Abstract
Purpose
Degenerative scoliosis usually begins at menopause and lateral rotatory olisthesis (LRO) might be a triggering factor in the onset of degenerative scoliosis in postmenopausal women. We set out to evaluate the influence of hormone replacement therapy (HRT) on degenerative scoliosis and on LRO.
Methods
A cross-sectional study was conducted in 146 postmenopausal women: 75 women had received HRT for more than 1 year (HRT > 1) and 71 women had never received HRT or less than 1 year (HRT < 1). Scoliotic curve, LRO, sacral slope, lordosis, kyphosis were measured. The excess risk of LRO associated with age, BMI, isometric strength of brachial biceps, bone mineral density, lean mass and HRT was evaluated using a multiple logistic regression model.
Results
No difference was found in sacral slope, lumbar lordosis or thoracic kyphosis between both groups or in the presence of scoliosis. The prevalence of LRO was significantly lower in HRT >1 than HRT <1 (8 vs. 30%) while the risk was dependent on age, HRT and their interaction. LRO increased with age only in HRT <1 (11% when aged ≤66 years vs. 39% when aged >66 years, p = 0.013), whereas the prevalence of LRO remained stable in HRT >1.
Conclusions
LRO was significantly lower in women who received HRT. The excess risk of LRO was dependent on both age and HRT status. These findings suggest that HRT might prevent the onset of LRO, and therefore might contribute to the prevention of low back pain.
Keywords: Lateral spondylolisthesis, Scoliosis, Hormone replacement therapy
Introduction
Scoliosis is a skeletal disease involving three-dimensional deformation of the spine leading to pain and disability in adults, thereby reducing mobility and impairing the quality of life of the patients [1, 2]. Scoliosis predominantly affects women, and an increase in prevalence is expected in relation to the ageing population. We and others have shown that scoliosis can be divided into two entities on the basis of its onset profile [3, 4]. Adult idiopathic scoliosis (AIS) occurs in the context of existing scoliosis developed during childhood or adolescence, which might progress after the end of bone maturation, whereas degenerative scoliosis (DS) is developed later in adulthood. In postmenopausal women, it is not possible to discriminate between the two profiles on the basis of clinical and radiological signs alone in the absence of medical history. This means that it is important to identify the early signs leading to the development of degenerative scoliosis.
Multiple factors predispose for degenerative scoliosis, and probably involve genetic, mechanical and biochemical components [5]. One factor, lateral rotatory olisthesis (LRO) also leads to the development of scoliosis, and pain of the spine [4, 6–8]. Therefore, understanding the factors that contribute to rotatory subluxation could help to identify patients at high risk of degenerative scoliosis and prevent back pain [1, 9]. Metabolic factors, such as oestrogen metabolism, may play a key role in spinal physiology. Although changes in oestrogen status influence the onset and progression of scoliosis, the impact of HRT on the prevalence of scoliosis has not yet been demonstrated [8, 10–13]. In adolescents, scoliosis is triggered at puberty, and its rate of progression slows at the end of bone maturation. Scoliosis is associated with lower serum oestradiol levels, which is also correlated with oestrogen receptor polymorphism [14]. In adults, it has been suggested that oestrogen deficiency might contribute to the occurrence of degenerative scoliosis, as it is observed mainly in postmenopausal women [4, 15]. Moreover, LRO is triggered by the menopause which, despite the absence of relevant data, suggests that oestradiol could play a role in the onset of scoliosis.
The contribution of bone to the development of scoliosis has been suggested, based on the fact that both bone loss and degenerative lesions are accentuated at the menopause. Bone mineral density (BMD) is low at the hip site in adults with lumbar scoliosis [16]. However, the lumbar BMD is increased in scoliosis, and the increase occurs predominantly on the concave side, due to bone accretion, which probably depends on the mechanical loads applied [17]. The influence of hormonal replacement therapy in oestrogen-dependent tissues and parameters, such as bone and muscle strength, has been studied separately, but the integrated effects of HRT on vertebral static parameters in adult scoliosis remains unknown. In our study we therefore set out to evaluate the role of HRT in degenerative scoliosis and LRO, and to assess the contributing factors that might trigger the development of static spine degeneration.
Methods
Participants
The subjects were all postmenopausal women and were referred to the outpatient clinic of rheumatology for back pain or to investigate the presence of osteoporosis. All the women who menopaused for more than 5 years were asked to participate at the study and most of them accepted. They were all affiliated to an insurance of mid-class workers. Economic status was roughly the same, living in the same district. All subjects underwent a medical examination, and answered questionnaires collecting medical history and treatments. They underwent thoracic and lumbar X-rays, and BMD measurement. They all provided written informed consent prior to their inclusion in the study according to the Declaration of Helsinki. The local ethics committee approved the study (No. 2001/70).
We selected all women who menopaused for more than 5 years. We excluded those who did not have total body bone density measurements and whose X-rays were not good enough to allow the correct measurement of spine indices. Subjects with history of spinal surgery, vertebral fractures and lumbo-sacral transitional vertebrae were rejected. 146 women fulfilled the above criteria for inclusion in this cross-sectional study. Two groups were then constituted according to the use of HRT:
71 subjects who had either never received HRT after the menopause or for a period less than 1 year (HRT < 1). The treatment was taken for symptoms of the menopause, mainly related to hot flushes, and was stopped few months later when the discomfort disappeared.
75 subjects who had received HRT for at least 1 year after going through the menopause (HRT > 1). HRT consisted of transdermal 17β oestradiol (0.75–1.5 mg/day) and progesterone (100 mg/day) for a mean duration of 8.7 ± 6.1 years (range 1–25 years).
Data sources
X-ray techniques
Coronal and lateral X-rays of thoracic and lumbar spine were performed in a standardized position for all patients. They were carried out with the patient standing 1 m away from the source. Thoracic radiographs were considered to be valid when T4–T12 were clearly visualized, while lumbar radiographs had to include T11 to sacrum.
Measurement of spinal indices
Spinal parameters were measured in the thoracic and lumbar radiographs by two experienced readers in blinded manner. The inter-reader concordance of measurements (expressed through an intra-class correlation coefficient) was 0.69 for kyphosis, 0.87 for lordosis and 0.93 for sacral slope.
Angle of sacral slope: measurement of the angle between the sacral plate and the horizontal plane.
Lumbar lordosis is the angle formed between sacral plate and the upper plate of the vertebra with the greatest horizontal slope.
Thoracic kyphosis (maximum kyphosis) is the angle between the upper plate of T4 and the lower plate of the vertebra with the greatest horizontal slope.
Cobb’s angle was measured in coronal X-rays as the angle between the two most tilted vertebrae of the scoliosis curves.
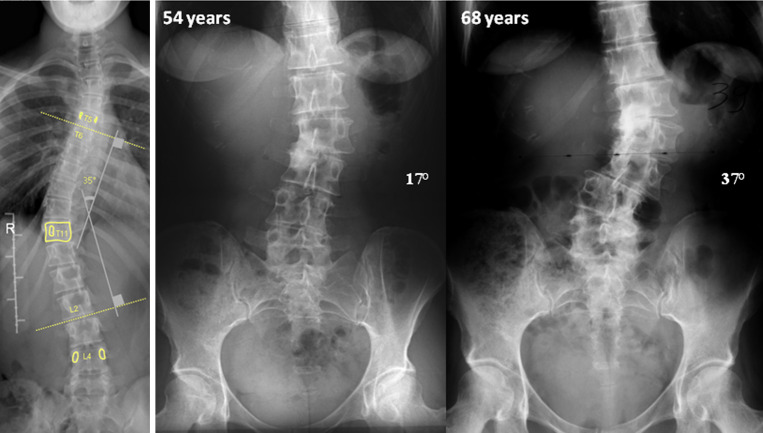
Scoliosis was defined as a Cobb angle >10° and vertebral rotation ≥5° using Perdriolle’s method (Fig. 1).
Rotatory lateral olisthesis (LRO) was defined as the presence of lateral olisthesis of two adjacent vertebrae in the frontal plain X-rays (Fig. 2).
Fig. 1.
Example of measure of Cobb Angle of a scoliosis curve
Fig. 2.
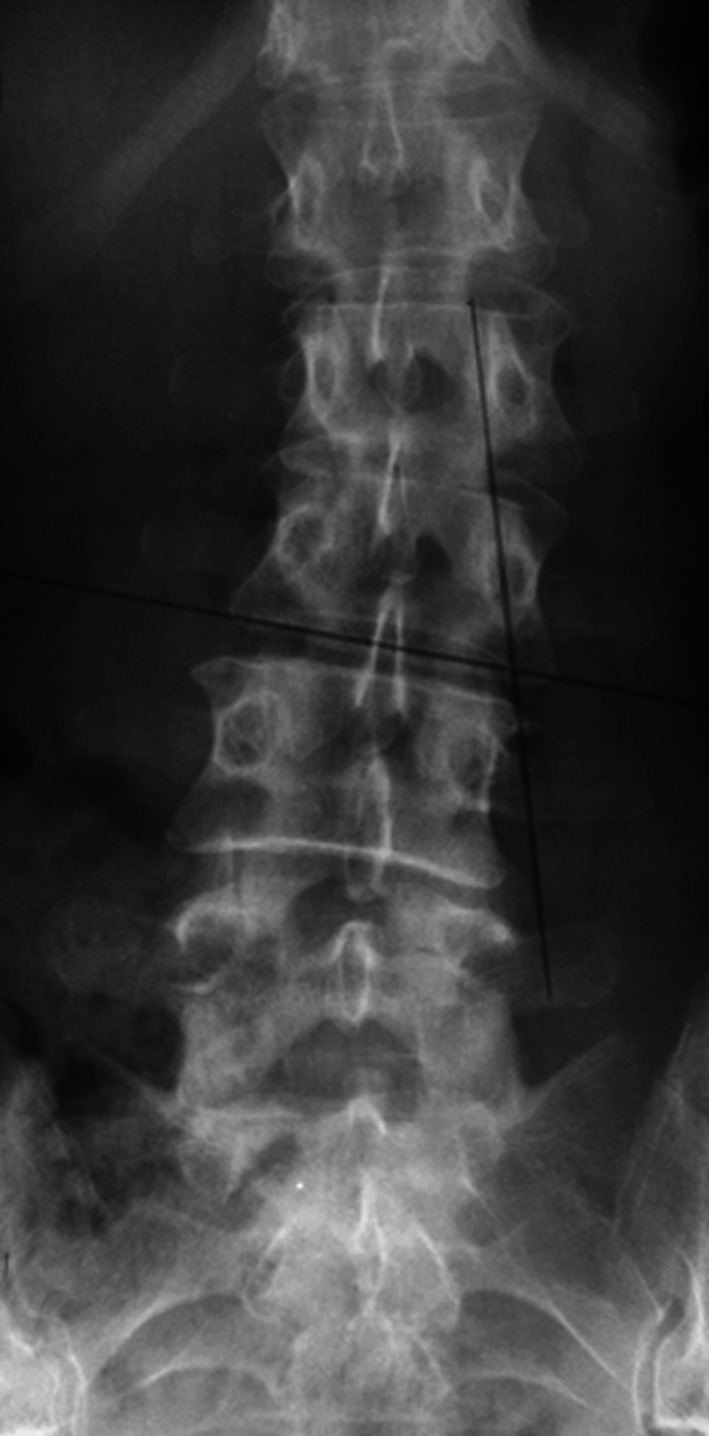
Example of degenerative progressive Scoliosis with progression of lateral listhesis
BMD and soft tissue body composition measurements
All the patients had a bone density measurement. Dual-energy X-ray absorptiometry (DEXA) (DPX-L, Lunar Corp., Madison, WI) was used to measure soft tissue body composition, and total and regional BMD. Scans were analysed with the manufacturer’s software. The scanner was calibrated daily using the standard calibration phantom supplied by the manufacturer. The coefficient of variation on the standard phantom was <1%. The total body acquisition mode yielded fat mass, lean mass and total BMD. Fat mass was expressed in terms of weight (g) and as a percentage of soft tissue body composition, lean mass was expressed in terms of weight (g), and BMD was expressed as weight/area (g/cm2). The BMD of the lumbar spine L2–4 and the non-dominant femoral sites were also measured independently.
Muscle strength test
A standard testing position was used for all subjects. They were instructed to sit upright in a chair with back support. The isometric strength of the biceps was tested by the maximum force the subject could pull to a flexion position of 90°. The results were expressed in kilograms (kg).
Statistical analysis
Participants were compared according to whether they had long-term HRT (>1) or had not received HRT or less than 1 year (<1) on the one hand, and the presence or absence of LRO on the other. Quantitative results (demographic, static and profile parameters) were expressed as mean ± SD, and the means were compared using a test of analysis of variance (Anova). Qualitative results (whether HRT had or had not been administered, the presence or absence of LRO, the presence or absence of scoliosis) were expressed as percentages, and frequencies were compared using Fisher’s exact test and Chi-squared test. All tests were two-sided. The level of significance was set to a p <0.05. After performing independent multiple comparisons, we calculated an experiment-wise error rate.
A multiple logistic regression model performed with the generalized linear model function (GLM) [18] was assessed in order to calculate the excess risk of LRO associated with specific clinical characteristics. We evaluated the LRO (adjusted for age, as a continuous response datum) as a function of HRT status (included as a binary variable) and muscle strength, total body lean and fat mass, and total body BMD (included as quantitative variables). As these quantitative variables were age-dependent, we first constructed a linear regression model for each of them according to age, and included residuals in the logistic regression model. The model included the individual factors and their pairwise combined effects (first-order interaction terms). To select the best minimal subset of explanatory variables to include in the logistic regression model, we conducted a stepwise model selection regression method. Variables were included in the final model if the p value was <0.10. Diagnostic tests were performed to detect non-normality and outliers. The best-fitting and less complex subsets of factors were selected using the lack-of-fit technique, which compares the maximum likelihood observed in two nested models. The comparison was assessed by the likelihood ratio test.
Because LRO predominantly affects the lumbar spine, a further logistic regression model was applied which included the BMD at the lumbar spine. In this model, the excess risk of LRO was determined by considering LRO as a function of HRT status and age, muscle strength, lean mass, fat mass and lumbar spine BMD (included as residuals resulting from linear regression models, in which age was the dependent variable).
Statistical analysis was performed using S-PLUS 6.1 software program for Windows (S-PLUS 6 for Windows, Insightful Corporation, Seattle, WA).
Results
Table 1 shows the demographic and clinical characteristics of the population. There were no significant differences in terms of age, BMI, muscle strength, or lean mass between the HRT >1 and HRT <1 women. Only fat mass was significantly higher in HRT <1 than in HRT >1 subjects (p = 0.04). The mean time since menopause was not significantly different in the two groups (p = 0.09). BMD at the lumbar spine and femoral neck was not different in HRT >1 and HRT <1 women, although there was a trend towards a higher Z-score in the HRT >1 women. The comparison of the radiological parameters according to HRT status (Table 1) showed that there was no significant difference in terms of sacral slope (p = 0.10), lumbar lordosis (p = 0.08) or thoracic kyphosis (p = 0.82). In the whole population, 47 women presented scoliosis, with a comparable prevalence in HRT >1 (25%) and HRT <1 (39%) (Fisher’s exact test: p = 0.09). Scoliosis was present in 28% of women aged below 66 years and in 38% in women aged above 66 years. In contrast, 27 women had LRO, and in these women there was a significant difference in the distribution of HRT status (p < 0.001), LRO being present in 8% of HRT >1 women (n = 6), but in 30% of HRT <1 women (n = 21). As independent comparisons were done at the 0.05 level, the experiment-wise error rate was 0.23 (i.e. the probability that at least one of them would result in a Type I error).
Table 1.
Demographic and radiological characteristics of the population (n = 146)
| Whole population (n = 146) (mean ± SD) | HRT > 1 (n = 75) (mean ± SD) | HRT < 1 (n = 71) (mean ± SD) | p value | |
|---|---|---|---|---|
| Demographic parameters | ||||
| Age (years) | 65.8 ± 5.7 | 65.0 ± 5.4 | 66.7 ± 5.9 | 0.07 |
| Weight (kg) | 61.9 ± 9.0 | 60.7 ± 8.5 | 63.2 ± 9.3 | 0.10 |
| Height (cm) | 158.7 ± 6.2 | 158.6 ± 6.4 | 158.8 ± 6.1 | 0.91 |
| BMI (kg/m²) | 24.6 ± 3.2 | 24.2 ± 3.4 | 25.0 ± 2.9 | 0.11 |
| Muscle strength (kg)a | 8.9 ± 2.6 | 9.0 ± 2.2 | 8.8 ± 3.0 | 0.65 |
| Lean mass (kg) | 37.8 ± 3.7 | 37.9 ± 3.6 | 37.8 ± 3.9 | 0.91 |
| Fat mass (kg) | 22.2 ± 7.5 | 20.8 ± 7.4 | 23.6 ± 7.5 | 0.04 |
| Femoral neck BMD (g/cm²) | 0.797 ± 0.119 | 0.810 ± 0.109 | 0.784 ± 0.128 | 0.22 |
| Z-score neck | 0.312 ± 0.917 | 0.365 ± 0.820 | 0.256 ± 1.012 | 0.47 |
| Lumbar spine BMD (g/cm²) | 1.059 ± 0.174 | 1.074 ± 0.170 | 1.043 ± 0.178 | 0.29 |
| Z-score lumbar spine | 1.126 ± 1.426 | 1.228 ± 1.365 | 1.019 ± 1.490 | 0.38 |
| Total body BMD (g/cm²) | 1.054 ± 0.092 | 1.068 ± 0.083 | 1.040 ± 0.098 | 0.09 |
| Gynaecological parameters | ||||
| Menopausal duration (years) | 15.8 ± 6.8 | 14.8 ± 6.3 | 16.8 ± 7.2 | 0.09 |
| HRT duration (years)b | 8.7 ± 6.1 | <1 | – | |
| Radiological parameters | ||||
| Sacral slope (°) | 37.4 ± 8.0 | 38.5 ± 7.2 | 36.3 ± 8.6 | 0.10 |
| Lumbar lordosis (°) | 56.8 ± 13.5 | 58.7 ± 9.7 | 54.8 ± 16.3 | 0.08 |
| Thoracic kyphosis (°) | 46.9 ± 15.5 | 47.2 ± 15.3 | 46.6 ± 15.7 | 0.82 |
| Scoliosis (% presence) | 32.2 | 25 | 39 | 0.09 |
| LRO (% presence)c | 18.5 | 8 | 30 | <0.001 |
p value tests the difference between HRT users (HRT > 1) and No HRT users (HRT < 1) in the population study, assessed by Anova (quantitative data) and a Fisher exact test (qualitative data)
aIsometric strength of brachial biceps
bAmong HRT users
cLateral rotatory olisthesis
We then compared the demographic, and radiological parameters, and HRT status factor in subjects without or with LRO (Table 2). Univariate analysis showed that except for age and HRT status, there were no significant differences between women with or without LRO for any of the other parameters. However, the Z-score at the lumbar spine was higher in the presence of LRO than in its absence (p = 0.05).
Table 2.
Comparison of demographic and radiological factors and HRT status in the women without lateral rotatory olisthesis (n = 119) and those with lateral rotatory olisthesis (n = 27)
| Without LRO (n = 119) (mean ± SD) | With LRO (n = 27) (mean ± SD) | p value | |
|---|---|---|---|
| Age (years) | 65.2 ± 5.6 | 68.8 ± 5.5 | <0.01 |
| Muscle strength (kg)a | 9.1 ± 2.5 | 8.2 ± 2.9 | 0.11 |
| Lean mass (kg) | 22.0 ± 6.9 | 23.0 ± 10.5 | 0.61 |
| Fat mass (kg) | 37.9 ± 35.4 | 37.3 ± 46.5 | 0.50 |
| Lumbar spine BMD (g/cm²) | 1.048 ± 0.164 | 1.115 ± 0.208 | 0.08 |
| Z-score lumbar spine | 1.017 ± 1.322 | 1.607 ± 1.766 | 0.05 |
| Total body BMD (g/cm²) | 1.056 ± 0.086 | 1.046 ± 0.120 | 0.63 |
| HRT use (%) | 58 | 22 | <0.001 |
p value tests the difference between without LRO group and with LRO group assessed by Anova (quantitative data) or a Chi-square test (qualitative data)
aIsometric strength of brachial biceps
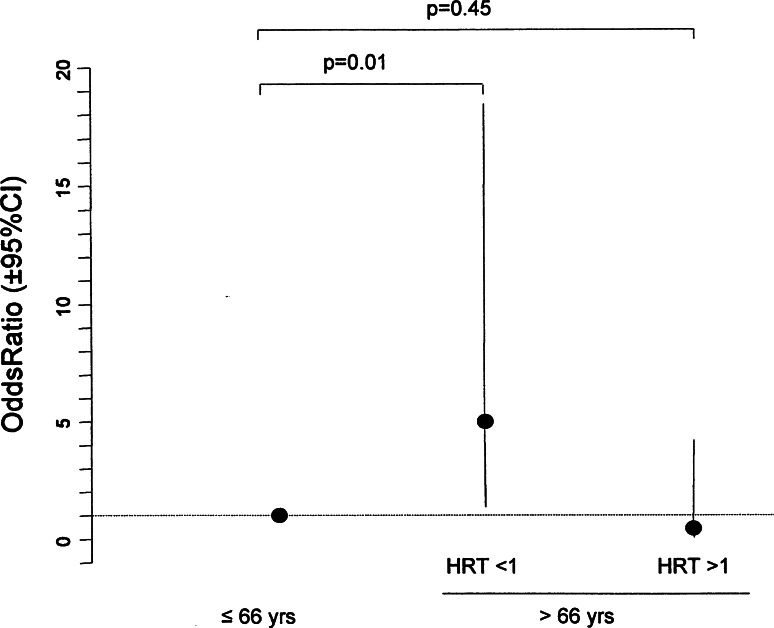
In order to evaluate the excess risk of LRO associated with the demographic and HRT status parameters, we used a multiple logistic regression model with stepwise model selection. This confirmed that only age and HRT had to be included in the final model. After comparing two nested designs [i.e. the model including an interaction (HRT status × age) vs. the simplest model without interaction], we found that both age and HRT status had dependent effects on the prevalence of LRO (p < 0.10 for age, HRT status and their interaction term, as described in the statistical methods for the selection of the stepwise model). However, LRO increased with age only in HRT <1 subjects (mean population study age ≤66 years, 11%; >66 years, 39%; p = 0.013), whereas in HRT >1 subjects, the prevalence of LRO remained stable (≤66 years, 9%; >66 years, 4%; p = 0.43). When women aged ≤66 years were used as the reference group (whatever the HRT used), the odds-ratio of having LRO in HRT <1 over 66 years of age was significantly increased (5, 95% CI 1.4–18.4, p = 0.01), whereas the odds-ratio of LRO in HRT >1 over 66 years of age was not significantly different (0.4, 95% CI 0.05–4.2, p = 0.45) (Fig. 3).
Fig. 3.
Logistic regression model: lateral rotatory olisthesis according to age in HRT <1 women and HRT >1 women. Odds and p values were calculated by logistic regression, as explained in the “Statistical analysis” section. The reference group for age was the group aged ≤66 years, regardless of their HRT status
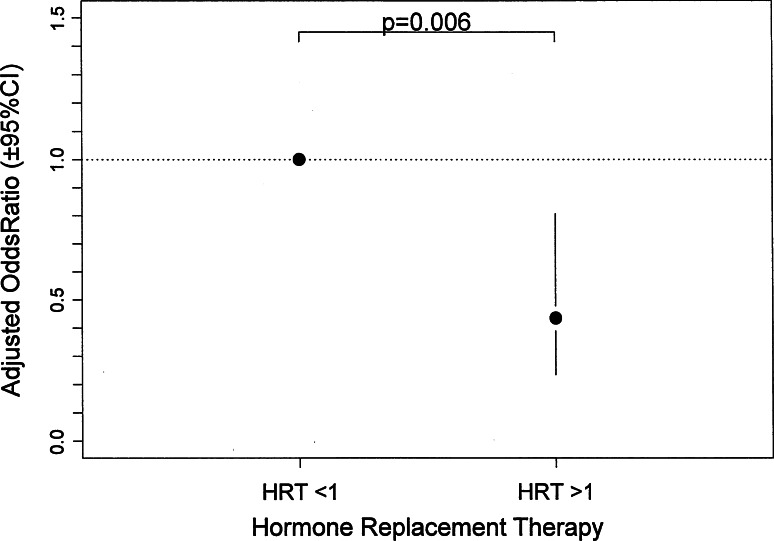
Although lumbar spine BMD was not significantly different in the univariate analysis between women with LRO and those without it (p = 0.08), we included this variable in the complete logistic regression model. After a parsimonious selection of variables by stepwise model selection, only HRT status, age and lumbar spine BMD were included in the final model (Table 3). Figure 4 shows that the risk of presenting LRO in HRT >1 women was significantly lower than that in HRT <1 women (0.43, 95% CI 0.23–0.81, p = 0.006), after an adjustment for age and lumbar spine BMD.
Table 3.
Model of multiple logistic regressions for lateral rotatory olisthesis in 146 postmenopausal women
| Coefficients | p valuesa | |
|---|---|---|
| Age | 0.5144 | 0.03 |
| HRT useb | −0.8930 | 0.006 |
| BMD lumbar spinec | 4.0084 | 0.03 |
| Muscular strength | 0.0275 | 0.90 |
| Fat mass | −0.0187 | 0.60 |
| Lean mass | −0.0285 | 0.72 |
The reference group for HRT status was the group of HRT <1 women. Age and bone mineral density at lumbar spine were included as quantitative variables
aLikelihood ratio test calculated to adjust the multiple regression using the lack-of-fit technique
bHormone replacement therapy
cBone mineral density
Fig. 4.
Logistic regression model: lateral rotatory olisthesis according to HRT status, adjusted for age and lumbar spine BMD. Odds and p values were calculated by logistic regression as explained in the “Statistical analysis” section. For the HRT >1 women, the odds ratio was adjusted for age and lumbar spine BMD. The reference group for HRT was the HRT <1 group
Discussion
In this cross-sectional study, we found that the prevalence of scoliosis was similar in women who had or had not received HRT. These results could indicate that HRT did not provide any protection against scoliosis. However, the presence of idiopathic scoliosis cannot be eliminated in the absence of previous X-rays. In post-menopausal adults, a spinal X-ray at a given time point does not discriminate between idiopathic and degenerative scoliosis, because the scoliosis observed could have developed before the menopause and this method is not appropriate for testing the effectiveness of hormone replacement therapy. In this retrospective study, the presence of LRO was markedly lower in women who had received HRT for at least 1 year (HRT > 1). When women with or without LRO were compared, those with LRO were slightly older, and their HRT status was significantly lower than that of women without LRO, while characteristics such as fat and lean mass and muscle strength were the same in the two groups. These data strongly suggest that HRT might provide some protection against LRO. Previous studies have shown that LRO is an early sign of degenerative alignment of the spine, and LRO triggers degenerative scoliosis with a small Cobb angle [4, 6, 8]. The lack of HRT might therefore precipitate the onset of LRO, which in turn could lead to the development of scoliosis, whereas HRT might reduce this risk. Moreover, the prevention of LRO as the first event in the development of scoliosis may be valuable not only for preventing scoliosis itself, but also for reducing lumbar back pain, as it has been shown that severe lateral olisthesis resulted in back pain and a lower quality of life [1, 9, 13, 19, 20]. We found that age is another risk factor for LRO. In the absence of HRT, a significantly higher risk is observed above the age of 66 years, while HRT reduces the risk of LRO regardless of age. Indeed, age might actually be an additional contributing factor for LRO in the absence of HRT. Other factors could have also influenced the development of LRO such as genetic determinants [5, 21] and intervertebral disc degeneration [22] which could be itself influenced by HRT as well as mechanical factors [23].
In order to investigate all the factors associated with LRO, we used a multivariate analysis including age, HRT status, as well as the factors which might be influenced by oestrogens, such as BMD, lean and fat mass, and muscle strength. BMD at the lumbar spine was included in the model because LRO is located at the mean lumbar vertebrae L3 or L4, as previously observed [14]. We found that age and HRT status were major determinants of LRO as well as of BMD at the lumbar spine, but not of the other parameters. Although higher BMD is expected in women with scoliosis, because of bone accretion secondary to the mechanical loading, we cannot exclude the contribution of bone to further mediating rotatory olisthesis. Oestrogens impact on several tissues that are responsible for the alignment of the spine as oestrogen receptors are expressed in many tissues. Various structural factors might be implicated, such as degenerative discs, facet–joint osteoarthritis, as well as ligaments and muscles [24, 25]. Oestrogens probably have anabolic effects on the muscles and ligaments of the spine. Oestrogen deficiency may induce lower mechanical resistance in spinal ligaments, and enthesis, which may play a role in muscle strength. HRT preserves muscle strength in postmenopausal women [24–29], and this is higher during the luteal phase [30]. HRT maintains the power output to a greater extent than isometric strength in postmenopausal women [31]. Using axial CT scans of the mid-thigh, Taaffe [32] showed that HRT preserves or improves skeletal muscle attenuation in early postmenopausal women, and has a positive effect on muscle performance. In our study, we did not observe any difference in muscle strength related to HRT, but our measurements were done at the biceps, which might not reflect muscle strength in the trunk.
The role played by bone in the onset of LRO has not been demonstrated so far, but it has recently been suggested that bone may contribute to scoliosis. Scoliosis is more frequent in osteoporotic patients than in osteopenic patients [16]. Lumbar bone loss was still observed in scoliotic patients below 60 years of age, and deformity worsened after HRT withdrawal, but not when bisphosphonates were used [17]. Mechanical strains transmitted to the bone might regulate bone turnover and modulate BMD. Therefore, high BMD might contribute to the development of LRO, rather than simply being a consequence. Nevertheless, our logistic regression model shows that after adjustment for age and BMD at the lumbar spine, the risk of having LRO is significantly lower in women with HRT >1 status, indicating that age and HRT status are the main factors involved. These effects further raise the question of the potential action of oestrogen receptor agonists in the development of LRO and of degenerative scoliosis.
In conclusion, we have shown that age and the absence of HRT are the main factors influencing LRO. The mechanical competence of oestrogen target tissues may contribute to the development of LRO, and may promote degenerative scoliosis. These findings shed a new light on the pathogenesis of spinal deformities. Other studies may be conducted in order to evaluate the contribution of oestrogens or of oestrogen receptor agonists in static indices of the spine.
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Schwab F, Dubey A, Gamez L, el-Fegoun AB, Hwang K, Pagala M, Farcy JP. Adult scoliosis: prevalence, SF-36 and nutritional parameters in an elderly volunteer population. Spine. 2005;30:1082–1085. doi: 10.1097/01.brs.0000160842.43482.cd. [DOI] [PubMed] [Google Scholar]
- 2.Daffner SD, Vaccaro AR. Adult degenerative lumbar scoliosis. Am J Orthop. 2003;32:77–82. [PubMed] [Google Scholar]
- 3.Schwab F, el-Fegoun AB, Gamez L, Goodman H, Farcy JP. A lumbar classification of scoliosis in the adult patient: preliminary approach. Spine. 2005;30:1670–1673. doi: 10.1097/01.brs.0000170293.81234.f0. [DOI] [PubMed] [Google Scholar]
- 4.Marty-Poumarat C, Scattin L, Marpeau M, Garreau de Loubresse C, Aegerter P. Natural history of progressive adult scoliosis. Spine. 2007;32:1227–1234. doi: 10.1097/01.brs.0000263328.89135.a6. [DOI] [PubMed] [Google Scholar]
- 5.Battié MC, Videman T, Levälahti E, Gill K, Kaprio J. Genetic and environmental effects on disc degeneration by phenotype and spinal level: a multivariate twin study. Spine (Phila Pa 1976) 2008;33:2801–2808. doi: 10.1097/BRS.0b013e31818043b7. [DOI] [PubMed] [Google Scholar]
- 6.Korovessis P, Piperos G, Sidiropoulos P, Dimas A. Adult idiopathic lumbar scoliosis. A formula for prediction of progression and review of the literature. Spine. 1994;190:1926–1932. doi: 10.1097/00007632-199409000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Piat C, Laredo JD, Tassin JL. Degenerative vertebral dislocation. Ann Radiol. 1995;38:214–220. [PubMed] [Google Scholar]
- 8.Murata Y, Takahashi K, Hanaoka E, Utsumi T, Yamagata M, Moriya H. Changes in scoliotic curvature and lordotic angle during the early phase of degenerative lumbar scoliosis. Spine. 2002;27:2268–2273. doi: 10.1097/00007632-200210150-00016. [DOI] [PubMed] [Google Scholar]
- 9.Jackson RP, Simmons EH, Stripinis D. Coronal and sagittal plane spinal deformities correlating with back pain and pulmonary function in adult idiopathic scoliosis. Spine. 1989;14:1391–1397. doi: 10.1097/00007632-198912000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Leboeuf D, Letellier K, Alos N, Edery P, Moldovan F. Do estrogens impact adolescent idiopathic scoliosis? Trends Endocrinol Metab. 2009;20:147–152. doi: 10.1016/j.tem.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Inoue M, Minami S, Nakata Y, et al. Association between estrogen receptor gene polymorphisms and curve severity of idiopathic scoliosis. Spine (Phila Pa 1976) 2002;27:2357–2362. doi: 10.1097/00007632-200211010-00009. [DOI] [PubMed] [Google Scholar]
- 12.Moe KE, Prinz LH, Larsen LH, Vitellio MV, Reed SO, Merriam GRT. Growth hormone in postmenopausal women after long term oral estrogen replacement therapy. J Gerontol Biol Sci. 1998;53:117–124. doi: 10.1093/gerona/53A.2.B117. [DOI] [PubMed] [Google Scholar]
- 13.Ploumis A, Liu H, Mehbod AA, Transfeldt EE, Winter RB. A correlation of radiographic and functional measurements in adult degenerative scoliosis. Spine (Phila Pa 1976) 2009;34:1581–1584. doi: 10.1097/BRS.0b013e31819c94cc. [DOI] [PubMed] [Google Scholar]
- 14.Esposito T, Uccello R, Caliendo R, et al. Estrogen receptor polymorphism, estrogen content and idiopathic scoliosis in human: a possible genetic linkage. J Steroid Biochem Mol Biol. 2009;116:56–60. doi: 10.1016/j.jsbmb.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Pritchett JW, Bortel DT. Degenerative symptomatic lumbar scoliosis. Spine (Phila Pa 1976) 1993;18:700–703. doi: 10.1097/00007632-199305000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Pappou IP, Girardi FP, Sandhu HS, et al. Discordantly high spinal bone mineral density values in patients with adult lumbar scoliosis. Spine (Phila Pa 1976) 2006;31:1614–1620. doi: 10.1097/01.brs.0000222030.32171.5f. [DOI] [PubMed] [Google Scholar]
- 17.Routh RH, Lee KM, Burshell AL, Nauman EA. The effects of anti-resorptive therapies and estrogen withdrawal in adult scoliosis measured by sub-segmental vertebral BMD analysis. Bone. 2009;45:193–199. doi: 10.1016/j.bone.2009.03.652. [DOI] [PubMed] [Google Scholar]
- 18.McCullagh P, Nelder JA. Generalized linear models. 2. London: Chapman and Hall; 1989. [Google Scholar]
- 19.Kostuik JP, Bentivoglio P. The incidence of low back pain in adult scoliosis. Acta Orthop Belg. 1981;47:548–559. [PubMed] [Google Scholar]
- 20.Velis KP, Thorne RP. Lateral spondylolisthesis in idiopathic scoliosis. Orthop Trans. 1979;3:282. [Google Scholar]
- 21.Aladin DM, Cheung KM, Chan D, Yee AF, Jim JJ, Luk KD, Lu WW. Expression of the Trp2 allele of COL9A2 is associated with alterations in the mechanical properties of human intervertebral discs. Spine (Phila Pa 1976) 2007;32:2820–2826. doi: 10.1097/BRS.0b013e31815b75c5. [DOI] [PubMed] [Google Scholar]
- 22.Keller TS, Colloca CJ, Harrison DE, Moore RJ, Gunzburg R. Muscular contributions to dynamic dorsoventral lumbar spine stiffness. Eur Spine J. 2007;16:245–254. doi: 10.1007/s00586-006-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson SJ, Steffen T. Biomechanics of the aging spine. Eur Spine J Suppl. 2003;2:S97–S103. doi: 10.1007/s00586-003-0621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips SK, Rook KM, Bruce NC, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci. 1993;84:95–98. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- 25.Phillips SK, Sanderson AG, Birch K, Bruce SK, Woledge RC. Changes in maximal voluntary force of human adductor pollicis muscle during the menstrual cycle. J Physiol. 1996;496:551–557. doi: 10.1113/jphysiol.1996.sp021706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heikkinen J, Kyllönen E, Kurttila-Matero E, Wilén-Rosenqvist G, Lankinen KS, Rita H, Väänänen HK. HRT and exercise: effects on bone density, muscle strength and lipid metabolism. A placebo controlled 2-year prospective trial on two estrogen–progestin regimens in healthy postmenopausal women. Maturitas. 1997;26:139–149. doi: 10.1016/S0378-5122(96)01098-5. [DOI] [PubMed] [Google Scholar]
- 27.Skelton DA, Phillips SK, Bruce SA, Naylor CH, Woledge RC. Hormone replacement therapy increases isometric muscle strength of adductor pollicis in post-menopausal women. Clin Sci (Lond) 1999;96:357–364. doi: 10.1042/CS19980318. [DOI] [PubMed] [Google Scholar]
- 28.Onambele NG, Skelton DA, Bruce SA, Woledge RC. Follow-up study of the benefits of hormone replacement therapy on isometric muscle strength of adductor pollicis in postmenopausal women. Clin Sci (Lond) 2001;100:421–422. doi: 10.1042/CS20000264. [DOI] [PubMed] [Google Scholar]
- 29.Greeves JP, Cable NT, Reilly T, Kingsland C. Changes in muscle strength in women following the menopause: a longitudinal assessment of the efficacy of hormone replacement therapy. Clin Sci. 1999;97:79–84. doi: 10.1042/CS19980406. [DOI] [PubMed] [Google Scholar]
- 30.Lemoine S, Granier P, Tiffoche C, Rannou-bekono F, Thieulant ML, Delamarche P. Estrogen receptor alpha mRNA in human skeletal muscles. Med Sci Sports Exerc. 2003;35:439–443. doi: 10.1249/01.MSS.0000053654.14410.78. [DOI] [PubMed] [Google Scholar]
- 31.Carville SF, Rutherford OM, Newham DJ. Power output, isometric strength and steadiness in the leg muscles of pre-and postmenopausal women: the effects of hormone replacement therapy. Eur J Appl Physiol. 2006;96:292–298. doi: 10.1007/s00421-005-0078-4. [DOI] [PubMed] [Google Scholar]
- 32.Taaffe DR, Sipila S, Cheng S, Puolakka J, Toivanen J, Suominen H. The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in postmenopausal women: a yearlong intervention. Clin Physiol Funct Imaging. 2005;25:297–304. doi: 10.1111/j.1475-097X.2005.00628.x. [DOI] [PubMed] [Google Scholar]