 3.86 | 1.10 | 13.50 | 0.034** |
| Previous lumbar Sx | 2.25 | 0.79 | 6.39 | 0.129 |
| Right side approach | 0.44 | 0.14 | 1.37 | 0.158 |
| Patient age | 1.03 | 0.98 | 1.09 | 0.298 |
| BMI ≥ 30 | 0.38 | 0.10 | 1.45 | 0.157 |
| 2 + Levels fused | 0.82 | 0.28 | 2.40 | 0.711 |
| Duration of surgery (min) | 1.00 | 1.00 | 1.01 | 0.034** |
| L4–L5 Level fused | 2.19 | 0.722 | 6.64 | 0.166 |
** Variable included in final model and statistically significant (p < 0.05)
Table 2.
Multivariate regression analysis of patients with lumbar plexus related motor deficits
| Adjusted odds ratio | 95% CI lower | 95% CI upper | p value | |
|---|---|---|---|---|
| Female sex | 2.14 | 0.44 | 10.46 | 0.349 |
| Previous lumbar Sx | 0.35 | 0.06 | 1.95 | 0.233 |
| Right side approach | 0.41 | 0.08 | 2.07 | 0.283 |
| Patient age | 1.03 | 0.96 | 1.10 | 0.441 |
| BMI ≥ 30 | 0.64 | 0.12 | 3.55 | 0.608 |
| 2 + Levels fused | 1.80 | 0.32 | 10.18 | 0.506 |
| Duration of surgery (min) | 1.01 | 1.01 | 1.01 | 0.009** |
| L4–L5 Level fused | 3.79 | 0.63 | 22.76 | 0.145 |
** Variable included in final model and statistically significant (p < 0.05)
Discussion
LLIF has become an increasingly common procedure to achieve arthrodesis and fusion of the anterior column of the lumbar spine as well as selective degenerative deformity correction, reduction of spondylolisthesis, and indirect foraminal decompression [1–3]. Despite its advantages over traditional fusion procedures, concerns remain among surgeons about its safety. Our study shows that 1.6% sensory deficits, 1.6% psoas mechanical deficits, and 2.9% lumbar plexus related deficits remained after 1 year. In this study we were able to correlate patient-, surgeon-, and technique-specific factors to postoperative deficits. Female sex was independent risk factors for psoas mechanical deficits and duration of surgery for both, psoas mechanical deficits as well as for lumbar plexus injury.
As Fig. 2 shows, the rate of anterior groin/thigh pain drops significantly over the follow-up time. This might be attributed to a transient irritation of the genitofemoral nerve damage which crosses at L2–3 [5, 9]. Surgeons have theorized that this may be related to direct cutaneous nerve neuropraxia or an indirect mechanism via a psoas muscle inflammatory response due to mechanical dissection. The incidence of this varies considerably in the literature from 1 to 75% [2, 10–12].
Several studies have reported on post-operative neurologic deficits following LLIF. However, the determination of absolute prevalence of deficits and their natural history as well as contributing factors remains incomplete and controversial in the literature. Overall, the number of post-operative neurologic events ranges in literature between 0.6 and 33.6% after LLIF [10, 11, 13–16]. In a study by Tohmeh et al. [12] 28% of patients experienced a new iliopsoas weakness and 18% claimed of sensory loss at the upper medial thigh although intra-operative real-time monitoring was used to prevent neurologic deficits after LLIF. In a prospective multicenter trial similar numbers were reported, with 33% experiencing some motor weakness after surgery [13]. The largest series in the literature, published by Rodgers et al. [11] reported on 0.7% of the patients having post-operative neurological deficits. Although most of the studies report the deficits to be transient and dissolve within the first months there are reports that 23% of the patients have some sensory or motor weakness remaining after 1-year follow-up [2]. Most of the above-mentioned studies do not assess the neurologic in great depth other than reporting the numbers or are limited to a small sample size. The variation in the literature can be attributed not only to the utilization of different techniques and variation in experience the technique for LLIF, but also to different ratio among of the approached levels (higher involvement of L4–L5 might increase the ratio). Previously, anatomic studies have suggested that the risk varies depending on the approached level [5–8]. It has been shown that there may be a theoretical increased risk of neurologic complication at the L4–5 level given the anatomic differences in the caudal levels [10]. At L4–5 it has been described that neurovascular structures are located in the operative zone in 44% of the cases and are therefore theoretically at increased risk for injury [17]. It has also been demonstrated that the total length of disc space, which is safe for cage placement decreases from L1–L2 to L4–L5 [7]. Another study by Regev confirmed that the safe surgical corridor decreases from 47.9% at L1–L2 to 13.1% at L4–L5 of the anterior-posterior diameter of the vertebra [8]. Additionally, surgeons should consider the risk of vascular injuries due to surgical proximity to major vessels, especially in right-sided approaches [18]. However, these studies were anatomic cadaveric studies which may or may not be reflective of in vivo operating conditions. Technical points during positioning include hip and knee flexion which impacts the position of the lumbar plexus and psoas muscle mass. Furthermore, parameters for safe retraction of psoas/lumbar plexus have not been defined. In our multi-variate regression analysis, operative involvement of L4–5 did not increase the risk of either a lumbar plexus motor deficit or psoas muscle mechanical deficit (Tables 1, 2). However, increased odds-ratios and a trend towards significance were observed for both and could indicate that our analysis might be underpowered. Operative time was a significant factor for both. Others have suggested that retraction against the transverse process, particularly at L4–5, could potentially be a source of ischemia and resultant neuropraxia. Therefore, it seems logical that operative time could be an additional risk factor for the development of iatrogenic neurologic complications.
The addition of neurologic monitoring systems give surgeons added confidence in safely navigating the safe working corridors of the psoas musculature en route to the intervertebral disc. However, aside from avoiding direct plexus injury, there have been controversies surrounding this technique. Tohmeh demonstrated that dynamically evoked, discrete-threshold electromyography reduces the risk of nerve injury during LLIF. However, even in this study, all cases with transient deficits had no concerning changes during intra-operative neuromonitoring assessment [12]. Furthermore, a case report by Houten shows two cases that had post-operative motor deficits following the transpsoas approach although the intra-operative monitoring did not detect anything [19]. These data further the notion that once a direct injury to nerve or plexus is avoided, ischemia (which may be silent on intra-operative neuromonitoring) is likely the greatest threat to neural health. This is consistent with the finding that operative time (and therefore retractor deployment related ischemia time) correlates with increased risk of neurologic injury.
It has been hypothesized that surgeon technique may affect outcomes. The psoas muscle should be dissected carefully and retractor placed as described by Ozgur [3]. Furthermore, patient positioning in the proper orientation (hips and knees flexed) could help avoid femoral nerve stretch and allows for more gentle dissection upon retractor employment. All of these factors could reduce the chances of neuropraxia [16]. Although Park et al. [6] showed that the lumbar plexus with hip flexion travels more anterior (non-significant) the advantages of a relaxed and therefore easier-to-dissect muscle seem to out weigh the change in position of the nerves. The deciding factor for the side of the approach should be the anatomy of the psoas muscle relative to the approached disc level as described by Kepler [9]. Other critical factors are the relationship of the pelvis to L4–5 level, the coronal angulation of the approached disc space and the side of the concavity of the curve. Additionally, table flexion might give the surgeon more room for the approach; however, it has been previously suggested that it might cause over stretch of the femoral nerve [16]. Special attention towards a safe approach should be considered in scoliosis cases. Regev et al. [8] concluded that the approach-safe zones in patients with scoliosis significantly decrease.
At this point it is worth mentioning that traditional spinal fusion procedures as posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF) are well established, but no such operation is without a risk of complication [20]. Especially permanent post-operative neurologic deficits occur in the other fusion options as well. Permanent motor deficits were reported as high as 6.1% for PLIF and 4.1% for TLIF [21, 22]. If considered that neurologic deficits after one-year are permanent, the rate of 2.9% for LLIF is overall less than traditional procedures as PLIF or TLIF. For anterior lumbar interbody fusion (ALIF) the risk for motor deficits decreases; however, issues as visceral and vascular injuries in the same or higher frequency arise [23]. In a multi-center database review by the scoliosis research society they found 2.5% of their patients with degenerative scoliosis with new neurological deficits. Although this number maybe slightly lower, it also included cauda equina and spinal cord injuries (none in our study).
Despite the novelty of the presented data in this study the limitations have to be considered and the findings should be interpreted accordingly. First, due to the retrospective nature of this study, the clinical follow-up was limited to 1 year and some patients were lost in follow-up. Although we present the largest series with 1-year clinical follow-up after LLIF the multi-variant analysis could only identify trends for some of the included variables. For example the involvement of L4–5 was not significant, although several anatomical studies suggest an increased risk [5–8]. Furthermore, with the increasing experience in LLIF the above-mentioned surgeon-specific factors causing post-operative deficits might decrease and therefore introduce another bias opportunity. Another limitation is that we do not know the exact retractor deployment time and took the operating time as reference for it. Furthermore, new occurring pain in the anterior thigh or groin region was only recorded if subjectively stated by the patient. No further pain scale was used to quantify the pain characteristics, which introduces another bias option. However, we believe that this incidence could be of special interest for the physician in the pre-operative consultation.
In conclusion, our data represent the first analysis of sensory and motor deficits following lateral lumbar interbody fusion. We were able to demonstrate that most of the sensory and motor deficits after LLIF are transient. Independent risk factors for psoas mechanical deficit were female sex and duration of surgery. For lumbar plexus related deficits, duration of surgery was found to be significant and trends in previous surgery as well as multi-level fusion observed. Especially with the increasing popularity of this procedure, we think that prospective studies are needed to confirm our findings and broaden the knowledge about the contributing factors to post-operative neurologic deficits following LLIF.
Acknowledgments
The authors would like to express their appreciation to Joe Nguyen for assistance in the statistical evaluation.
Conflict of interest
None.
References
- 1.Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976) 2010;35(26 Suppl):S331–S337. doi: 10.1097/BRS.0b013e3182022db0. [DOI] [PubMed] [Google Scholar]
- 2.Sharma AK, Kepler CK, Girardi FP, Cammisa FP, Huang RC, Sama AA. Lateral lumbar interbody fusion: clinical and radiographic outcomes at 1 year: a preliminary report. J Spinal Disord Tech. 2011;24:242–250. doi: 10.1097/BSD.0b013e3181ecf995. [DOI] [PubMed] [Google Scholar]
- 3.Ozgur BM, Aryan HE, Pimenta L, et al. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435–443. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Berjano P, Lamartina C. Minimally invasive lateral transpsoas approach with advanced neurophysiologic monitoring for lumbar interbody fusion. Eur Spine J. 2011;20(9):1584–1586. doi: 10.1007/s00586-011-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moro T, Kikuchi S, Konno S, et al. An anatomic study of the lumbar plexus with respect to retroperitoneal endoscopic surgery. Spine. 2003;28:423–428. doi: 10.1097/01.BRS.0000049226.87064.3B. [DOI] [PubMed] [Google Scholar]
- 6.Park DK, Lee MJ, Lin EL, et al. The relationship of intrapsoas nerves during a transpsoas approach to the lumbar spine. J Spinal Disord Tech. 2010;23:223–228. doi: 10.1097/BSD.0b013e3181a9d540. [DOI] [PubMed] [Google Scholar]
- 7.Benglis DM, Vanni S, Levi AD, et al. An anatomical study of the lumbosacral plexus as related to the minimally invasive transpsoas approach to the lumbar spine. J Neurosurg Spine. 2009;10:139–144. doi: 10.3171/2008.10.SPI08479. [DOI] [PubMed] [Google Scholar]
- 8.Regev GJ, Chen L, Dhawan M, et al. Morphometric analysis of the ventral nerve roots and retroperitoneal vessels with respect to the minimally invasive lateral approach in normal and deformed spines. Spine (Phila Pa 1976) 2009;34:1330–1335. doi: 10.1097/BRS.0b013e3181a029e1. [DOI] [PubMed] [Google Scholar]
- 9.Kepler CK, Bogner EA, Herzog RJ et al (2010) Anatomy of the psoas muscle and lumbar plexus with respect to the surgical approach for lateral transpsoas interbody fusion. Eur Spine J (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 10.Tormenti MJ, Maserati MB, Bonfield CM, Okonkwo DO, Kanter AS. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus. 2010;28:E7. doi: 10.3171/2010.1.FOCUS09263. [DOI] [PubMed] [Google Scholar]
- 11.Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine (Phila Pa 1976) 2011;36:26–32. doi: 10.1097/BRS.0b013e3181e1040a. [DOI] [PubMed] [Google Scholar]
- 12.Tohmeh AG, Rodgers WB, Peterson MD. Dynamically evoked, discrete-threshold electromyography in the extreme lateral interbody fusion approach. J Neurosurg Spine. 2011;14:31–37. doi: 10.3171/2010.9.SPINE09871. [DOI] [PubMed] [Google Scholar]
- 13.Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine (Phila Pa 1976) 2010;35(26 Suppl):S322–S330. doi: 10.1097/BRS.0b013e3182022e04. [DOI] [PubMed] [Google Scholar]
- 14.Papanastassiou ID, Eleraky M, Vrionis FD. Contralateral femoral nerve compression: an unrecognized complication after extreme lateral interbody fusion (XLIF) J Clin Neurosci. 2011;18:149–151. doi: 10.1016/j.jocn.2010.07.109. [DOI] [PubMed] [Google Scholar]
- 15.Pimenta L, Oliveira L, Schaffa T, Coutinho E, Marchi L. Lumbar total disc replacement from an extreme lateral approach: clinical experience with a minimum of 2 years’ follow-up. J Neurosurg Spine. 2011;14:38–45. doi: 10.3171/2010.9.SPINE09865. [DOI] [PubMed] [Google Scholar]
- 16.Youssef JA, McAfee PC, Patty CA, Raley E, DeBauche S, Shucosky E, Chotikul L. Minimally invasive surgery: lateral approach interbody fusion: results and review. Spine (Phila Pa 1976) 2010;35(26 Suppl):S302–S311. doi: 10.1097/BRS.0b013e3182023438. [DOI] [PubMed] [Google Scholar]
- 17.Uribe JS, Arredondo N, Dakwar E, et al. Defining the safe working zones using the minimally invasive lateral retroperitoneal transpsoas approach: an anatomical study. J Neurosurg Spine. 2010;13:260–266. doi: 10.3171/2010.3.SPINE09766. [DOI] [PubMed] [Google Scholar]
- 18.Hu WK, He SS, Zhang SC, Liu YB, Li M, Hou TS, Ma XL, Wang J. An MRI study of psoas major and abdominal large vessels with respect to the X/DLIF approach. Eur Spine J. 2011;20(4):557–562. doi: 10.1007/s00586-010-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houten JK, Alexandre LC, Nasser R, Wollowick AL (2011) Nerve injury during the transpsoas approach for lumbar fusion. J Neurosurg Spine 27. (Epub ahead of print) [DOI] [PubMed]
- 20.Mura PP, Costaglioli M, Piredda M, Caboni S, Casula S. TLIF for symptomatic disc degeneration: a retrospective study of 100 patients. Eur Spine J. 2011;20(Suppl 1):S57–S60. doi: 10.1007/s00586-011-1761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes B, Rodts GE, Jr, Haid RW, Jr, Subach BR, McLaughlin MR. Allograft implants for posterior lumbar interbody fusion: results comparing cylindrical dowels and impacted wedges. Neurosurgery. 2002;51:1191–1198. doi: 10.1097/00006123-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Villavicencio AT, Burneikiene S, Bulsara KR, Thramann JJ. Perioperative complications in transforaminal lumbar interbody fusion versus anterior-posterior reconstruction for lumbar disc degeneration and instability. J Spinal Disord Tech. 2006;19:92–97. doi: 10.1097/01.bsd.0000185277.14484.4e. [DOI] [PubMed] [Google Scholar]
- 23.Fantini GA, Pappou IP, Girardi FP, Sandhu HS, Cammisa FP., Jr Major vascular injury during anterior lumbar spinal surgery: incidence, risk factors, and management. Spine (Phila Pa 1976) 2007;32:2751–2758. doi: 10.1097/BRS.0b013e31815a996e. [DOI] [PubMed] [Google Scholar]