Abstract
Interhemispheric competition between homologous areas in the human brain is believed to be involved in a wide variety of human behaviors from motor activity to visual perception and particularly attention. For example, patients with lesions in the posterior parietal cortex are unable to selectively track objects in the contralesional side of visual space when targets are simultaneously present in the ipsilesional visual field, a form of visual extinction. Visual extinction may arise due to an imbalance in the normal interhemispheric competition. To directly assess the issue of reciprocal inhibition, we used fMRI to localize those brain regions active during attention-based visual tracking and then applied low-frequency repetitive transcranial magnetic stimulation over identified areas in the left and right intraparietal sulcus to asses the behavioral effects on visual tracking. We induced a severe impairment in visual tracking that was selective for conditions of simultaneous tracking in both visual fields. Our data show that the parietal lobe is essential for visual tracking and that the two hemispheres compete for attentional resources during tracking. Our results provide a neuronal basis for visual extinction in patients with parietal lobe damage.
INTRODUCTION
Theories of visual attention have postulated the presence of two networks in the brain, located in the right and left parietal cortices, which drive attention to the corresponding contralateral hemispace. There has been a long-standing debate about the extent of interhemispheric competition between these two networks (Mesulam, 1999; Kinsbourne, 1977). In particular, an activation-orienting hypothesis has been suggested in which each hemisphere generates a contralateral attentional bias when stimulated by an external stimulus by inhibiting its contralateral counterpart (Reuter-Lorenz, Kinsbourne, & Moscovitch, 1990). In a seminal paper studying cats, Sprague (1966) introduced the concept of inhibition between homologous areas in the two hemispheres, but similar effects have rarely been reported in humans studies. However, evidence for interhemispheric competition has come from neuropsychological studies on parietal neglect patients, which suggest that visual extinction is likely caused by an imbalance between homologous areas in the two hemispheres (Corbetta, Kincade, Lewis, Snyder, & Sapir, 2005; Vandenberghe et al., 2005; Driver & Vuilleumier, 2001; Vuilleumier, Hester, Assal, & Regli, 1996). More specifically, a unilateral cerebral lesion may have the effect of disinhibiting the contralateral healthy hemisphere. Consequently, an exaggerated physiological contralateral bias becomes evident when two visual stimuli are simultaneously presented one in each hemifield. Under these conditions, the contralesional stimulus is extinguished by the ipsilesional stimulus (Duncan et al., 1999). Recent fMRI (Geng et al., 2006; Corbetta et al., 2005; Fink, Driver, Rorden, Baldeweg, & Dolan, 2000) and animal (Rushmore, Valero-Cabre, Lomber, Hilgetag, & Payne, 2006) studies support this notion. However, there is no direct evidence in healthy observers that homologous areas in both hemispheres interact in a mutually inhibitory manner under conditions of sustained attention. Understanding this mutual inhibition could have considerable implications for treatment and rehabilitative strategies for patients with parietal lobe lesions (Fecteau, Pascual-Leone, & Theoret, 2006; Oliveri & Caltagirone, 2006).
A particularly important aspect of sustained attention is the ability to keep track of multiple moving targets simultaneously. Tracking enables us to compute spatial relations between objects, to navigate in our environment while avoiding moving obstacles, and to monitor multiple moving objects, such as keeping track of our children at the playground. Studies investigating attentive tracking have suggested competing interactions between attentional resources in the two hemispheres. Patients with right parietal lesions are unable to track two targets when presented in the left and right visual fields simultaneously, while they perform normally when tracking one target either in the left or in the right visual field (Battelli et al., 2001). Although the deficit is more pronounced in the contralesional visual field, ipsilesional deficits in patients with right parietal lesion have also been reported (Battelli, Cavanagh, Martini, & Barton, 2003; Duncan et al., 1999). This finding is likely related to visual extinction, a common neuropsychological deficit after lesion to the parietal lobes, characterized by an inability to detect a contralesional target when another target is present in the ipsilesional field (Driver & Vuilleumier, 2001).
Within the parietal lobe, the intraparietal sulcus (IPS) may be particularly important for attentive tracking. fMRI studies have shown that the IPS is active during visual tracking (Culham et al., 1998), and the activity in the IPS (among other areas) varies with the change in attentional load (i.e., activity is higher as the number of targets to be tracked is increased). In contrast, the nonparietal area MT+, although strongly activated during visual tracking, does not change activity with attentional load, suggesting that it is activated by the motion per se but not by attentional allocation (Culham, Cavanagh, & Kanwisher, 2001). These results suggest that IPS activity is more directly involved in tracking performance.
The present study provides a direct measurement of the interaction between homologous brain areas using TMS as a “virtual lesion” technique to test the role of a brain area in a specific task. We demonstrate that a TMS-induced temporary inactivation over the IPS causes extinction-like behavior in normal subjects during attentional tracking. Experiment 1 shows that the IPS is directly involved in visual tracking, and that the two hemispheres are in competition when attention is split between the two hemifields. Experiment 2 rules out an alternative explanation based on the number of items tracked.
EXPERIMENT 1: AN “EXTINCTION” EFFECT DURING FULL-FIELD ATTENTIONAL TRACKING
Methods
Observers
We tested a total of 11 healthy participants (3 authors and 8 naïve observers) in Experiments 1 and 2. All participants were 26 to 38 years of age, and had normal or corrected-to-normal vision. All participants were checked for TMS exclusion criteria (Wassermann, 1998) and gave written informed consent to the study which had been approved by the Beth Israel Deaconess Medical Center's Institutional Review Boards. The study was conducted in the Harvard–Thorndike General Clinical Research Center at BIDMC in order to provide the safest environment for the subjects. Eight individuals (4 men and 4 women) participated in Experiment 1, including two authors and six naïve observers.
Stimuli
Stimuli were displayed on a Macintosh G4 laptop using Matlab (MathWorks) in conjunction with the Psychophysics Toolbox (Brainard, 1997; Pelli, 1997). The displays consisted of a central fixation point (a black circle, radius = 0.15°) and eight moving items (black circles, radius = 0.3°) presented on a gray background. Four of the circles moved within a 9.4° × 9.4° region inset 2° to the left of fixation, and the other four moved within an equal size region inset 2° to the right of fixation. Items moved at a constant speed, repelled each other to maintain a minimum center to center spacing of 1.5° and “bounced” off of the invisible edges of the square region in which they moved.
Procedure
In the half-field condition, subjects tracked two target circles (in either the left or in the right visual field), and in the full-field condition, subjects tracked four target circles (2 on each side simultaneously) while keeping their eyes fixated on the central fixation point throughout each trial in both conditions. These conditions were first equated for difficulty by adjusting the target speed in a preliminary threshold session.
Threshold session
On the first day of testing, we equated all conditions for difficulty by determining the speed at which observers could perform the task with 75% accuracy. At the beginning of each trial, the fixation point was presented for 1 sec, then eight circles appeared (4 on the left, 4 on the right), and a subset blinked off and on at 2 Hz for 2 sec to identify them as targets for tracking. Then all of the circles moved without crossing the midline for 3 sec. After the items stopped, one of them was highlighted in red (50/50 target or distractor). The observer then indicated by keypress whether the red item was a target or distractor, with a response time cutoff of 3 sec. After the response, the fixation point turned green for a correct response or red for an incorrect response for 1 sec. The next trial began immediately following this feedback.
Subjects first performed a practice block (16 trials) in which the circles moved at 5 deg/sec to learn the task, and then a test block in which the circles moved at one of eight different speeds (5–26 deg/sec) on each trial, with the speeds randomly interleaved over 7 blocks of 32 trials each. It took approximately 45 min to complete this session. This threshold procedure was used to identify the speed at which two targets or four targets could be tracked with 75% accuracy. Different threshold speeds were obtained for each individual subject, and different speeds were obtained for two half-field targets and for four full-field targets.
Main experiment
In the main experiment, the response cutoff time was reduced to 2.5 sec and the feedback duration was reduced to 0.5 sec, so that the duration of tracking could be increased to 6 sec. A 3-sec duration was used in the threshold session to reduce the duration of that session.
There were two conditions presented randomly intermixed within each block of trials. In the half-field condition, two targets were presented either in the left or the right hemifield, whereas in the full-field condition, four targets were presented with two in each hemifield (Figure 1A). As described above, these conditions were matched for difficulty by setting the speed to the 75% accuracy threshold for each individual subject in each condition. In each block of trials, observers completed 48 trials (24 per condition) at their threshold speed. There were two experimental sessions (on separate days), each consisting of four blocks of trials, with a 20-min rest period in between each block. The first session (Day 1) had the following four blocks: (1) baseline, (2) TMS Site 1, (3) TMS Site 2, (4) baseline. The second session (Day 2) had the following four blocks: (1) baseline, (2) TMS Site 3, (3) TMS Site 4, (4) baseline. The four TMS sites were the left IPS, right IPS, left MT+, and right MT+. Within a session, subjects received TMS over one IPS site on one hemisphere, and one MT+ site on the opposite hemisphere, with the brain area (IPS/MT+) and hemisphere (left/right) orders counterbalanced across subjects.
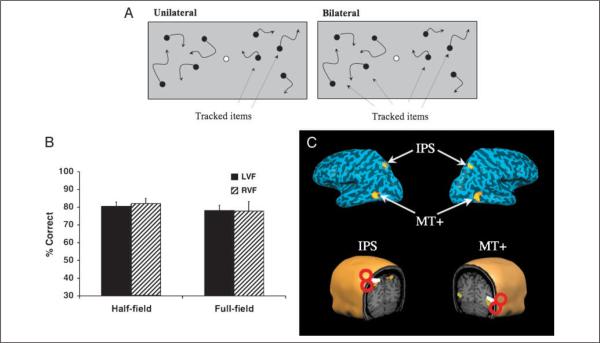
Figure 1.
(A) Schematic representation of the two task conditions. Half-field: The subject tracked two targets presented either on the right or left of fixation (white circle). Full-field: The subject tracked four targets presented in the left and right visual field simultaneously. Solid arrows indicate motion and they were not present during the actual task. Dotted arrows point to the targets (not present during the task). (B) Baseline data before TMS. Half-field and full-field percent correct performance (average of 8 subjects) for left (black bars) and right (hatched bars) visual fields in the two conditions: half-field and full-field. (C) Top: Activations are depicted on inflated left and right hemispheres of a single representative subject: intraparietal sulcus (IPS) and middle temporal areas (MT+). Bottom: The four magnetic stimulation sites identified in the fMRI experiment on the scalp of each subject. Red circles and white handles are a cartoon representation of the TMS coil.
fMRI Protocol and Stimulation Site Reconstruction
Subjects were scanned on a 3-T Phillips Intera scanner at the Boston University Center for Biomedical Imaging. Functional imaging was conducted using an echo-planar imaging sequence (TR = 2000 msec, TE = 30 msec, flip angle = 90°, FOV = 192 mm, matrix size = 64 × 64). Scanned volumes were 30 oblique slices (3 × 3 mm in-plane resolution, 3 mm thick; interslice gap of 0.3 mm) covering the parietal and occipital lobes. The first four images of each 580 acquisition functional run were discarded to allow for T1 equalization. High-resolution T1-weighted structural scans (1 × 1 × 1 mm) were acquired for each subject to verify the anatomical locations of selected ROIs.
IPS and MT+ localizer
IPS and MT+ were localized using a block-design multiple-object tracking experiment similar to that used by Culham et al. (2001). Subjects were shown eight randomly moving dots presented in a fixed window approximately 15° × 15° of visual angle. Subjects were instructed to either passively view or actively track a varying number of dots (1, 2, or 4) cued at the beginning of the trial. Each of the four conditions was repeated four times in each run. Trials were 24 sec in length followed by a 12-sec rest period.
Acquired data were analyzed using the Brainvoyager QX (version 1.6). Functional data were motion corrected, slice scan time corrected, spatial smoothed (4 mm FWHM), and coregistered to high-resolution anatomical data. A model of the expected response of IPS and MT+ was generated using previously reported response properties for the two ROIs (Culham et al., 2001). ROIs were defined as a significant (p < .001, uncorrected) cluster of voxels in the parietal and medial temporal lobes. For all subjects, the most robust activation during multiple object tacking was observed in the parietal lobe bilaterally extending between the IPS and the postcentral sulcus.
High-resolution functional images were overlaid onto the anatomical images, obtained from the same fMRI experimental session using a frameless stereotaxy system (BrainSight, Rogue Research, Montreal, Canada). A three-dimensional anatomical reconstruction was used to visualize and measure the Talairach coordinates of the projected cortical target of the IPS and MT+ stimulation sites in all subjects (Figure 1C, example of one representative subject). The projected target of stimulation over the IPS corresponded to the posterior extent of the IPS, the same region implicated in visual tracking responsive in the fMRI study we conducted beforehand and corresponded well to previously reported results (Culham et al., 1998). The MT+ stimulation site projected onto the ascending extent of the lateral occipital sulcus, corresponding to reported location of MT+ as determined through ours and previous neuroimaging studies (Grossman, Battelli, & Pascual-Leone, 2005; Seiffert, Somers, Dale, & Tootell, 2003; Huk, Dougherty, & Heeger, 2002; Sunaert, Van Hecke, Marchal, & Orban, 1999). Although fMRI revealed some individual variability in the functional activation during active tracking, for stimulation over the IPS, the average center of stimulation in stereotaxic space for all subjects corresponded to the following Talairach x, y, and z values: −24, −62, and 50 for left and 25, −60, and 50 for the right hemisphere, respectively (Talairach & Tournoux, 1988). For MT+, the average coordinates were −36, −64, and 13 for the left and 46, −63, and 3 for the right hemisphere, respectively.
TMS Protocol
Subject performance was calibrated individually prior to any brain stimulation, as described above (Threshold Session). TMS was delivered using a MagStim stimulator (MagStim, Whitland, UK) and a commercially available 70-mm figure-of-eight MagStim stimulation coil. We applied a 10-min train of repetitive low-frequency (1 Hz) stimulation at 75% maximum stimulator output over one of the four brain sites, right IPS or MT+ and left IPS or MT+. Each subject underwent two testing sessions separated by at least 24 hr. To aid in brain site localization, subjects wore a Lycra swimmer's cap on which the reference points for simulation were marked (see previous section for localization technique). The coil was held with the handle pointing backward toward the back of the head and positioned perpendicular to the stimulated region.
Immediately following the repetitive stimulation over the targeted brain site, subjects performed the visual tracking task (same task as the prestimulation baseline). The time required to perform the psychophysical task (approximately 10 min) is within that for which rTMS has been shown to have lasting effects in parietal regions (Hilgetag, Theoret, & Pascual-Leone, 2001). After completion of the task, observers rested for 15 min to allow complete recovery from the stimulation. Stimulation was then applied to the other brain site in the opposite hemisphere, and the subject again performed the task. The order of brain site stimulation was counterbalanced across observers, which allowed us to control for any effects of “double-dose” of stimulation (there were none).
Following the second stimulation, task completion, and a 15-min recovery period, participants repeated the task to obtain a final poststimulation baseline. The same procedure was used in the second testing session.
Results
Only a slight reduction in speed (on average, 2 deg/sec) was necessary for subjects to track four targets in each field as well as two targets presented within a single half-field (t < 1 for targets tested in left and right visual fields, respectively; mean speed: full-field = 13.5 deg/sec; half-field = 15.5 deg/sec; Figure 1B). This is consistent with previous work demonstrating that it is possible to track four targets in separate hemifields with little or no cost over tracking two targets within a single hemifield, suggesting that tracking is functionally independent in the two visual fields (Alvarez & Cavanagh, 2005). The tracking task was then run at these threshold speeds throughout the entire TMS experiment.
Previous studies using off-line TMS with low-frequency stimulation have shown that the behavioral effect after TMS can last, on average, half as long as the time of the stimulation (Robertson, Theoret, & Pascual-Leone, 2003). Because we stimulated for 10 min, we divided our post-TMS data into two halves with the first and the last 5 min after stimulation analyzed separately. Of principal interest was whether performance in the contralateral visual field (opposite TMS) was different than in the ipsilateral visual field (same side as TMS), and in particular, whether there was a contralateral deficit (worse performance in the contralateral field).
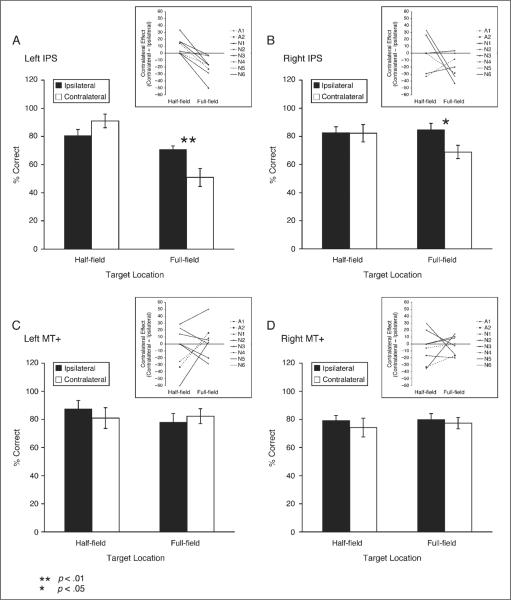
TMS over the left IPS induced a significant contralateral impairment in the full-field tracking condition (track four targets, two in each hemifield), but only in the first 5 min post-TMS. Ipsilateral and contralateral performance in the full-field condition were significantly different from each other for the first 5 min after TMS [t(7) = 3.72, p < .01], but not in the last 5 min after TMS (t < 1). Performance returned to baseline level for the last 5 min post-TMS, indicating full recovery. In the half-field condition (track two targets in one hemifield), there was a significant trend for an improvement in the contralateral field [t(7) = 2.47, p < .05] in the first 5 minutes, but not in the last 5 min (t < 1) (Hilgetag et al., 2001).
TMS over the right IPS also induced a contralateral deficit in the full-field condition. Although the magnitude of the effect was smaller [left IPS vs. right IPS for full-field contralateral condition, t(7) = 3.35, p < .05], the effect had the same time course as TMS over the left IPS, showing an effect only in the first 5 min. In the full-field condition, ipsilateral and contralateral performance with TMS over the right IPS were significantly different from each other in the first 5 min after TMS [t(7) = 2.48, p < .05; Figure 2B], but not in the last 5 min after TMS (t < 1). Performance returned to baseline for the last 5 min after TMS over the right IPS, indicating full recovery. TMS over the left or right visual motion area MT+ did not induce any significant changes in performance in either the half-field or full-field condition (Figure 2C and D).
Figure 2.
Subjects' performance following left (A) and right (B) IPS stimulation, and following left (C) and right (D) MT+ stimulation for the first 5 min post-TMS. Black bars indicate ipsilateral performance (same side as TMS) and white bars contralateral performance (opposite side of TMS). Error bars represent one within-subject standard error of the mean, calculated with Cousineau's (2005) modification of Loftus and Masson's (1994) method. Asterisks indicate where performance was significantly different between the contralateral and ipsilateral conditions (only during full-field tracking following left or right IPS stimulation). Insets show individual-subject difference scores (contralateral – ipsilateral), where positive values indicate a relative advantage in the contralateral field, and negative values indicate a relative deficit in the contralateral field. Authors are shown with dashed lines (A = 2), and naïve observers with solid lines (N = 6).
EXPERIMENT 2: CONTROL FOR THE NUMBER OF OBJECTS TRACKED
Although we carefully matched the half-field and full-field conditions in terms of attentional load by equating task performance, the total number of targets tracked was greater in the full-field condition than in the half-field condition of Experiment 1. Thus, it remains possible that the number of targets tracked, independent of attentional load, accounts for the contralateral deficit observed in the full-field condition of Experiment 1. To address this possibility, we required observers to track either two or four targets all within the same half of the visual field. If increasing the number of targets from two to four results in a contralateral deficit during half-field tracking, then it would seem that it is the number of targets that is important, not whether tracking occurs in one hemifield or both hemifields simultaneously. However, if we do not see a contralateral deficit for tracking four half-field targets, it would support the conclusion that the large contralateral deficit observed in the full-field condition of Experiment 1 was due to simultaneous tracking in both halves of the visual field.
Methods
All aspects of the method were identical to Experiment 1, except as noted here.
Observers
Eight individuals participated. Three were authors (two also participated in Experiment 1), and five were naïve subjects (three also participated in Experiment 1). We had functional localizers for all the subjects for the left IPS (the only site we stimulated in Experiment 2).
Stimuli and Procedure
The following modifications were made to the displays to allow for the presentation of eight items in each visual field so that up to four items could be tracked within a single half of the visual field. The displays consisted of a central fixation point (a black circle, radius = 0.15°) and 16 moving items (black circles, radius = 0.3°) presented on a gray background. Eight of the circles moved within a 17.1° × 17.1° region inset 2° to the left of fixation, and the other eight moved within an equal size region inset 2° to the right of fixation. Participants tracked either two or four targets in the right visual field or in the left visual field (i.e., always within half of the visual field). The same speed threshold procedure as in Experiment 1 was used to estimate the speed thresholds for tracking two or four circles within a hemifield. Within a trial, the items moved at a constant speed (eight speeds from 2 to 23 deg/sec for the threshold session, and at about 75% accuracy threshold speed for each individual participant in the main experimental session). Items repelled each other (minimum center to center spacing = 2.58) and “bounced” off of the invisible edges of the square region in which they moved.
We tested only the half-field condition, and the targets were always presented either in the left or in the right visual field, and separate 75% correct speed thresholds were measured for tracking two and tracking four targets to equate the two conditions for difficulty or attentional load.
TMS Protocol
Each subject underwent one testing session during which we applied TMS over the left IPS at the same intensity and frequency as in Experiment 1.
Results
A significant reduction in speed (9 deg/sec on average) was necessary for subjects to track four targets in a half-field as well as two targets within a half-field [2 half-field targets = 17.8 deg/sec; 4 half-field = 8.8 deg/sec; t(7) = 10.27, p < .001]. This is consistent with previous work, which suggests that targets within a hemifield compete for attentional resources (Alvarez & Cavanagh, 2005), and that more targets can be tracked when the target speed is reduced (Alvarez & Franconeri, 2007). The tracking task was then run at these threshold speeds throughout the entire TMS experiment.
With the speed adjustment, baseline performance in the main experiment was well matched for tracking two or four targets within a hemifield (79% for 2 targets, 79% for 4 targets, t < 1). We applied TMS to the left IPS, where we saw the largest, most robust contralateral effect in Experiment 1, and the question was, do we see a large contralateral deficit whenever four targets are tracked, even if all of the targets are within a single half of the visual field? The answer appears to be no (Figure 3). None of the ipsilateral versus contralateral differences were significant in the first 5 min or in the second 5 min after stimulation (all ts < 1, all ps > .59). Particularly notable is the lack of difference in the first 5 min for the contralateral condition (Figure 3, compare white bars for 2 and 4 targets): Performance was comparable for two half-field targets and four half-field targets (77% vs. 77%, t < 1). This is in stark contrast to the results of Experiment 1, where in the first 5 min the difference in the contralateral condition between two half-field targets and four full-field targets was large and significant [91% vs. 51%, respectively; t(7) = 3.76, p < .01]. Given that a contralateral deficit does not arise under a single hemifield load of four targets, the contralateral deficit for four full-field targets in Experiment 1 does not appear to be due to the number of targets. Instead, it appears to be due to the full-field, bilateral nature of the tracking task in that condition.
Figure 3.
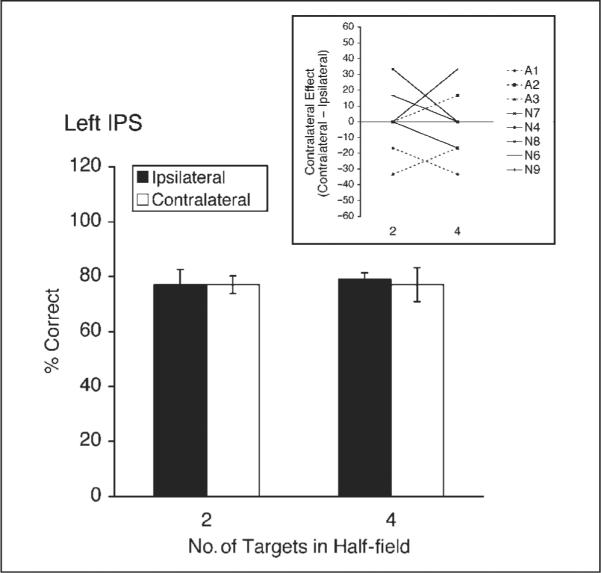
Subjects' performance for two and four targets in one-half of the visual field following left IPS stimulation in Experiment 2, for the first 5 min after stimulation. Black bars indicate ipsilateral performance (same side as TMS) and white bars contralateral performance (opposite side of TMS). Error bars represent one within-subject standard error of the mean, calculated with Cousineau's (2005) modification of Loftus and Masson's (1994) method. The inset shows individual-subject difference scores (contralateral – ipsilateral), where positive values indicate a relative advantage in the contralateral field, and negative values indicate a relative deficit in the contralateral field. Authors are shown with dashed lines (A = 3), and naïve observers with solid lines (N = 5).
DISCUSSION
Our results show that low-frequency rTMS over the IPS impairs performance on attention-based visual tracking of moving stimuli, whereas TMS over the motion area MT+ does not alter behavioral performance. These findings are consistent with those of Culham et al. (1998), who used fMRI to show that the IPS is selectively activated during attentive tracking, whereas activity in MT+ does not distinguish between active tracking and passive viewing of the same moving stimuli. An alternative explanation of our results could be a disruption of eye movements after TMS over the IPS. However, this is very unlikely, as a disruption of eye movements should have had a similar effect for both the full-field and half-field tracking condition, but this was not the case in our experiment. Several fMRI studies have also shown that MT+ is robustly activated when subjects are asked to perform visual pursuit tasks (Dukelow et al., 2001; Barton et al., 1996), however, only the IPS is selectively activated during active tracking (Culham et al., 1998), and this ability was affected by TMS over the IPS in Experiment 1.
A Neural Model for Attentive Tracking
Although we found that the IPS plays a selective role during attentive tracking, TMS had an effect only during full-field tracking, and not during half-field tracking. This difference cannot be due to differences in task difficulty between the full-field and half-field tasks because we psychophysically measured the subjects' speed sensitivity before the TMS experiment and equated the two conditions for difficulty. Moreover, Experiment 2 ruled out the number of targets tracked as a possible explanation of the deficit we found during full-field tracking as TMS had no effect when subjects were asked to track four targets within a single hemifield. So what is the difference between the half-field and full-field conditions?
Previous cognitive research on attentive tracking has found evidence for independent tracking of targets in the left and right visual hemifields (Alvarez & Cavanagh, 2005), as if independent resources were operating in each visual field. Similar findings using different experimental paradigms have also been found in normal subjects (Kingstone, 2004; Corballis & Gratton, 2003) as well as in commissurotomy patients (Holtzman & Gazzaniga, 1982, 1985). Although Alvarez and Cavanagh (2005) did not make any claims about the underlying neural basis of hemifield independence, the most obvious possibility was that the left and right cerebral hemispheres operate independently in this task. From this view, the left parietal lobe would be solely dedicated to tracking targets in the right visual field, and the right parietal lobe would only track targets in the left visual field. The current study rules out this simple possibility and suggests that the functional independence in tracking between the hemifields must arise from a more complex network of competitive interactions between the left and right cerebral hemispheres.
The current results, and the functional independence observed in attentive tracking (Alvarez & Cavanagh, 2005), can both be accounted for by the following neural model of attentive tracking. Each IPS is able to control the selection of targets in both the ipsilateral and contralateral visual field. However, in normal conditions, inhibitory connections between the left and right IPS reduce the amount of ipsilateral processing performed by each hemisphere.
For example, when a single target was being tracked in the right visual field, the left IPS would strongly inhibit the right IPS. In the baseline condition of our experiment (without TMS), the left IPS would serve as the primary control center for tracking the target, and would actively inhibit the right IPS. There would be little or no change from this situation following TMS to the right IPS because the right IPS is already inhibited without TMS. However, following TMS to the left IPS, the right IPS would be released from inhibition and would then serve as the primary control center for tracking the target, whereas the left IPS would make a reduced contribution.
When tracking targets in both visual fields, each IPS would inhibit ipsilateral processing of the other IPS. Thus, in the baseline condition, the left IPS would be the control center for tracking the target in the right visual field, and the right IPS would be the control center for tracking the target in the left visual field, where neither IPS would contribute to tracking in the ipsilateral field. Following TMS to the left IPS, the amount of inhibition on the right IPS would be reduced. However, given that there are targets in both visual fields, the right IPS is dedicated to tracking the target in the left visual field, and therefore, cannot compensate for the reduced functioning of the left IPS. Behaviorally, this results in an inability to track the target in the right visual field. In the case of TMS to the right IPS, the pattern would be reversed.
This model also accounts for the functional independence observed in the Alvarez and Cavanagh (2005) study. Under normal conditions, due to inhibitory connections between the left and right IPS, each IPS contributes primarily to tracking targets in the contralateral visual field. Thus, when tracking a target in the left visual field (controlled primarily by left IPS), there is little to no cost to adding a second target to the right visual field (controlled by the right IPS), and vice versa.
This model of attentive tracking has two key components: (1) The IPS in each hemisphere can control the selection of targets in either the ipsilateral or the contralateral visual field, and (2) under normal conditions inhibitory processes reduce the amount of ipsilateral processing by each IPS. Future research will be required to determine exactly how these inhibitory effects are realized. There could be direct links between the left and right IPS via the corpus callosum, in which case the inhibition would be from IPS to IPS, and would result in reduced activity in the inhibited IPS. Alternatively, there could be projections from lower-level visual areas to the contralateral IPS, and the inhibition could occur by limiting the amount of communication over these connections. In this case, the amount of IPS activity would not be reduced by inhibition, but activity in areas which communicate to the contralateral IPS would be reduced (Geng et al., 2006).
MT+ and Attentive Tracking
Several recent studies on nonhuman primates have all shown that neurons in middle temporal area (MT) are modulated by attention (Recanzone & Wurtz, 2000; Treue & Martinez Trujillo, 1999; Treue & Maunsell, 1996). However, the attentional effects are always relatively small (Seidemann & Newsome, 1999; Ferrera & Lisberger, 1997; Newsome, Wurtz, & Komatsu, 1988) and the attentional modulation strength depends on stimulus attributes such as contrast and size (Pack, Hunter, & Born, 2005; Martinez-Trujillo & Treue, 2002). In particular, neurons in area MT fire more strongly to large low-contrast stimuli (Pack et al., 2005), whereas in our study, we used small high-contrast stimuli and this could have contributed to a moderate involvement of the MT+ area in our visual tracking task.
We are not claiming that MT+ is not involved in tracking motion, however, it might well be that its contribution is solely due to the presence of a strong motion signal, as has be argued previously (Culham et al., 2001).
The Neuronal Basis of Visual Extinction
The contralateral deficit we found in our study appears to manifest only under conditions in which there are competing tasks in the two hemifields, as in extinction with neglect patients (Mesulam, 1999).
In our experiment, there was a trend for TMS to exert a stronger effect over the left IPS than over the right. Indeed, for left TMS, subjects' performance dropped to chance level in the full-field condition during the first 5 min after TMS. Our results lend support to Kinsbourne's (1977) biased hemispheric-competition model of visuo-spatial attention, which postulated a stronger contralateral bias of the left hemisphere than the right hemisphere. In addition, many studies on visual neglect have emphasized that the rightward bias of the left attentional system is stronger than the leftward bias of the right attentional system (Bartolomeo & Chokron, 2002). Our results are consistent with this view because TMS over the left IPS caused a more severe temporary drop in contralateral performance than TMS over the right IPS. Finally, recent studies have suggested a left hemisphere superiority for selective attention (Chokron, Brickman, Wei, & Buchsbaum, 2000; Tabert et al., 2000). Our experiment required subjects to perform an endogenous, top–down selective attentional task and this might account for the more severe impairment we found after TMS over the left IPS.
Conclusions
In conclusion, our results demonstrate the direct involvement of the parietal lobe, particularly the IPS, in sustained attention. In addition, the selective effect of TMS during full-field tracking provides strong evidence for inhibition by the opposite hemisphere when both visual fields demand attention simultaneously. These results could have important implications in designing rehabilitation strategies for stroke recovery (Fecteau et al., 2006).
Acknowledgments
This work was supported by NEI EY15960 to L. B., by NIH/NIMH F31-MH069095 and NIH/NEI F32-EY016982 to G. A. A., by the Netherlands Organization for Scientific Research (NWO Pioneer) to T. C., by RO1-EY12091, R21-EY0116168 and K24 RR018875 to A. P. L., and the Harvard-Thorndike General Clinical Research Center at Beth Israel Deaconess Medical Center (NCRR MO1 RR01032).
REFERENCES
- Alvarez GA, Cavanagh P. Independent resources for attentional tracking in the left and right visual hemifields. Psychological Science. 2005;16:637–643. doi: 10.1111/j.1467-9280.2005.01587.x. [DOI] [PubMed] [Google Scholar]
- Alvarez GA, Franconeri SL. How many objects can you attentively track?: Evidence for a resource-limited tracking mechanism. Journal of Vision. 2007;7:1–10. doi: 10.1167/7.13.14. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P, Chokron S. Orienting of attention in left unilateral neglect. Neuroscience and Biobehavioral Reviews. 2002;26:217–234. doi: 10.1016/s0149-7634(01)00065-3. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Simpson T, Kiriakopoulos E, Stewart C, Crawley A, Guthrie B, et al. Functional MRI of lateral occipitotemporal cortex during pursuit and motion perception. Annals of Neurology. 1996;40:387–398. doi: 10.1002/ana.410400308. [DOI] [PubMed] [Google Scholar]
- Battelli L, Cavanagh P, Intriligator J, Tramo MJ, Henaff MA, Michel F, et al. Unilateral right parietal damage leads to bilateral deficit for high-level motion. Neuron. 2001;32:985–995. doi: 10.1016/s0896-6273(01)00536-0. [DOI] [PubMed] [Google Scholar]
- Battelli L, Cavanagh P, Martini P, Barton JJ. Bilateral deficits of transient visual attention in right parietal patients. Brain. 2003;126:2164–2174. doi: 10.1093/brain/awg221. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Chokron S, Brickman AM, Wei T, Buchsbaum MS. Hemispheric asymmetry for selective attention. Brain Research, Cognitive Brain Research. 2000;9:85–90. doi: 10.1016/s0006-8993(99)02169-1. [DOI] [PubMed] [Google Scholar]
- Corballis PM, Gratton G. Independent control of processing strategies for different locations in the visual field. Biological Psychology. 2003;64:191–209. doi: 10.1016/s0301-0511(03)00109-1. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nature Neuroscience. 2005;8:1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson's method. Tutorial in Quantitative Methods for Psychology. 2005;1:42–45. [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RB. Cortical fMRI activation produced by attentive tracking of moving targets. Journal of Neurophysiology. 1998;80:2657–2670. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavanagh P, Kanwisher NG. Attention response functions: Characterizing brain areas using fMRI activation during parametric variations of attentional load. Neuron. 2001;32:737–745. doi: 10.1016/s0896-6273(01)00499-8. [DOI] [PubMed] [Google Scholar]
- Driver J, Vuilleumier P. Perceptual awareness and its loss in unilateral neglect and extinction. Cognition. 2001;79:39–88. doi: 10.1016/s0010-0277(00)00124-4. [DOI] [PubMed] [Google Scholar]
- Dukelow SP, DeSouza JF, Culham JC, van den Berg AV, Menon RS, Vilis T. Distinguishing subregions of the human MT+ complex using visual fields and pursuit eye movements. Journal of Neurophysiology. 2001;86:1991–2000. doi: 10.1152/jn.2001.86.4.1991. [DOI] [PubMed] [Google Scholar]
- Duncan J, Bundesen C, Olson A, Humphreys G, Chavda S, Shibuya H. Systematic analysis of deficits in visual attention. Journal of Experimental Psychology: General. 1999;128:450–478. doi: 10.1037//0096-3445.128.4.450. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Pascual-Leone A, Theoret H. Paradoxical facilitation of attention in healthy humans. Behavioural Neurology. 2006;17:159–162. doi: 10.1155/2006/632141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera VP, Lisberger SG. Neuronal responses in visual areas MT and MST during smooth pursuit target selection. Journal of Neurophysiology. 1997;78:1433–1446. doi: 10.1152/jn.1997.78.3.1433. [DOI] [PubMed] [Google Scholar]
- Fink GR, Driver J, Rorden C, Baldeweg T, Dolan RJ. Neural consequences of competing stimuli in both visual hemifields: A physiological basis for visual extinction. Annals of Neurology. 2000;47:440–446. [PubMed] [Google Scholar]
- Geng JJ, Eger E, Ruff CC, Kristjansson A, Rotshtein P, Driver J. On-line attentional selection from competing stimuli in opposite visual fields: Effects on human visual cortex and control processes. Journal of Neurophysiology. 2006;96:2601–2612. doi: 10.1152/jn.01245.2005. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Battelli L, Pascual-Leone A. Repetitive TMS over posterior STS disrupts perception of biological motion. Vision Research. 2005;45:2847–2853. doi: 10.1016/j.visres.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Theoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced “virtual lesions” of human parietal cortex. Nature Neuroscience. 2001;4:953–957. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- Holtzman JD, Gazzaniga MS. Dual task interactions due exclusively to limits in processing resources. Science. 1982;218:1325–1327. doi: 10.1126/science.7146915. [DOI] [PubMed] [Google Scholar]
- Holtzman JD, Gazzaniga MS. Enhanced dual task performance following corpus commissurotomy in humans. Neuropsychologia. 1985;23:315–321. doi: 10.1016/0028-3932(85)90018-1. [DOI] [PubMed] [Google Scholar]
- Huk AC, Dougherty RF, Heeger DJ. Retinotopy and functional subdivision of human areas MT and MST. Journal of Neuroscience. 2002;22:7195–7205. doi: 10.1523/JNEUROSCI.22-16-07195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingstone A. Hemispheric performance in object-based attention. Psychonomic Bulletin & Review. 2004;11:84–91. doi: 10.3758/bf03206465. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. Hemi-neglect and hemisphere rivalry. Advances in Neurology. 1977;18:41–49. [PubMed] [Google Scholar]
- Loftus GR, Masson ME. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo J, Treue S. Attentional modulation strength in cortical area MT depends on stimulus contrast. Neuron. 2002;35:365–370. doi: 10.1016/s0896-6273(02)00778-x. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: Parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. Journal of Neurophysiology. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Caltagirone C. Suppression of extinction with TMS in humans: From healthy controls to patients. Behavioural Neurology. 2006;17:163–167. doi: 10.1155/2006/393924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack CC, Hunter JN, Born RT. Contrast dependence of suppressive influences in cortical area MT of alert macaque. Journal of Neurophysiology. 2005;93:1809–1815. doi: 10.1152/jn.00629.2004. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Recanzone GH, Wurtz RH. Effects of attention on MT and MST neuronal activity during pursuit initiation. Journal of Neurophysiology. 2000;83:777–790. doi: 10.1152/jn.2000.83.2.777. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Kinsbourne M, Moscovitch M. Hemispheric control of spatial attention. Brain and Cognition. 1990;12:240–266. doi: 10.1016/0278-2626(90)90018-j. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Theoret H, Pascual-Leone A. Studies in cognition: The problems solved and created by transcranial magnetic stimulation. Journal of Cognitive Neuroscience. 2003;15:948–960. doi: 10.1162/089892903770007344. [DOI] [PubMed] [Google Scholar]
- Rushmore RJ, Valero-Cabre A, Lomber SG, Hilgetag CC, Payne BR. Functional circuitry underlying visual neglect. Brain. 2006;129:1803–1821. doi: 10.1093/brain/awl140. [DOI] [PubMed] [Google Scholar]
- Seidemann E, Newsome WT. Effect of spatial attention on the responses of area MT neurons. Journal of Neurophysiology. 1999;81:1783–1794. doi: 10.1152/jn.1999.81.4.1783. [DOI] [PubMed] [Google Scholar]
- Seiffert AE, Somers DC, Dale AM, Tootell RB. Functional MRI studies of human visual motion perception: Texture, luminance, attention and after-effects. Cerebral Cortex. 2003;13:340–349. doi: 10.1093/cercor/13.4.340. [DOI] [PubMed] [Google Scholar]
- Sprague JM. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science. 1966;153:1544–1547. doi: 10.1126/science.153.3743.1544. [DOI] [PubMed] [Google Scholar]
- Sunaert S, Van Hecke P, Marchal G, Orban GA. Motion-responsive regions of the human brain. Experimental Brain Research. 1999;127:355–370. doi: 10.1007/s002210050804. [DOI] [PubMed] [Google Scholar]
- Tabert MH, Chokron S, Tang CY, Wei T, Brickman AM, Buchsbaum MS. Visual target detection paradigm for the study of selective attention. Brain Research, Brain Research Protocols. 2000;6:80–85. doi: 10.1016/s1385-299x(00)00040-4. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-Dimensional proportional system: An approach to cerebral imaging. Stuttgart, Germany: Georg Thieme Verlag. 1988 [Google Scholar]
- Treue S, Martinez Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Geeraerts S, Molenberghs P, Lafosse C, Vandenbulcke M, Peeters K, et al. Attentional responses to unattended stimuli in human parietal cortex. Brain. 2005;128:2843–2857. doi: 10.1093/brain/awh522. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Hester D, Assal G, Regli F. Unilateral spatial neglect recovery after sequential strokes. Neurology. 1996;46:184–189. doi: 10.1212/wnl.46.1.184. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalography and Clinical Neurophysiology. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]