Abstract
Objective
Spasticity with increased tone and spasms is frequent in patients after spinal cord injury (SCI). Damage to descending corticospinal pathways that normally exert spinal segmental control is thought to play an important causal role in spasticity. The authors examined whether the modulation of excitability of the primary motor cortex with high-frequency repetitive transcranial magnetic stimulation (rTMS) could modify lower limb spasticity in patients with incomplete SCI.
Methods
Patients were assessed by the Modified Ashworth Scale, Visual Analogue Scale, and the Spinal Cord Injury Spasticity Evaluation Tool (SCI-SET) and neurophysiologically with measures of corticospinal and segmental excitability by the Hmax/Mmax, T reflex, and withdrawal reflex. Fifteen patients received 5 days of daily sessions of active (n = 14) or sham (n = 7) rTMS to the leg motor area (20 trains of 40 pulses at 20 Hz and an intensity of 90% of resting motor threshold for the biceps brachii muscle).
Result
A significant clinical improvement in lower limb spasticity was observed in patients following active rTMS but not after sham stimulation. This improvement lasted for at least 1 week following the intervention. Neurophysiological studies did not change.
Conclusions
High-frequency rTMS over the leg motor area can improve aspects of spasticity in patients with incomplete SCI.
Keywords: spasticity, repetitive TMS, spinal cord injury, neurophysiological examination
Introduction
Spasticity is generally conceptualized as a symptom of the upper motor neuron syndrome, characterized by an exaggeration of the stretch reflex, spasms, and resistance to passive movement across a joint, secondary to hyperexcitability of spinal reflexes.1–6 In patients with clinical signs of spasticity after spinal cord injury (SCI), complete loss of descending corticospinal projections is rare. Preserved yet altered pro-priospinal and supraspinal input to a given segmental level may explain why spasticity is a frequent consequence of SCI.1 One of the hypotheses on the pathophysiology of spasticity highlights the causal role of long-term reductions in segmental inhibition rather than primary increases in excitation.4
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive technique that induces changes in cortical excitability at the site of stimulation and transsynaptically at distant sites. Modulation of excitability at the directly targeted brain region depends on the rTMS parameters and can result in either transient facilitation or suppression. These effects may outlast the duration of the stimulation trains for minutes or even hours.7–12 Previous studies have shown that high-frequency rTMS applied over the primary motor cortex can reduce H-reflex size in healthy subjects13–15 and reduce spasticity in patients with multiple sclerosis (MS)16 or cerebral palsy and spastic quadriplegia.17
We hypothesized that increasing the excitability of the primary motor cortex would modify descending cortico-spinal influences, increase corticospinal inhibitory input, reduce segmental spinal excitability, and thus reduce limb spasticity in patients with incomplete SCI. To test this hypothesis, we applied repeated daily sessions of real or sham high-frequency rTMS in 15 patients with incomplete SCI. We assessed spasticity with various clinical scales, as well as monitored the impact of the intervention on segmental excitability using various neurophysiological measures.
Methods
The study was a randomized, double-blind, sham-controlled trial. We recruited 15 patients with SCI. Patients were randomized to undergo either real or sham rTMS. Of the 7 patients who underwent sham rTMS first, 6 were subsequently crossed over to undergo active rTMS following a ≥2-week washout period. Therefore, we obtained data on the effects of active rTMS in a total of 14 patients (8 who only underwent active rTMS and 6 who had previously undergone sham rTMS and then crossed over). All courses of rTMS consisted of 5 consecutive daily sessions of active or sham rTMS. Patients and investigators (except the rTMS operator) were blind to the form of stimulation.
Patients
We included patients with (a) incomplete SCI classified as grades C or D according to the American Spinal Cord Injury Association Impairment scale18; (b) spasticity affecting predominantly lower limb with a Modified Ashworth Scale (MAS)19 score, which reflects resistance to stretch ≥1.5; (c) stable medical treatment for at least 1 week before and 1 week after the stimulation; (d) no joint-related limitation of passive range of movement; and (e) a written informed consent for the study, which had been approved by the institutional review board. All patients were naive to rTMS and unaware of the purpose of the study. Demographic and clinical characteristics of the patients are summarized in Table 1. The mean age was 36.2 ± 15.8 years (range = 15–68 years), and the mean time since SCI was 7.3 ± 3.9 months. In all patients spasticity affected both legs symmetrically, thus our study was limited to 1 leg (the right leg in all patients) except MAS, which was evaluated in both legs.
Table 1.
Demographic Patient Characteristics, Clinical Level of the Lesion, and Medication
| Stimulation | Patients | Sex | Age (Years) | Level of Lesion | ASIA | Time Since Injury (Months) | Etiology of Lesion | MAS | Antispastic Medication |
|---|---|---|---|---|---|---|---|---|---|
| Active | 1a | Male | 54 | C6 | C | 8 | Trauma | 3 | Baclofen, tizanidine |
| Active | 2a | Male | 32 | T4 | C | 9 | Myelitis | 3 | Baclofen |
| Active | 3a | Female | 43 | T4 | C | 17 | Tumor | 3 | Baclofen, tizanidine |
| Active | 4a | Male | 53 | C4 | C | 7 | Trauma | 3 | Baclofen |
| Active | 5a | Male | 18 | T1 | D | 6 | Tumor | 2 | Baclofen |
| Active | 6a | Male | 21 | C5 | D | 11 | Trauma | 3 | Baclofen |
| Active | 7 | Male | 48 | T4 | C | 5 | Trauma | 3 | Baclofen |
| Active | 8 | Male | 31 | T11 | C | 4 | Myelitis | 3 | Baclofen |
| Active | 9 | Male | 29 | T12 | C | 2 | Trauma | 3 | None |
| Active | 10 | Male | 21 | T2 | C | 3 | Trauma | 3 | None |
| Active | 11 | Male | 29 | T11 | D | 7 | Trauma | 3 | None |
| Active | 12 | Male | 29 | T4 | D | 8 | Myelitis | 4 | Baclofen |
| Active | 13 | Male | 52 | C6 | D | 8 | Trauma | 3 | Baclofen |
| Active | 14 | Female | 15 | T5 | C | 13 | Trauma | 4 | Baclofen |
| Sham | 1a | Male | 54 | C6 | C | 6 | Trauma | 3 | Baclofen, tizanidine |
| Sham | 2a | Male | 32 | T4 | C | 6 | Myelitis | 3 | Baclofen |
| Sham | 3a | Female | 43 | T4 | C | 15 | Tumor | 3 | Baclofen, tizanidine |
| Sham | 4a | Male | 53 | C4 | C | 4 | Trauma | 3 | Baclofen |
| Sham | 5a | Male | 18 | T1 | D | 4 | Tumor | 2 | Baclofen |
| Sham | 6a | Male | 21 | C5 | D | 9 | Trauma | 3 | Baclofen |
| Sham | 7 | Male | 68 | T12 | C | 3 | Trauma | 2 | None |
Abbreviations: C, cervical; T, Thoracic; ASIA, American Spinal Cord Injury Association Impairment Scale; MAS, Modified Ashworth Scale.
The 6 patients who received first sham stimulation and then received active stimulation after a washout of at least 2 weeks.
Clinical Evaluation of Spasticity
The assessment was done on the right knee. We used MAS; a Visual Analogue Scale (VAS) of 10 cm in length for self-evaluation for spasms, stiffness, and/or clonus during daily activities and walking; the Modified Penn Spasm Frequency Scale (MPSFS); the Spinal Cord Assessment Tool for Spasticity (SCAT); and the Spinal Cord Injury Spasticity Evaluation Tool (SCI-SET).
Neurophysiological Evaluation
All tests were conducted with the patient lying in the supine position. Routine electrodiagnostic equipment (Medelec Synergy, Oxford Instruments, Surrey, UK) was used.
H reflex
The soleus (SOL) compound motor action potential (CMAP=M) and the H reflex were evoked by electrical stimulation (1-ms rectangular pulse) through a bipolar electrode fixed over the posterior tibial nerve at the popliteal fossa and recorded with bipolar Ag–AgCl surface electrodes placed over the SOL muscle. The reference electrode was placed 2 cm distal from the active electrode. The electromyographic (EMG) signal was amplified (1 mV/Division) and band-pass filtered (2–10.000 Hz). The intensity of the stimulation was progressively increased (0.3 mA) to obtain the maximal peak-to-peak amplitudes of H reflex and M wave.
T reflex
The T reflex was recorded in the SOL muscle with Achilles tendon tapping by an electrical hammer (Kawe Reflex Hammer, Trömner, Germany), while the foot was slightly dorsiflexed. The EMG signal was amplified (1 mV/D) and band-pass filtered (2–10.000 Hz).
Withdrawal reflex
Rectified surface EMG was obtained from tibialis anterior (TA) and SOL muscles using bipolar Ag–AgCl surface electrodes. The EMG signal was amplified (0.5 mV/D) and band-pass filtered (50–1.000 Hz). Withdrawal reflex was evoked by electrical stimulation (1-ms rectangular pulse) through a bipolar electrode fixed over the posterior tibial nerve at the medial malleolar fossa. Pain threshold was established for each subject, and the threshold was used for train stimulation (5 pulses; 100 Hz).
rTMS Protocol
Patients received 5 consecutive daily rTMS sessions applied in the morning (between 9 and 12 AM). We used a MagStim Super Rapid magnetic stimulator (Magstim Company, Whitland, UK) equipped with a commercially available double cone coil (each wing measuring 110 mm in diameter) that was held over the vertex. All rTMS sessions were conducted with the patient lying supine.
For active (real) rTMS we applied 2-second-long bursts at 20 Hz (40 pulses/burst) with intertrain intervals of 28 seconds, for a total of 1600 pulses over 20 minutes. The intensity of stimulation was set as 90% of the resting motor threshold (RMT) intensity for induction of motor-evoked potentials (MEPs) in the right biceps brachii (BB) muscle. This muscle was spared in all patients. For motor threshold determination the double cone coil was held lateral to the vertex over the optimal scalp position overlying the left hemisphere from which single-pulse TMS evoked responses of maximal amplitude in the right BB. Motor threshold was defined as the intensity that evoked MEPs of >50 μV peak-to-peak amplitude in 5 of 10 consecutive stimulations.
For sham stimulation, the double cone coil was held over the vertex (just as in the active TMS condition), but it was disconnected from the main stimulator unit. Instead, a second coil (8-shaped) was connected with the MagStim stimulator and discharged under the patient’s pillow. Thus, no current was induced in the brain, and even though the patients did not experience a tapping sensation on their scalp, they were exposed to a similar clicking noise. All 7 patients in the sham stimulation group reported that they thought they had got active stimulation when explicitly asked at the end of the trial.
Experimental Design
The experimental protocol included the following steps:
Baseline clinical and neurophysiological evaluation
First rTMS session followed by clinical and neurophysiological reevaluation
Daily rTMS sessions for 4 more days
Clinical and neurophysiological evaluation after completion of the fifth consecutive daily rTMS session
Follow-up clinical evaluation 1 week after the rTMS course
Baseline neurophysiological evaluation was done immediately before the rTMS intervention. The neurophysiologic evaluations during the rTMS course were done immediately before and after the first rTMS session and immediately after the last session.
Data Analysis
The H reflex, T reflex, and M potential amplitudes were measured from peak to peak. The Hmax/Mmax ratio was calculated by dividing the maximal amplitude of the H wave by that of the M wave. In the withdrawal reflex, we measured the latency and area under the curve in TA and SOL muscles.
Data are presented as mean ± standard deviation. Friedman test was used for multiple group comparisons, and Wilcoxon test was used for post hoc comparisons of clinical scales and neurophysiological baseline data with the results after the first session of rTMS, the last session of rTMS, and 1 week after rTMS. Mann–Whitney U test was used to compare data between different groups of patients (eg, patients with traumatic vs nontraumatic SCI).
For all tests, significance level was set as P < .05 with Bonferroni correction for multiple comparisons.
Results
All patients tolerated the stimulation without complications, and no adverse effects were reported, except for 3 patients who complained of twitching facial muscles during the first session of active stimulation. In the real TMS group, 8 patients spontaneously reported improved sleep quality and longer uninterrupted sleep hours for several days following the 5 sessions of stimulation. None of the patients in the sham stimulation group reported such impressions, even when explicitly asked about them.
The mean rTMS intensity used was 41.9 ± 6.0% of maximal stimulator output. Only in 3 patients were we able to elicit MEPs in the right TA, in whom the motor threshold was 60%, 90%, and 98%. In the other 12 patients we were not able to elicit MEPs in TA despite 100% of TMS intensity.
Clinical Scales
Active stimulation
Spasticity was significantly reduced at the end of the first and the last rTMS sessions as measured by MAS from both lower extremities when compared with the baseline condition (Wilcoxon test; P < .006). These effects were maintained 1 week after the end of the rTMS course (Wilcoxon test; P = .049). MPSFS in patients undergoing active stimulation was reduced significantly after the last session (Wilcoxon test; P = .01). Spasm frequency and severity according to MPSFS reduced significantly after the first and the last rTMS sessions when compared with the baseline condition (Wilcoxon test; P < .01 for each comparison). Just 2 patients reported pain related to spasticity in MPSFS, which disappeared after the last rTMS session. There was significant reduction in the spasticity according to SCAT at the end of the first and the last sessions (Wilcoxon test; P < .04), and the effect was maintained 1 week after active stimulation (Wilcoxon test; P = .049).
In patients who underwent active stimulation following a course of sham stimulation, spasticity was significantly reduced at the end of the first and the last rTMS sessions as measured by MAS when compared with the baseline condition. These effects were maintained 1 week after the end of the rTMS course (Wilcoxon test; P < .02 for all comparisons). Indeed, we found no difference in the effects of real rTMS on spasticity between patients who underwent sham rTMS first and those who did not.
The improvement in spasticity measured by MAS was not significantly different between patients with traumatic SCI and those with nontraumatic SCI (Mann–Whitney U test; P = .2).
Sham stimulation
There was no significant effect of sham stimulation on spasticity at any time points measured by MAS, MPSFS, or SCAT (Friedman test; P > .1; Table 2). Spasm frequency and severity according to MPSFS did not change significantly after the first and the last rTMS sessions when compared with the baseline condition (Wilcoxon test; P > .2 for each comparison). Two patients from the sham group reported pain related to spasticity in MPSFS, which did not change after the last rTMS session.
Table 2.
Changes in Spasticity in Patients With Sham and Active Stimulation Before, After First Session, After Last Session, and 1 Week After Stimulation
| Stimulation Type | Before Stimulation | After First Session | After Last Session | One Week After Stimulation | Pa |
|---|---|---|---|---|---|
| MAS of right knee | |||||
| Active | 2.9 (1.2) | 2.0 (1.4) | 1.4 (1.4) | 2.2 (1.0) | <.001 |
| Sham | 2.6 (0.9) | 2.7 (0.8) | 2.6 (0.9) | 2.5 (1.0) | .82 |
| MAS of left knee | |||||
| Active | 2.7 (1.0) | 2.1 (1.1) | 1.3 (1.4) | 2.1 (1.2) | <.009 |
| Sham | 2.4 (1.3) | 2.6 (1.1) | 2.7 (1.0) | 2.6 (1.1) | .89 |
| MPSFS | |||||
| Active | 8.3 (3.8) | 6.8 (3.7) | 5.25 (3.6) | 6.2 (4.4) | .01 |
| Sham | 8.8 (3.9) | 7.0 (3.7) | 6.3 (4.5) | 7.3 (3.5) | .44 |
| SCAT | |||||
| Active | 5.9 (2.3) | 4.2 (1.8) | 4.6 (1.8) | 4.2 (2.2) | .01 |
| Sham | 5.2 (1.9) | 4.7 (2.3) | 4.5 (2.1) | 4.5 (2.1) | .18 |
Abbreviations: MAS, Modified Ashworth Scale; MPSFS, Modified Penn Spasm Frequency Scale; SCAT, Spinal Cord Assessment Tool for Spasticity.
Standard deviations are in parentheses.
P value refers to the results of Friedman’s test.
Significance level p < .05.
Subjective Reports
Active stimulation
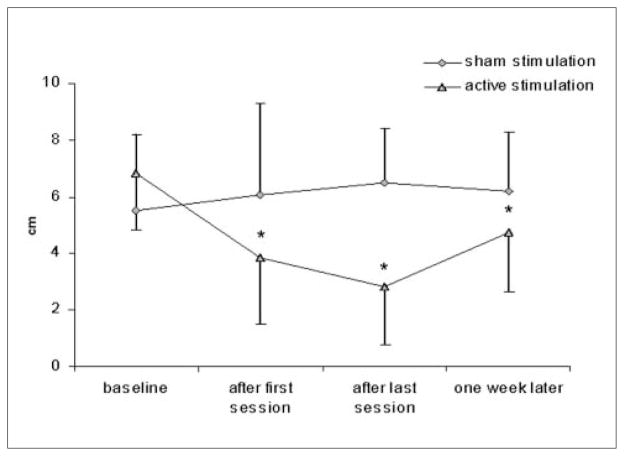
Thirteen of the 14 patients reported significantly less spasticity according to VAS at the end of the first and the last rTMS sessions when compared with the baseline condition (Wilcoxon test; P < .002). This effect was maintained 1 week after rTMS in 12 of 14 patients (Wilcoxon test; P = .004; Figure 1). These findings did not differ between patients who underwent sham rTMS first and then active rTMS and those who underwent only active rTMS.
Figure 1.
Visual analogue scale
Note: Patients with active stimulation reported significant improvement in spasticity after the last session and 1 week follow-up when compared with the baseline condition measured by the visual analogue scale.
*P < .005 (Wilcoxon test).
The percentage improvement in spasticity measured by VAS was not significantly different between patients with traumatic SCI and those with nontraumatic SCI (Mann–Whitney U test; P = .8).
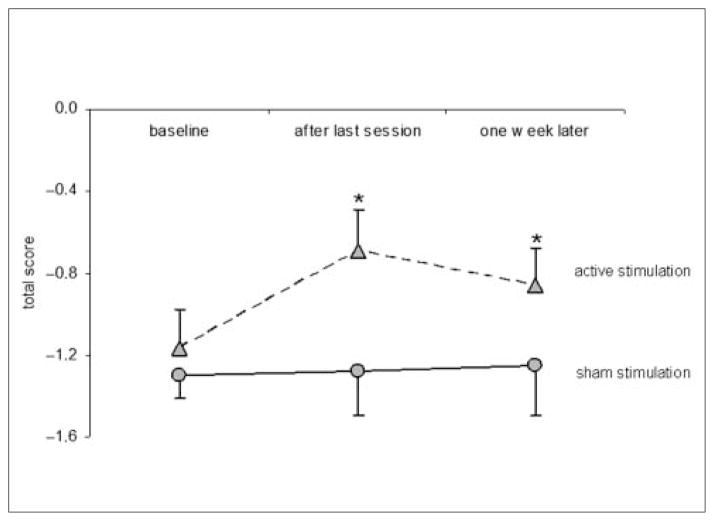
According to the SCI-SET, spasticity was reduced significantly after 5 days of rTMS (Wilcoxon test; P = .003), and this improvement remained statistically significant 1 week after rTMS (Wilcoxon test; P = .005; Figure 2).
Figure 2.
Spinal Cord Injury Spasticity Evaluation Tool (SCI-SET) results
Note: Patients with active stimulation reported significant improvement in spasticity after last session and 1 week follow-up when compared with the baseline condition measured by the SCI-SET. *P = .006 (Wilcoxon test).
Sham stimulation
None of the 7 patients reported significant improvement in their spasticity according to VAS (Friedman test; P = .8; Figure 1). There was no significant effect of sham stimulation on spasticity at any time point as measured by SCI-SET (Friedman test; P = .28; Figure 2).
Neurophysiological Recordings
Active or sham stimulation
Regardless of whether patients underwent active or sham rTMS, the Hmax/Mmax ratio, the T reflex, and the area under the curve in TA and SOL muscles in the withdrawal reflex were not significantly different after the first or the last rTMS sessions when compared with the baseline condition (Friedman test: P > .3 for active rTMS; P > .5 for sham rTMS groups; Table 3).
Table 3.
Mean and Standard Deviation of the Neurophysiologic Data According to Type and Time of the Stimulationa
| Stimulation Type | Before Stimulation (Baseline) | After First Session | After Last Session | Pb |
|---|---|---|---|---|
| Hmax/Mmax ratio | ||||
| Active | 0.55 (0.28) | 0.48 (0.32) | 0.55 (0.26) | .36 |
| Sham | 0.54 (0.29) | 0.50 (0.26) | 0.53 (0.25) | .31 |
| T reflex (mV) | ||||
| Active | 2.7 (1.4) | 2.6 (1.6) | 2.3 (1.2) | .67 |
| Sham | 2.0 (1.0) | 2.2 (1.4) | 2.2 (1.3) | 1.0 |
| Withdrawal reflex area (mV ms) | ||||
| TA | ||||
| Active | 9.7 (16.5) | 13 (21.4) | 11.5 (15.8) | .69 |
| Sham | 7.8 (11.8) | 7.0 (4.9) | 7.9 (2.1) | .82 |
| SOL | ||||
| Active | 3.0 (4.6) | 2.7 (4.4) | 3.4 (5.0) | .87 |
| Sham | 3.6 (3.2) | 5.2 (6.3) | 5.1 (4.1) | .85 |
Abbreviations: TA, tibial anterior muscle; SOL, soleus muscle.
Standard deviations are in parentheses.
P value refers to the results of Friedman’s test.
Discussion
The main finding of the present study is that high-frequency rTMS over the leg primary motor cortex modulates spasticity in patients with incomplete SCI and the effect is maintained for at least 1 week after 5 days of daily rTMS sessions. The clinical changes were not accompanied by measurable neurophysiologic changes (Hmax/Mmax, T reflex, and withdrawal reflex).
Our findings are consistent with reported results on the effects of rTMS on spasticity in other conditions. Centonze et al16 observed an improvement in lower limb spasticity with 5 Hz rTMS stimulation at 100% RMT over the leg primary motor cortex in patients with MS. After 2 weeks of daily rTMS sessions, the authors observed a durable improvement in lower limb spasticity.16 Also in MS patients, Nielsen et al20 applied repetitive magnetic stimulation over the thoracic spine and found clinical improvement in spasticity. Finally, Valle et al,17 in a study of children with spastic quadriplegia, found a clinically significant rTMS-induced reduction of spasticity (as assessed by various scales).
All our patients showed a significant and consistent clinical improvement in spasticity with active rTMS. However, we failed to demonstrate neurophysiologic changes following the rTMS intervention. Reduction of Hmax/Mmax amplitude ratio following rTMS has been demonstrated in normal subjects for the upper extremity11,15 and the lower extremity.13 We had hypothesized a neurophysiologic impact of the rTMS in our patients also. Nonetheless, clinical benefits in the absence of significant neurophysiological changes are consistent with the complex pathophysiology of spasticity in SCI. There are arguably different types of spasticity depending, for example, on the exact site of insult along the neuroaxis.4 Patients with spastic paresis are similar in some ways (ie, they are spastic and paretic), but many variations occur, depending on the damage to different regions of the central nervous system and depending on the nature of that damage.2,4,21 The lack of neurophysiologic changes in our patients may be due to a number of factors:
The cause of spasticity was different in our patients than in others in whom there has been an association described between clinical and neurophysiologic effects of rTMS. In the patients in whom an association was found between clinical improvement and reduction of Hmax/Mmax amplitude ratio following motor cortex rTMS,16,20 spasticity was due to MS,16,20 where lesions can be scattered throughout various regions of the central nervous system. Also in MS patients, but targeting the thoracic spinal cord, Nielsen et al20 found clinical improvement in spasticity associated with a reduction of Hmax/Mmax.
The stimulation intensity applied during active rTMS in our study was relatively low (90% RMT of BB, because of absent MEP in lower extremity in most patients or very high RMT in the other 3 patients), and neurophysiologic changes may be minimal or rather transient in such a setting.
Antispasticity medication (baclofen and tizanidine) are potent neuromodulators that might have influenced the effect of rTMS in some of our patients. We did not find a discernible impact of medications on our clinical or neurophysiologic measure, but our sample size is too small to reliably assess this issue.
Finally, it is worth considering that the neurophysiology of spasticity is complex, and the measures we applied may have missed the critical substrate for the clinical impact of rTMS. Spasticity is a motor disorder characterized by brisk tendon jerks and a velocity-dependent elastic muscle hypertonia during stretch, affecting certain muscle groups preferentially.2 Although the spinal segmental stretch reflex arc contains monosynaptic connections with motor neurons by spindle primary afferents coming from that muscle and synergistic muscles (via Ia fibers), most excitatory activity in the stretch reflex is mediated via oligosynaptic and polysynaptic pathways. Interneurons play a much larger role than direct connections between first-order sensory neurons and motor neurons.2–6,21 Secondary changes in mechanical muscle fiber properties might also contribute to spasticity,6 although they will be unlikely to change with rTMS. Furthermore, muscle hypertonia and exaggerated reflexes are 2 distinct features of the upper motor neuron syndrome that may be independently present following SCI.
When applied to the motor cortex, high-frequency rTMS tends to provoke a lasting facilitation of corticospinal excitability.22 The magnitude and duration of the after effects seems to depend on the total number of stimuli, and longer periods of rTMS induce a more consistent and persistent change in corticospinal excitability. In our patients with incomplete SCI, the clinical effect of rTMS on spasticity lasted for at least 1 week following the 5-day stimulation course. Previous studies have demonstrated that cumulative plastic changes can be produced by rTMS, in healthy participants23 and in patients with Parkinson disease24 and MS.16 Some authors have suggested that after repeated daily sessions of repetitive magnetic stimulation over the spinal cord20,25 or rTMS in MS,16 long-lasting modulation of spinal circuits may be related to long-lasting depression-like mechanisms. High-frequency rTMS at subthreshold intensity can lead to an increase in regional glucose metabolism immediately after the end of rTMS, suggesting the possibility of an rTMS-induced increase in overall neuronal activity in the stimulated M1.26 We hypothesize, therefore, that in our patients rTMS induced amelioration of spasticity through enhancement of descending corticospinal projections and there may be some effect to local interneurons of the spinal cord, similar to the impact of magnetic stimulation over the spinal cord.20 The pyramidal tract has widespread terminations in the spinal gray matter, thereby controlling motor neurons through not only monosynaptic but also nonmonosynaptic connections, involving local interneurons and sensory afferents.
Further studies systematically exploring the effects induced by repeated sessions of high-frequency rTMS on the corticospinal tract excitability in spasticity from different origin are necessary to provide further mechanistic insights, assess further the clinical benefit, and ultimately examine whether rTMS might offer therapeutic benefit for patients with spasticity.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research and/or authorship of this article:
This work was supported in part by grants from the Foundation La Marató TV3 (071931), the Cátedra BBVA (CAT06/023), CNRS UMR 5105 LPNC & INSERM Unit S975-ICM, Paris, France, the FIS (PI082004), and the National Institutes of Health (K24 RR018875 and UL1 RR025758).
Footnotes
Declaration of Conflicting Interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Frigon A, Rossignol S. Functional plasticity following spinal cord lesions. Prog Brain Res. 2006;157:231–260. doi: 10.1016/s0079-6123(06)57016-5. [DOI] [PubMed] [Google Scholar]
- 2.Elbasiouny SM, Moroz D, Bakr MM, Mushahwar VK. Management of spasticity after spinal cord injury: Current techniques and future directions [published online ahead of print September 1, 2009] Neurorehabil Neural Repair. doi: 10.1177/1545968309343213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbruzzese G. The medical management of spasticity. Eur J Neurol. 2002;9:30–34. doi: 10.1046/j.1468-1331.2002.0090s1030.x. [DOI] [PubMed] [Google Scholar]
- 4.Adams MM, Hicks AL. Spasticty after spinal cord injury. Spinal Cord. 2005;43:577–586. doi: 10.1038/sj.sc.3101757. [DOI] [PubMed] [Google Scholar]
- 5.Decq P. Pathophysiology of spasticity. Neurochirurgie. 2003;49:163–184. [PubMed] [Google Scholar]
- 6.Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007;6:725–733. doi: 10.1016/S1474-4422(07)70193-X. [DOI] [PubMed] [Google Scholar]
- 7.Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin Neurophysiol. 2001;112:1367–1377. doi: 10.1016/s1388-2457(01)00585-5. [DOI] [PubMed] [Google Scholar]
- 8.Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- 9.Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;5:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- 11.Berardelli A, Inghilleri M, Rothwell JC, et al. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res. 1998;122:79–84. doi: 10.1007/s002210050493. [DOI] [PubMed] [Google Scholar]
- 12.Romero JR, Anschel D, Sparing R, Gangitano M, Pascual-Leone A. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin Neurophysiol. 2002;113:101–107. doi: 10.1016/s1388-2457(01)00693-9. [DOI] [PubMed] [Google Scholar]
- 13.Perez MA, Lungholt BK, Nielsen JB. Short-term adaptations in spinal cord circuits evoked by repetitive transcranial magnetic stimulation: possible underlying mechanisms. Exp Brain Res. 2005;162:202–212. doi: 10.1007/s00221-004-2144-2. [DOI] [PubMed] [Google Scholar]
- 14.Quartarone A, Bagnato S, Rizzo V, et al. Distinct changes in cortical and spinal excitability following high-frequency repetitive TMS to the human motor cortex. Exp Brain Res. 2005;161:114–124. doi: 10.1007/s00221-004-2052-5. [DOI] [PubMed] [Google Scholar]
- 15.Valero-Cabre A, Oliveri M, Gangitano M, Pascual-Leone A. Modulation of spinal cord excitability by subthreshold repetitive transcranial magnetic stimulation of the primary motor cortex in humans. Neuroreport. 2001;12:3845–3848. doi: 10.1097/00001756-200112040-00048. [DOI] [PubMed] [Google Scholar]
- 16.Centonze D, Koch G, Versace V, et al. Repetitive transcranial magnetic stimulation of the motor cortex ameliorates spasticity in multiple sclerosis. Neurology. 2007;68:1045–1050. doi: 10.1212/01.wnl.0000257818.16952.62. [DOI] [PubMed] [Google Scholar]
- 17.Valle AC, Dionisio K, Pitskel NB, et al. Low and high frequency repetitive transcranial magnetic stimulation for the treatment of spasticity. Dev Med Child Neurol. 2007;49:534–538. doi: 10.1111/j.1469-8749.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 18.Marino RJ, Barros T, Biering-Sorensen F, et al. ASIA Neurological Standards Committee 2002. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26(suppl 1):S50–S56. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- 19.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen JF, Sinkjaer T, Jakobsen J. Treatment of spasticity with repetitive magnetic stimulation: a double-blind placebo-controlled study. Mult Scler. 1996;2:227–232. doi: 10.1177/135245859600200503. [DOI] [PubMed] [Google Scholar]
- 21.Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord: Its Role in Motor Control and Movement Disorders. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- 22.Peinemann A, Reimer B, Loer C, et al. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiol. 2004;115:1519–1526. doi: 10.1016/j.clinph.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Baumer T, Lange R, Liepert J, et al. Repeated premotor rTMS lead to cumulative changes of motor cortex excitability in humans. Neuroimage. 2003;20:550–560. doi: 10.1016/s1053-8119(03)00310-0. [DOI] [PubMed] [Google Scholar]
- 24.Buhmann C, Gorsler A, Baumer T, et al. Abnormal excitability of premotor-motor connections in de novo Parkinson’s disease. Brain. 2004;127:2732–2746. doi: 10.1093/brain/awh321. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen JF, Sinkjaer T. Long-lasting depression of soleus motoneurons excitability following repetitive magnetic stimuli of the spinal cord in multiple sclerosis patients. Mult Scler. 1997;3:18–30. doi: 10.1177/135245859700300103. [DOI] [PubMed] [Google Scholar]
- 26.Siebner HR, Peller M, Willoch F, et al. Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology. 2000;54:956–963. doi: 10.1212/wnl.54.4.956. [DOI] [PubMed] [Google Scholar]