Abstract
Genetically engineered cells with mutations of relevance to electroporation, cell membrane permeabilization by electric pulses, can become a promising new tool for fundamental research on this important biotechnology. Listeria monocytogenes mutants lacking DltA or MprF and assayed for sensitivity to the cathelicidin like anti-microbial cationic peptide (mCRAMP), were developed to study the effect of cell wall charge on electroporation. Working in the irreversible electroporation regime (IRE), we found that application of a sequence of 50 pulses, each 50 μs duration, 12.5 kV/cm field, delivered at 2 Hz led to 2.67±0.29 log reduction in wild-type L. monocytogenes, log 2.60±0.19 in the MprF-minus mutant, and log 1.33±0.13 in the DltA-minus mutant. The experimental observation that the DltA-minus mutant was highly susceptible to cationic mCRAMP and resistant to IRE suggests that the charge on the bacterial cell wall affects electroporation and shows that this approach may be promising for fundamental studies on electroporation.
Keywords: Irreversible electroporation, Membrane charge, Electroporation threshold, Listeria monocytogenes, Lipoteichoic acid
1. Introduction
Electroporation is a physico–chemical process during which the cell membrane is permeabilized by externally applied pulse electric fields (PEF) [1]. Presumably, the cell membrane permeabilization increases due to the formation of nanoscale defects – pores – in the membrane [2]. The permeabilized cells either survive after pore reseal or die [3,4]. The process during which the cells survive is called reversible electroporation and the process under which the cells die is known as irreversible electroporation.
In fact, reversible electroporation has become an important tool in biotechnology and medicine in the last thirty years [5]. It affords the introduction of external substances into cells while keeping cells alive. Examples of reported applications of reversible electroporation are gene delivery to cells [1] and tissues [6], and the introduction of drugs into cells [7]. Moreover, reversible electroporation is a basis for a cancer treatment therapy known as “electrochemotherapy”, in which otherwise impermeable cytotoxic chemicals are delivered to cancerous tumors to induce tumor death [8].
Cell inactivation by pulse electric field is an emerging technique in food, biotechnology and medicine. The classical study on the effect of PEF on microbial inactivation was performed by Sale and Hamilton [9–11]. Since then, numerous publications investigated the possibility to apply PEF as an alternative non-thermal pasteurization and storage technology for foods [12–14] and drugs [15]. In medicine, irreversible electroporation has emerged as a soft tissue ablation technique [16]. During PEF tissue ablation of multi-cell structures, such as blood vessels and nerves, the structures remain intact and neighboring cells are not affected [17].
Although the phenomenon of electroporation has been a subject of research for five decades, an established molecular mechanism is yet to be discovered. In order to characterize the cells response to PEF treatment, semi-empirical mathematical models are used [18–21]. Moreover, process optimization is performed for each specific product and cell type. It was shown in numerous experiments that successful PEF cell inactivation depends on process, product and cell characteristics [13]. Indeed, the efficiency of electroporation depends on process parameters such as electric field strength (E), pulse duration (t), number of pulses (N), pulse shape, and temperature. In addition, it depends on biological cell size, geometry, lipid membrane structure and growth rate. Moreover, the following medium properties were shown to be important for electroporation achievement: conductivity, water activity and pH [13].
The precise mechanism of electroporation is not yet fully understood; and therefore electroporation protocols are usually developed through lengthy trial and error procedures. It is important to point out that a single and uniform PEF protocol for all classes of bacteria and cells has not yet been found. Moreover, reported strain specific PEF resistance variations present a substantial difficulty for robust process design. Indeed, significant variations in PEF resistance were reported for different strains of L. monocytogenes [22] and Escherichia coli [23].
Theoretical models of electroporation indicate that critical electroporation potential threshold and pore formation density are affected by transmembrane potential [24–28]. The thermodynamic theory of electroporation as a function of buffer strength was developed by Neumann and Kakorkin [24]. In that work the authors showed that in general, increasing positive surface charge density on the membrane/buffer interface reduces the Gibbs free energy for pore formation [24,25]. In a cell contribution of electrochemical potential and surface charge contribute to the total transmembrane potential:
| (1) |
Where Δφm is a total transmembrane potential; Δφec is a membrane electrochemical potential, derived in equilibrium from the Nernst/Goldman equation equation for resting potential [29]; Δφec potential contribution of surface charge group explicit equation for which appears in [25].
Once the external electric field is applied the transmembrane potential is given by Eq. (2) (based on [25]):
| (2) |
Where E is the strength of the electric field; f(σ)is a conductivity function; a is a cell radius; θ is the angle of the radial direction vector. θ is zero (and cos θ=1) when the vector that coincides with the direction of the electrical field.
It is reported that in excess of a critical Δφm of – 1 V rapid electrical breakdown and local conformational changes of bilayer structures occur, thus causing the electroporation phenomenon [30].
In a series of studies, Cheng introduced a biomechanical model of electroporation, which connects the membrane critical electroporation threshold with membrane physical properties such as thickness and charge [26–28]. In those studies Cheng proposed an inverse correlation between membrane charge and electroporation thresholds. Decreasing membrane charge from one side possibly leads to the decreased transmembrane potential. Weaver and Mintzer showed that transmembrane potential diminishes the energy barrier to pore formation [31]. It was also shown that pore formation frequency increases with increased transmembrane potential [2]. The developed models led us to the hypotheses that differences in surface charge between various bacterial strains lead to variation in PEF resistance.
To the best of our knowledge the previous works investigated the impact of the surface charge on electroporation threshold through the variations in the surrounding media pH and ion strength [32]. Gambaro et al. investigated the impact of the surrounding media pH and ion strength on electroporation threshold in lipid bilayers composed of egg lecithin and cholesterol [32]. In that work the minimum threshold for electroporation was achieved at pH 3. Multiple experimental studies in the food sterilization field showed that decreasing the medium pH increases the vulnerability of both Gram-positive and -negative bacteria as well as yeast to electroporation. Increased pulsed electric field disinfection efficiency at lower pH was also reported with E.coli [33], Saccharomyces cerevisiae, Lactobacillus plantarum LA 10–11, Listeria innocua NCTC 11289, L. innocua NCTC 11289 [34], Bacillus subtilis, L monocytogenes, Staphylococcus aureus, Salmonella serotype Senftenberg 775 W and Yersinia enterocolitica [35]. Gomez et al. reported on inactivation of L. monocytogenes by PEF in media of different pH [36,37]. In that work five orders of magnitude difference in bacterial inactivation was achieved by reducing the buffer pH from 7 to 3.5 [37].
The goal of this work is to investigate, on the molecular level, if specific charge modifications of L. monocytogenes cell wall constituents lead to variation in PEF resistance.
L monocytogenes is a ubiquitous, Gram-positive, facultative anaerobic, food-borne pathogen and the causative agent of listeriosis [38]. Gram-positive bacteria possess a highly variable protective surface structure, which includes thick layers of peptidoglycan interlaced with additional glycopolymers that form the cell wall. One of the best studied glycopolymers is techoic acid, which may be attached either to the peptidoglycan or to membrane lipids [39].
Production of cationic antimicrobial peptides is an innate host immune defense that many Gram-positive bacteria avoid by increasing cell surface electronegativity [40,41]. One mechanism to increase cell surface charge is modification of techoic acids by d-ala esterification, which is carried out through the action of the dlt operon in a variety of bacteria including L. monocytogenes [42–46]. Inactivation of dltA has been shown to inhibit d-ala esterification resulting in susceptibility to cationic peptides in many Gram-positives including L. monocytogenes [42–48]. Another mechanism to increase cell surface charge is lysinylation of membrane phosphatidyl glycerol, which is carried out through the action of mprF in several Gram-positives, including L. monocytogenes [49,50].
In this work we showed experimentally, for the first time, that cell wall charge can impact the irreversible electroporation threshold in L. monocytogenes. Wild-type (wt), MprF-minus, and DltA-minus L. monocytogenes were differentially susceptible to antimicrobial cationic peptide activity, suggesting decreased cell surface charge. A DltA-minus mutant, which showed the largest sensitivity to cationic peptide activity, showed the largest resistance to pulse electric field treatment in milk. Our findings are in agreements with previously developed biophysical models [24–28], and may contribute to the fundamental knowledge in the field of electroporation and suggest that developing of functional cells with defined mutations may become a useful tool for electroporation research in medicine and biotechnology.
2. Methods
2.1. Bacterial strains
L. monocytogenes strain 10403S, which is naturally resistant to streptomycin, was used in this study. Stationary-phase bacterial cultures were prepared by inoculation of Brain Heart Infusion (BHI) broth with a single colony and overnight incubation at 30 °C.
2.2. L. monocytogenes mutations
In-frame internal deletion of dltA from the bacterial chromosome was generated by allelic exchange as previously described [51]. Briefly, 500 nucleotide flanking regions of dltA were amplified and ligated by SOE PCR with the following primers: 5′-ATT AGT CGA CCG TAA AAG TTT GAT TTA CTA TAT CCA AAA T-3′, 5′-AAT AAA GAA TTG TGC CGT ATG GTA AAC TCA AAT TCT TTC TAT GAT ACT CGT TGT CAT TAT-3′, 5′-ATA ATG ACA ACG AGT ATC ATA GAA AGA ATT TGA GTT TAC CAT ACG GCA CAA TTC TTT ATT-3′, and 5′-ATT ACT GCA GAG GCA ATA GTG AAC TTG CTT TTA AAC CAT T-3′. The resulting fragment was ligated into pKSV7, a shuttle vector capable of replication in E. coli and temperature-sensitive replication in L. monocytogenes and other Gram-positives, with SalI and PstI. The resulting construct, ΔdltApKSV7, was conjugated into L. monocytogenes and integrated into the L. monocytogenes genome by homologous recombination between the bacterial chromosome and homologous sequences on the plasmid. Integration mutants were enriched for by growth at 40°C, a non-permissive temperature for plasmid replication, and isolated in the presence of chloramphenicol. Mutants that spontaneously excised the integrated plasmid were enriched for by growth at 30°C in the absence of chloramphenicol selection and chloramphenicol-sensitive revertants were isolated and in-frame internal deletion of dltA was confirmed by PCR.
A transposon insertion in mprF was isolated in a previous genetic screen using a Himar1 mariner based transposon library [52]. Briefly, Himar1 is a transposable element of the Tc1/mariner superfamily. A transposon library was previously constructed using the vector pJZ037 that contained the Himar1 transposase under the control of the pSpac(hy) promoter, the transposon containing the EM resistance gene (ermAM) flanked on both sides by T7 promoters oriented outwards and inverted repeats. L.monocytogenes transposon insertion mutants were selected in the presence of erm and screened on blood-agar plates. A mutant was isolated with a transposon insertion in mprF [52]. The schematic representation of the mutations appears in Fig. 1.
Fig. 1.
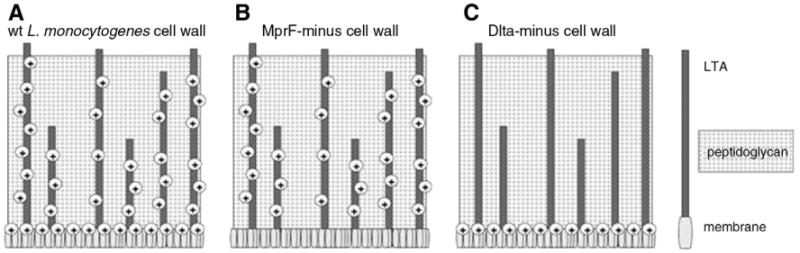
Schematic representation of the impact on cell wall constituent charge of L. monocytogenes mutants A. Wild type, B. MprF-minus, C. DltA-minus mutants.
2.3. Cationic peptides
Overnight stationary-phase cultures were backdiluted 1:40 and grown shaking at 37°C for 4 h to mid-log phase and optical density (OD) 600 nm was monitored at 15-minute intervals with a Spectra-Max 340PC384 Absorbance Microplate Reader (Molecular Devices, Sunnyvale, CA). At mid-log phase, 10 μg/ml mouse cathelicidin like anti-microbial peptide (mCRAMP) (Anaspec, Freemont, Ca) was added and OD 600 nm was monitored for an additional 5 h. Five repeats were performed for each protocol.
2.4. Irreversible electroporation protocol
Streptomycin (Sigma-Aldrich,USA) was added to pasteurized 2% fat milk purchased in a local store to a concentration of 60 μg/ml. The milk was contaminated with DltA-minus, MprF-minus, or wt L. monocytogenes to ∼7*106 CFU/ml. In this work we treated 90 μl of the contaminated milk immersed in 1 mm gap cuvette (Genesee Scientific,San Diego,CA ) by applying a sequence of 50 pulses, each 50 μs duration, 12.5 kV/cm field, delivered at 2 Hz using ECM 830 square pulse generator (BTX, Harvard Apparatus,MA, USA). Temperature was measured in the cuvette using a Neoptix Reflex® signal conditioner with a 1-mm probe covered with polyimide (Neoptix, Québec, Canada). Five repeats were performed for each protocol.
2.5. Bacterial count
Bacterial CFUs were determined by the spread counting method. The milk samples were diluted in Dulbecco's phosphate-buffered saline to eliminate effects of media on cell growth [53] and were plated on Luria-Bertani Miller (LB) agar plates and incubated at 37 °C for 24 h. Three dilutions were plated for each sample and CFUs were quantified the following day.
2.6. Statistics
Statistical analyses were performed using the Microsoft Office Excel 2007 external package, as well as student t-tests with unequal variances.
3. Results
L. monocytogenes mutations
DltA and MprF deficient L. monocytogenes mutants were obtained by internal in-frame deletion and transposon-mediated mutagenesis respectively. Briefly, dltA was deleted from the bacterial chromosome by use of pKSV7, a Gram-positive thermosensitive suicide vector that allows allelic exchange deletion mutagenesis [51]. A construct, ΔdltApKSV7, that contained 500 nucleotide flanking regions of dltA was introduced into L. monocytogenes by conjugation. A mutant that underwent allelic exchange and no longer contained dltA was isolated and confirmed by PCR. MprF-minus L. monocytogenes was acquired by transposon mutagenesis in a previous study using a Himar1 mariner based transposon library [52]. Briefly, the vector pJZ037 was introduced into L. monocytogenes by electroporation to create a transposon library and a subsequent blood agar screen led to the identification and isolation of a transposon insertion mutation within mprF that had completely disrupted the gene [52]. The schematic representation of the mutations appears in Fig. 1.
3.1. Cationic peptides
To determine whether mutations in L. monocytogenes dltA, and mprF affected electronegativity of the bacterial cell surface (Fig. 1), we assayed for sensitivity to cationic peptides. Wild-type, DltA-minus and MprF-minus L. monocytogenes were grown in BHI and mCRAMP was added at mid-log phase. No growth defect was observed in untreated DltA-minus and MprF-minus L. monocytogenes cultures (Fig. 2). Wild-type L. monocytogenes was unaffected by mCRAMP (Fig. 2A). Addition of mCRAMP at mid-log phase inhibited growth of MprF-minus L. monocytogenes (Fig. 2B). Upon addition of mCRAMP, OD 600 nm slightly decreased from 155 min to 285 min, after which logarithmic growth resumed and MprF-minus cultures nearly reached stationary phase. Addition of mCRAMP to DltA-minus L. monocytogenes resulted in decrease of OD 600 nm, indicating death (Fig. 2C). These results indicated that DltA-minus and MprF-minus L. monocytogenes are differentially sensitive to the cationic peptide mCRAMP suggesting the cell surface of DltA-minus is more electronegative than MprF-minus and wild-type L. monocytogenes.
Fig. 2.
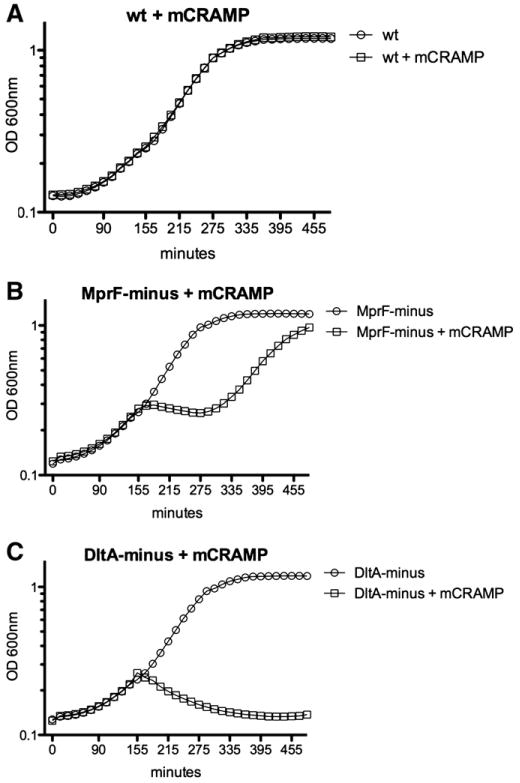
Cationic peptide effect on L. monocytogenes mutants. At mid-log phase, 10 μg/ml mouse cathelicidin like anti-microbial peptide (mCRAMP) was added to A. wt, B. MprF-minus, C. DltA-minus L. monocytogenes. OD 600 nm was monitored for an additional 5 h. Five repeats were performed for each protocol.
3.2. PEF inactivation of L. monocytogenes
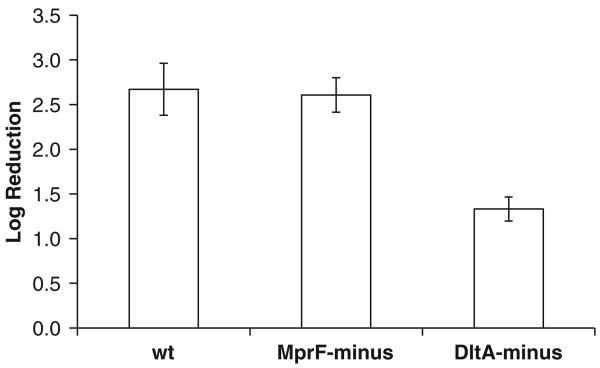
Application of 50 pulses, each50 μs duration, 12.5 kV/cm field, delivered at 2 Hz led to 2.67 ± 0.29 log reduction of wt L. monocytogenes, log 2.60 ± 0.19 MprF-minus L. monocytogenes, and log 1.33±0.13 DltA-minus L. monocytogenes (Fig. 3). The temperature change increase was 7.6±0.4 °C with a maximum temperature no more than 34 °C.
Fig. 3.
PEF inactivation of various L. monocytogenes mutants. 90 μl of the contaminated milk immersed in 1 mm gap cuvette were treated by applying a sequence of 50 pulses, each 50 μs duration, 12.5 kV/cm field, delivered at 2 Hz. Five repeats were performed for each treatment.
4. Discussion
During the last three decades, electroporation has become a very important medical and biomedical technology. While the exact molecular mechanisms by which electroporation is produced is not known, ad-hoc experimental studies have generated numerous practical protocols for inducing reversible and irreversible electroporation in various types of cells and electroporation medium. Previous experimental observations on the medium pH impact and theoretical studies suggested an important role of surface charge on the critical electric field needed for electroporation. This study has employed bacterial genetics to directly demonstrate the relationship of Gram-positive bacterial cell wall constituent charge with the effect of externally applied electric field on microbe survival. Moreover, we suggest that a fundamental understanding of the process of electroporation can be advanced by analyzing bacterial cells with mutations relevant to the process of electroporation and characterizing subsequent differences in their sensitivity to externally applied electric field.
The results from Fig. 2 indicate that resistance of L. monocytogenes mutants to mCRAMP is dependent on cell wall modifications induced by the action of specific genes. Lysinylation of membrane phosphatidyl glycerol through the action of mprF and d-ala esterification of techoic acids by the dlt operon provide cell wall constituents with positive charge, which likely repel positively-charged mCRAMP from the cell membrane. While wt L. monocytogenes was unaffected by mCRAMP, MprF and DltA-minus mutants were differentially sensitive to the cationic peptide.
The DltA-minus mutant showed high sensitivity to mCRAMP and failed to grow in its presence. In fact, there was a rapid and continuous decrease in OD 600 nm following addition of mCRAMP, which indicates bacterial killing. In contrast, growth of MprF-minus L. monocytogenes was inhibited initially by addition of mCRAMP, but growth resumed to near wt rate and cultures eventually reached near stationary phase OD 600 nm. This indicates MprF-minus was not significantly killed by mCRAMP and growth was only temporally inhibited, suggesting that MprF-minus was affected by mCRAMP activity, but not nearly to the extent of DltA-minus L. monocytogenes.
This dramatic difference suggests that d-ala esterified techoic acid charge provides a much greater contribution to the total cell wall charge than lysinylation of membrane phosphatidyl glycerol. However, it is possible that in the absence of MprF there is compensation by the dlt operon and L. monocytogenes and expression is increased to accelerate or increase esterification of teichoic acid to increase the total cell surface charge.
The theoretical considerations (Eq. 2) and experimental results from Fig. 2 lead to an explanation of the results shown on Fig. 3. Wt L. monocytogenes was not affected by mCRAMP (Fig. 3A) suggesting that its cell wall is positively charged, due to the contribution of both lysinylation of membrane phosphatidyl glycerol and d-ala-esterification of the techoic acids. DltA-minus L. monocytogenes was highly susceptible to mCRAMP (Fig. 2C) indicating a significant decrease in cell surface charge. We found that this decrease in cell surface charge has led to an increase in resistance to PEF treatment (Fig. 3). MprF-minus L. monocytogenes proliferated and reached the “plateau” phase in the presence mCRAMP (Fig. 2B) and showed the similar resistance to PEF treatment as the wt (Fig. 3).
Each of the three components: electrochemical potential, surface charge potential and externally electric field induced potential impact the total transmembrane potential (Eq. (2)). Electroporation commences once the total transmembrane potential is higher than a specific threshold [30]. J. Teissié et al. suggested that the cell permeabilization is not homogeneous since the distribution of the potential depends on the specific location on the membrane [54]. The specific area which undergoes electropermeabilization is calculated from Eq. (3) as follows [54,55]:
| (3) |
Where Smis the portion of the total membrane on which electroporation takes place, φcis the critical tranmembrane potential which leads to electropermeabilization. The size and number of pores (defects) which can appear only inside Sm is controlled only by the number of pulses and pulse duration [54].
Based on Eqs. (2) and (3) we hypothesize that given the same external electric field, large cell wall positive charge leads to a larger surface area inside which pore formation occurs. This consequently leads to the larger probability of cell death in the case of IRE. Results from Fig. 2 and other studies [42,44–46] suggest that DltA-minus L. monocytogenes has much less positive charge than wt or MprF-minus. According to the proposed mechanism this leads to the smallest area of the cell membrane that can be permeabilized, causing the higher resistivity to PEF. In contrast to the DltA-minus mutant, MprF-minus survived the cationic peptide treatment and showed similar vulnerability to PEF as wt. The similar inactivation pattern may be explained by the contribution of the ratio of lysinylation of membrane phosphatidyl glycerol and d-ala esterification of lipoteichoic acid to the total positive charge of the cell wall, for which additional studies are needed. We suspect that the PEF strength used in this study was not sufficient to show the differences in resistance between MprF-minus and wt L. monocytogenes likely due to similar electroporation surface area Sm which possibly predefines the probability for cell death.
Our results are consistent with membrane lipid studies. The effects of lipid head charge and tail composition on critical break voltage were investigated on black lipid membranes(BLM) [56]. In that work it was shown that breakdown voltage is similar for BLM made of lipids with phosphatidylserine headgroups and BLM made of lipids with phosphatidylcholine headgroups, and was not dependent on buffer strength [56]. In contrast, another study showed that increased membrane surface tension by repulsion of polar lipid heads reduces the electroporation potential threshold. Moreover, asymmetry is an additional factor which impacts the critical electroporation potential due to the complementary effect of the internal electric field [57]. The presence of charged LTA likely contributed to membrane charge asymmetry and thus to the additional effect of internal electric field.
The study in this paper has also a practical value. Ingestion of L. monocytogenes by immunocompromised individuals and pregnant women results in severe disease and can lead to death or spontaneous abortion, respectively. Outbreaks of human listeriosis occur due to contaminated food, often soft cheeses, milk, and meat, making L. monocytogenes a serious threat to food safety [58–60]. L. monocytogenes possess various attributes which enable resistance to low pH, high salt concentration, temperature and allow replication at refrigeration temperatures, which make them well equipped to survive food safety processing techniques [60]. PEF were applied among other non-thermal methods for L. monocytogenes inactivation in milk [61–64]. Differences in PEF resistance were previously reported for various strains of L. monocytogenes [22]. In this work we tested the hypotheses that cell wall charge impacts the resistance of bacteria to PEF treatment. Our experimental results show that cell wall constituents' charge affects the irreversible electroporation threshold. Natural variation in the cell wall charge, which is possible within a bacterial population, should be taken into account in the electroporation planning.
5. Conclusions
To summarize, we demonstrated that the charge of cell wall constituents impacts the pulse electric field resistance of various L. monocytogenes strains. Decreasing the cell wall charge through inhibition of d-ala esterification of LTA caused significant increase in the resistance of L. monocytogenes to PEF treatment. Our approach may lead to the development of new synthetic biology based cell modifications that will elucidate the electroporation mechanism.
Acknowledgments
We thank Prof. Daniel Portnoy from UC Berkeley for providing assistance concerning the microbiological aspects of this project. The work was partly supported by NIH grants PO1 A1063302 and PO1 A127655 to DP. AG and BR acknowledge AngioDynamics Inc. for the gift supporting the electroporation research.
Footnotes
Author contributions: AG conceived the experiments, performed electroporation experiments, analyzed the data and drafted the manuscript, CR constructed the mutants, verified the cell wall charge and drafted the manuscript, BR supervised the work and drafted the manuscript
References
- 1.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1:841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver JC, Chimadzev YA. Theory of electroporation: a review. Bioelectrochem Bioenerg. 1996;41:135–160. [Google Scholar]
- 3.Somolinos M, Mañas P, Condón S, Pagán R, García D. Recovery of Saccharomyces cerevisiae sublethally injured cells after pulsed electric fields. Int J Food Microbiol. 2008;125:352–356. doi: 10.1016/j.ijfoodmicro.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Saulis G. Pore disappearence in a cell after electroporation. Theoretical simulation and comparison with experiments. Biophys J. 1997;73:1299–1309. doi: 10.1016/S0006-3495(97)78163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pakhomov AG, Miklavcic D, Markov MS. Advanced Electroporation Techniques in Biology and Medicine. CRC Press; 2010. [Google Scholar]
- 6.Titomirov AV, Sukharev S, Kistanova E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim Biophys Acta. 1991;1088:131–134. doi: 10.1016/0167-4781(91)90162-f. [DOI] [PubMed] [Google Scholar]
- 7.Okino M, Mohri H. Effects of a high-voltage electrical impulse and an anticancer drug on in vivo growing tumors. Jpn J Cancer Res. 1987;78:1319–1321. [PubMed] [Google Scholar]
- 8.Mir LM, Gehl J, Sersa G, Collins CG, Garbay JR, Billard V, Geertsen PF, Rudolf Z, O'Sullivan GC, Marty M. Standard operating procedures of the electro-chemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. Eur J Cancer. 2006;4:14–25. [Google Scholar]
- 9.Sale AJ, Hamilton WA. Effect of high electric field on micro-organisms. I. Killing of bacteria and yeast. Biochim Biophys Acta. 1967;148:781–788. doi: 10.1016/0005-2736(68)90030-8. [DOI] [PubMed] [Google Scholar]
- 10.Sale AJ, Hamilton WA. Effect of high electric field on micro-organisms. II. Mechanism of action of the lethal effect. Biochim Biophys Acta. 1967;148:788–800. doi: 10.1016/0005-2736(68)90030-8. [DOI] [PubMed] [Google Scholar]
- 11.Sale AJ, Hamilton WA. Effects of high electric fields on microorganisms. III. Lysis of erythrocytes and protoplasts. Biochim Biophys Acta. 1968;163:37–43. doi: 10.1016/0005-2736(68)90030-8. [DOI] [PubMed] [Google Scholar]
- 12.Lelieved HLM, Notermans S, de Haan SWH. Food Preservation by Pulsed Electric Fields. CRC; Cambridge, England: 2007. From Research to Application. [Google Scholar]
- 13.Golberg A, Fischer Y, Rubinsky B. The use of irreversible electroporation in food preservation. In: Rubinsky B, editor. Irreversible Electroporation. Springer Berlin Heidelberg; 2010. pp. 273–312. [Google Scholar]
- 14.Golberg A, Kandel J, Belkin M, Rubinsky B. Intermittently delivered pulsed electric fields for sterile storage of turbid media. IEEE Trans Plasma Sci. 2010;38:3211–3218. [Google Scholar]
- 15.Golberg A, Belkin M, Rubinsky B. Irreversible electroporation for microbial control of drugs in solution. AAPS PharmSciTech. 2009 doi: 10.1208/s12249-009-9277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubinsky B. Irreversible Electroporation. Springer; 2010. [Google Scholar]
- 17.Onik G, Mikus P, Rubinsky B. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat. 2007;6:295–300. doi: 10.1177/153303460700600405. [DOI] [PubMed] [Google Scholar]
- 18.Peleg M. A model of microbial survival after exposure to pulsed electric field. J Sci Food Agric. 1995;67:93–99. [Google Scholar]
- 19.Peleg M, Cole MB. Reinterpretation of microbial survival curves. Crit Rev Food Sci Nutr. 1998;38:353–380. doi: 10.1080/10408699891274246. [DOI] [PubMed] [Google Scholar]
- 20.Peleg M, Cole MB. Estimating the survival of Clostridium botulinum spores during heat treatments. J Food Prot. 2000;63:190–195. doi: 10.4315/0362-028x-63.2.190. [DOI] [PubMed] [Google Scholar]
- 21.Golberg A, Rubinsky B. A statistical model for multidimensional irreversible electroporation cell death in tissue. Biomed Eng Online. 2010;9:13. doi: 10.1186/1475-925X-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lado BH, Yousef AE. Selection and identification of a Listeria monocytogenes target strain for pulsed electric field process optimization. Appl Environ Microbiol. 2003;69:2223–2229. doi: 10.1128/AEM.69.4.2223-2229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somolinos M, García D, Mañas P, Condón S, Pagán R. Effect of environmental factors and cell physiological state on pulsed electric fields resistance and repair capacity of various strains of Escherichia coli. Int J Food Microbiol. 2008;124:260–267. doi: 10.1016/j.ijfoodmicro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 24.Neumann E, Kakorin S. Electroporation of curved lipid membranes in ionic strength gradients. Biophys Chem. 2000;85:249–271. doi: 10.1016/s0301-4622(00)00112-5. [DOI] [PubMed] [Google Scholar]
- 25.Neumann E, Kakorin S, Toensing K. Principles of membrane electroporation and transport of macromolecules. In: Jaroszeski MJ, Heller R, Gilbert R, editors. Electrically Mediated Delivery of Molecules to Cells. Vol. 37. Humana Press, Inc.; Totowa, NJ: 2000. pp. 1–35. [DOI] [PubMed] [Google Scholar]
- 26.Cheng K. A biomechanical model of electroporation of a biological membrane. Proceedings of the 1997 Sixteenth Southern Biomedical Engineering Conference; 1997. pp. 70–73. [Google Scholar]
- 27.Cheng K. Development of biomechanical models of electroporation. presented at 4th Southern Biomedical Engineering Conference; Shreveport, Louisiana. 1995. [Google Scholar]
- 28.Cheng K. Physical models of mechanism of electroporation. Biophys J. 1995;68:A221. [Google Scholar]
- 29.Junge D. Nerve and Muscle Excitation. 2nd. Sinauer Associates; Sunderland, MA: 1981. [Google Scholar]
- 30.Neumann E. Gene delivery by membrane elecroporation. In: Lynch PT, Davet MR, editors. Electrical Manipulation of Cells. Chapman and Hall; New York: 1996. pp. 157–184. [Google Scholar]
- 31.Weaver JC, Mintzer RA. Decreased bilayer stability due to transmembrane potentials. Phys Lett. 1981;86A:57–59. [Google Scholar]
- 32.Gambaro M, Gliozzi A, Robello M. Effect of surface charges on the electroporation process in lipid bilayers. Prog Colloid Polym Sci. 1991;84:189–193. [Google Scholar]
- 33.Liu X, Yousef AE, Chism GW. Inactivation of E. coli 0157:H7 by the combination of organic acids and pulsed electric field. J Food Saf. 1997;16:287–299. [Google Scholar]
- 34.Wouters PC, Dutreux N, Smelt JPPM, Lelieveld HLM. Effects of pulsed electric fields on inactivation kinetics of Listeria innocua. Appl Environ Microbiol. 1999;65:5364–5371. doi: 10.1128/aem.65.12.5364-5371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia D, Gomez N, Raso J, Pagan R. Bacterial resistance after pulsed electric fields depending on the treatment medium pH. Innov Food Sci Emerg Technol. 2005;6:388–395. [Google Scholar]
- 36.Gómeza N, Garcíaa D, Álvareza I, Condóna SS, Raso J. Modelling inactivation of Listeria monocytogenes by pulsed electric fields in media of different pH. Int J Food Microbiol. 2004;103:199–206. doi: 10.1016/j.ijfoodmicro.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 37.Gómez N, García D, Álvarez I, Raso J, Condón S. A model describing the kinetics of inactivation of Lactobacillus plantarum in a buffer system of different pH and in orange and apple juice. J Food Eng. 2005;70:7–14. [Google Scholar]
- 38.Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol. 2008;6:276–287. doi: 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- 40.Hancock RE, Scott MG. The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci U S A. 2000;97:8856–8861. doi: 10.1073/pnas.97.16.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peschel A. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 2002;10:179–186. doi: 10.1016/s0966-842x(02)02333-8. [DOI] [PubMed] [Google Scholar]
- 42.Abachin E, Poyart C, Pellegrini E, Milohanic E, Fiedler F, Berche P, Trieu-Cuot P. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol Microbiol. 2002;43:1–14. doi: 10.1046/j.1365-2958.2002.02723.x. [DOI] [PubMed] [Google Scholar]
- 43.Collins LV, Kristian SA, Weidenmaier C, Faigle M, Van Kessel KP, Van Strijp JA, Gotz F, Neumeister B, Peschel A. Staphylococcus aureus strains lacking D-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J Infect Dis. 2002;186:214–219. doi: 10.1086/341454. [DOI] [PubMed] [Google Scholar]
- 44.Kristian SA, Datta V, Weidenmaier C, Kansal R, Fedtke I, Peschel A, Gallo RL, Nizet V. D-alanylation of teichoic acids promotes group a streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J Bacteriol. 2005;187:6719–6725. doi: 10.1128/JB.187.19.6719-6725.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neuhaus FC, Baddiley J. A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in Gram-positive bacteria, Microbiol. Mol Biol Rev. 2003;67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Gotz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 47.Mandin P, Fsihi H, Dussurget O, Vergassola M, Milohanic E, Toledo-Arana A, Lasa I, Johansson J, Cossart P. VirR, a response regulator critical for Listeria monocytogenes virulence. Mol Microbiol. 2005;57:1367–1380. doi: 10.1111/j.1365-2958.2005.04776.x. [DOI] [PubMed] [Google Scholar]
- 48.Vadyvaloo V, Arous S, Gravesen A, Hechard Y, Chauhan-Haubrock R, Hastings JW, Rautenbach M. Cell-surface alterations in class IIa bacteriocinresistant Listeria monocytogenes strains. Microbiology. 2004;150:3025–3033. doi: 10.1099/mic.0.27059-0. [DOI] [PubMed] [Google Scholar]
- 49.Peschel A, Jack RW, Otto M, Collins LV, Staubitz P, Nicholson G, Kalbacher H, Nieuwenhuizen WF, Jung G, Tarkowski A, van Kessel KP, van Strijp JA. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J Exp Med. 2001;193:1067–1076. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thedieck K, Hain T, Mohamed W, Tindall BJ, Nimtz M, Chakraborty T, Wehland J, Jansch L. The MprF protein is required for lysinylation of phospholipids in listerial membranes and confers resistance to cationic antimicrobial peptides (CAMPs) on Listeria monocytogenes. Mol Microbiol. 2006;62:1325–1339. doi: 10.1111/j.1365-2958.2006.05452.x. [DOI] [PubMed] [Google Scholar]
- 51.Camilli A, Tilney LG, Portnoy DA. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zemansky J, Kline BC, Woodward JJ, Leber JH, Marquis H, Portnoy DA. Development of a mariner-based transposon and identification of Listeria monocytogenes determinants, including the peptidyl-prolyl isomerase PrsA2, that contribute to its hemolytic phenotype. J Bacteriol. 2009;191:3950–3964. doi: 10.1128/JB.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.European Pharmacopoeia, Test for efficiency of antimicrobial preservations. 2005;(5.1.3) [Google Scholar]
- 54.Teissié J, Golzio M, Rols MP. Mechanisms of cell membrane electropermeabilization: a minireview of our present (lack of ?) knowledge. Biochim Biophys Acta. 2005;1724:270–280. doi: 10.1016/j.bbagen.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Scwister K, Deuticke B. Formation and properties of aqueous leaks induced in human erythrocytes by electrical breakdown. BBA - Biomembranes. 1985;816:322–348. doi: 10.1016/0005-2736(85)90501-2. [DOI] [PubMed] [Google Scholar]
- 56.Diederich A, Winterhalter M. Influence of surface charges on the rupture of black lipid membranes. Phys Rev E. 1998;58:4883–4889. [Google Scholar]
- 57.Gencoa I, Gliozzi A, Relinia A, Robelloa M, Scalas E. Electroporation in symmetric and asymmetric membranes. BBA - Biomembranes. 1992;1149:10–18. doi: 10.1016/0005-2736(93)90019-v. [DOI] [PubMed] [Google Scholar]
- 58.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Foodrelated illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Denny J, McLauchlin J. Human Listeria monocytogenes infections in Europe—an opportunity for improved European surveillance. Eurosurveillance. 2008;13:1–5. [PubMed] [Google Scholar]
- 60.Ryser ET, Marth EH. Listeria, Listeriosis, and Food Safety. Third. CRC Press; Boca Raton: 2007. [Google Scholar]
- 61.Álvarez I, Pagán R, Condón S, Raso J. Environmental factors influencing the inactivation of Listeria monocytogenes by pulsed electric fields. Lett Appl Microbiol. 2002;20:691–700. doi: 10.1046/j.1472-765x.2002.01221.x. [DOI] [PubMed] [Google Scholar]
- 62.Lado BH, Bomser JA, Dunne PC, Yousef AE. Pulsed electric field alters molecular chaperone expression and sensitizes Listeria monocytogenes to heat. Appl Environ Microbiol. 2004;70:2289–2295. doi: 10.1128/AEM.70.4.2289-2295.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reina LD, Jin ZT, Yousef AE, Zhang QH. Inactivation of Listeria monocytogenes in milk by pulsed electric field. J Food Prot. 1998;61:1203–1206. doi: 10.4315/0362-028x-61.9.1203. [DOI] [PubMed] [Google Scholar]
- 64.Fleischman GJ, Ravishankar S, Balasubramaniam VM. The inactivation of Listeria monocytogenes by pulsed electric field (PEF) treatment in a static chamber. Food Microbiol. 2004;21:91–95. [Google Scholar]