Abstract
The endocannabinoid system has recently emerged as a vital component of the stress response and is an appealing target for the treatment of mood and anxiety disorders. Additionally, corticolimbic endocannabinoid signaling is important for stress-induced regulation of emotional behavior. However, the mechanism by which this occurs remains elusive. Combining biochemical and behavioral analyses within the forced swim test, we examined whether stress-induced regulation of endocannabinoid signaling in the medial prefrontal cortex contributes to behavioral responses to stress, and whether these responses are dependent on serotonergic neurotransmission. Forced swim stress produced a rapid and pronounced reduction in medial prefrontal anandamide content, but had no effect on 2-arachidonoylglycerol content within this region. Local administration of an inhibitor of anandamide hydrolysis (0.01 μg) into the ventromedial region of the prefrontal cortex decreased passive coping responses and increased active behavioral strategies, a phenomenon which was blocked by local antagonism of the CB1 receptor. Furthermore, local inhibition of anandamide hydrolysis within the medial PFC increased the firing rate of serotonergic neurons within the dorsal raphe, suggesting that prefrontal cortical endocannabinoid signaling may modulate stress coping behaviours through a regulation of serotonergic neurotransmission. Accordingly, serotonin depletion prevented the ability of FAAH inhibition within the medial PFC to promote active stress coping responses. Collectively, these data argue that stress-induced changes in endocannabinoid signaling within the medial PFC modulate stress-coping behaviors through a regulation of serotonergic neurotransmission and provide a neuroanatomical framework by which we may understand the mechanisms subserving the antidepressant potential of the endocannabinoid system.
Keywords: endocannabinoid, forced swim test, in vivo electrophysiology, mass spectrometry, prefrontal cortex, serotonin
1. Introduction
The endocannabinoid system has recently surfaced as a promising therapeutic candidate for the treatment of emotional disorders that are precipitated by stress, and converging evidence has revealed a complex bidirectional relationship between endocannabinoid signaling and the neural stress circuit (Hill and McEwen, 2010; Riebe and Wotjak, 2011). Interactions with this circuit occur via stress-induced regulation of the endocannabinoid ligands N-arachidonylethanolamide (anandamide; AEA) and 2-arachidonoylglycerol (2-AG) (Hill and McEwen, 2010; Patel and Hillard, 2008). These neuroactive lipid signaling molecules activate presynaptic cannabinoid receptors (CB1), which results in the inhibition of excitatory, inhibitory, and monoaminergic neurotransmitter release throughout stress-responsive corticolimbic brain regions including the prefrontal cortex, amygdala, hippocampus, and hypothalamus (Freund et al., 2003). Following CB1 receptor activation, these endocannabinoids are then metabolized by their respective degradative enzymes; fatty acid amide hydrolase (FAAH) metabolizes AEA while monoacylglycerol lipase is responsible for the degradation of 2-AG (Ahn et al. 2008). Alterations within any of the components of the endocannabinoid system can have profound consequences for proper stress responses (Hill et al., 2010b; Riebe and Wotjak, 2011; Finn, 2010).
Given the extensive presence of endocannabinoid signaling within corticolimbic circuits and its sensitivity to regulation by stress, it is not surprising that endocannabinoid signaling is involved in the regulation of emotional behavior. Accordingly, facilitation of endocannabinoid signaling through the inhibition of AEA hydrolysis by FAAH has been shown to promote active coping responses to stress and reduce anxiety-like responses to aversive environmental stimuli (Gobbi et al., 2005; Haller et al., 2009; Patel and Hillard, 2006; Bortolato et al., 2007; Moreira et al., 2008; reviewed in Zanettini et al., 2011 and Hill et al., 2009a). One putative mechanism subserving the ability of FAAH inhibition to modulate stress coping behavior is that stress exposure rapidly activates FAAH, which downregulates AEA signaling in corticolimbic structures such as the amygdala (Hill et al., 2009b). As such, this rapid loss in AEA/CB1 signaling in response to stress could be a contributing factor to stress-induced alterations in emotional behavior. It is plausible that this endogenous mechanism may also dictate responses to stress within the prefrontal cortex, a structure known to critically mediate stress coping strategies and emotional behavior, although this hypothesis has yet to be empirically validated.
Accordingly, to investigate whether stress-induced regulation of prefrontocortical endocannabinoid signaling contributes to changes in emotional behavior, we coupled biochemical analyses of endocannabinoid signaling following exposure to swim stress with local pharmacological manipulation of AEA hydrolysis. Since recent studies have demonstrated that the ventromedial subregion of the prefrontal cortex (vmPFC) is particularly essential for the modulation of emotional behavior by cannabinoid signaling (Bambico et al., 2007; Rubino et al., 2008), our focus was on this brain region. Moreover, given the established role of serotonin (5-HT) in promoting active coping responses to swim stress (Cryan et al., 2005), we also sought to determine whether the ability of prefrontocortical endocannabinoid signaling to modulate coping behaviors in the forced swim test is dependent on 5-HT neurotransmission.
2. Experimental Procedures
2.1. Subjects
Male Sprague Dawley rats (Charles River Breeding, Montreal, Canada; 300-350 g) were pair-housed unless they had been implanted with cannulae, in which case they were individually housed in a room maintained at a temperature of 21±1°C with ad libitum food and water access. All experimental testing was conducted in accordance with the guidelines of the Canadian Council of Animal Care and was approved by the Animal Care Committee of the University of British Columbia.
2.2. Biochemical Studies
The medial PFC was rapidly harvested and frozen as previously described (Hill et al., 2010a) at four time points: no stress (D1 basal), immediately following the initial 15 min swim stress exposure (D1 stress), 24 h following the first swim exposure (D2 basal) and immediately following the 5 min swim stress exposure (D2 stress). The medial PFC was dissected as a tissue block that was anatomically defined as the area dorsal to the anterior olfactory nucleus, ventral to the motor cortex and medial to the corpus callosum and claustrum formation. All cohorts for biochemical analyses consisted of 7-8 rats per group. For endocannabinoid analysis, lipid extraction was carried out as described previously (Hill et al., 2010a) and contents of AEA and 2-AG were determined using isotope-dilution, liquid chromatography/mass spectrometry as described previously (Patel et al., 2005).
2.3. Behavioral Studies
Cylindrical glass containers (diameter 35 cm and height 45 cm) filled to 30 cm with water at a temperature of 24±1°C were used for all swim stress exposures. For both biochemical and behavioral studies, two swim sessions were employed. The first session was of 15 min duration and the second session of 5 min duration was performed 24 h thereafter. Consistent with the analysis of stress coping behaviors in the forced swim test (Porsolt et al., 2001), the first 15-min session was treated as an exposure session, while the second 5-min session was treated as the test session. Stress coping behaviors were analyzed during the 5-min session on day 2. In each study, animals were subjected to forced swim stress 1 hour after intra-PFC drug administration. Both active (swimming and struggling) and passive coping responses (immobility) were recorded and scored by trained observers blinded to treatment conditions (see McLaughlin et al., 2007 for a description of scoring criteria for each behavioral component).
In order to analyze the effects of intra-PFC inhibition of FAAH on coping behaviors in the forced swim test, cannulae were implanted into the vmPFC (coordinates from bregma: anterior/posterior = +3.0mm, medial/lateral = ±0.7mm, dorsal/ventral = -3.4mm from dura). Four steel screws and dental acrylic were used to permanently affix the guide cannulae to the skull. Behavioral testing began 7–10 days after the implantation surgery.
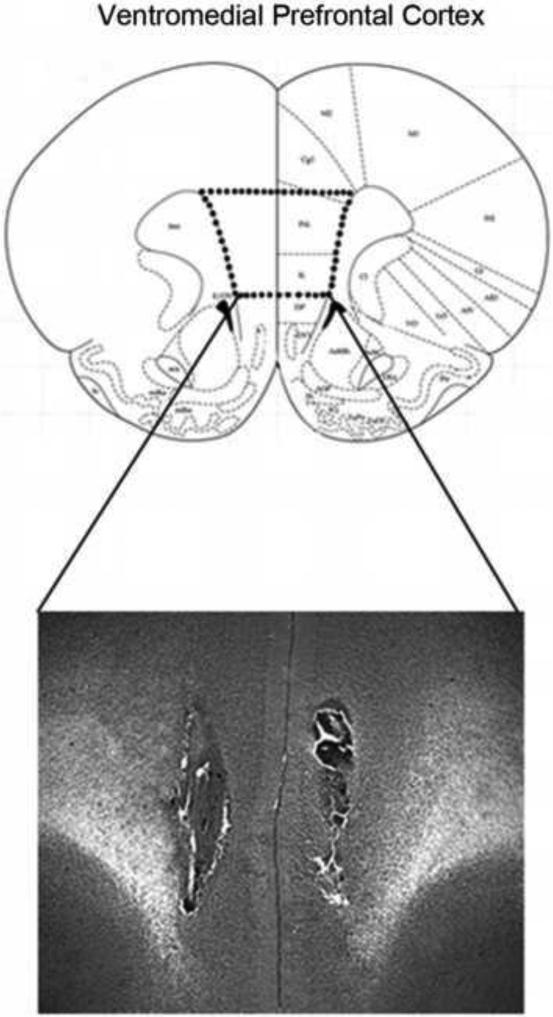
The FAAH inhibitor URB597 (Cayman Chemical; Ann Arbour, MI, USA), or its vehicle (1:9 dimethyl sulfoxide: 0.9 % saline; injection volume of 200 nl for all infusions), was infused at a dose of 0.01 μg bilaterally into the vmPFC prior to day 1 swim exposure, prior to day 2 swim exposure, or prior to both sessions. This dose was based on that demonstrated by Rubino and colleagues to increase AEA content and influence anxiety-like behavior (Rubino et al., 2008). To determine whether the behavioral effect of intra-vmPFC administration is a CB1 receptor-dependent phenomenon, a separate group of rats received a cocktail of URB597 and the CB1 receptor antagonist AM251 prior to day 1 and day 2 swim exposure (0.01 μg URB597: 0.28 ng AM251). Another cohort of rats was used to determine whether 5-HT activity might mediate the effects of intra-vmPFC URB597 administration. Vehicle or p-chlorophenylalanine (pCPA; 350 mg/kg, i.p.), a selective inhibitor of 5-HT synthesis, was administered 72 and 48 h before URB597 administration and subsequent swim exposure. All cohorts for behavioral testing consisted of 7-8 rats per group. Following behavioral testing, placements were verified employing the stereotaxic atlas of Paxinos and Watson (1998). Histological analysis revealed that approximately 90% of cannulae placements were in boundaries of the region of interest (Fig. 1). Subjects with cannulae outside of the desired subregion were excluded from subsequent analyses.
Figure 1.
Representative bilateral cannula placement for rats receiving microinfusions into the ventromedial region of the prefrontal cortex. Placements were confirmed according to the atlas of Paxinos and Watson (1998).
2.4. Electrophysiology Studies
In vivo extracellular single-unit recordings of putative dorsal raphe 5-HT neurons were performed, as previously described (Bambico et al., 2007), to examine whether the firing rate of this subpopulation of neurons is affected by bilateral intra-vmPFC administration of 0.01μg URB597. Rats were anaesthetized with chloral hydrate (400 mg/kg, i.p.) and mounted in a stereotaxic frame. Extracellular single-unit recordings were performed using single-barreled glass micropipettes pulled from 2 mm Stoelting capillary glass on a Narashige (Tokyo, Japan) PE-21 pipette puller and preloaded with fiberglass strands to promote capillary filling with 2% Pontamine Sky Blue dye in sodium acetate (0.5 M, pH 7.5). The micropipette tips were broken down to diameters of 1–3 μm. Electrode impedances ranged from 2 to 4 MΩ. A burr hole was drilled on the cranial midline subtending regions above the entire rostrocaudal medial extent of the dorsal raphe, a region considered richest in 5-HT neurons (Descarries et al., 1982). The electrode was lowered 0.5–1.0 mm posterior to the interaural line on the midline and 2.5–3.5 mm from the dura mater, just beneath the Sylvian aqueduct. The first putative 5-HT neurons were encountered immediately within this region, and 5-HT neuron-containing coordinates stretch from 5.0 to 6.5 mm ventral to the dura mater. Single-unit 5-HT activity was recorded as discriminated action potentials amplified by a Tennelec (Oakridge, TN) TB3 MDA3 amplifier, postamplified and filtered by a Realistic 10 band frequency equalizer, digitalized by a CED1401 interface system (Cambridge Electronic Design, Cambridge, UK), processed on-line, and analyzed off-line by Spike2 software version 5.05 for Windows PC (Microsoft, Seattle, WA). Under physiological conditions, 5-HT neurons exhibit characteristic electrophysiological properties distinguishable from non-5-HT neurons. These 5-HT neurons exhibit a slow (0.1–4 Hz) and a prominently regular firing rate (coefficient of variation (COV), ranges from 0.12 to 0.87), a broad biphasic (positive–negative) or triphasic waveform (0.8–3.5 ms; 1.4 ms first positive and negative deflections) (Allers and Sharp, 2003; Bambico et al., 2007; Baraban and Aghajanian, 1980). When the regularity of firing was apparently altered, putative 5-HT neurons were distinguished based on firing rate, spike shape, and duration, which are reliable markers for 5-HT neurons. Cannulae aimed at the vmPFC were also bilaterally implanted as described above. Once a stably firing 5-HT neuron was found and 1–3 min of baseline activity was established, microinfusion of URB597 or vehicle was administered directly into the vmPFC and changes in the neuronal discharge pattern of dorsal raphe 5-HT neurons were monitored over the following 120 min. At the end of each experiment, the recording site was marked by iontophoretic ejection (5–10 μA, negative current for 10 min) of Pontamine Sky Blue for histological verification. Data were presented as mean firing rate (Hz) +/- SEM.
2.5. Statistics
A one-way analysis of variance was used to analyze the effects of swim stress exposure on PFC tissue content of AEA and 2-AG as well as the effects of intra-vmPFC drug administration on behavioral parameters during swim sessions. An independent t-test was used to compare firing rates of 5-HT neurons following intra-vmPFC URB597 or vehicle injections. All post-hoc analyses were performed using Dunnett's tests. All statistical significance levels were set at 0.05.
3. Results
There was a significant effect of swim stress exposure on AEA content within the medial PFC [F (3, 30) = 11.23, p < 0.001], with post-hoc analysis revealing that relative to D1 basal, AEA content was significantly reduced following both D1 and D2 stress (both p's < 0.001). There was no difference between basal D1 and basal D2 AEA content, although exposure to the 5 min swim stress session on D2 reduced AEA content relative to D2 basal levels (p < 0.02; Fig. 2a). Consistent with these changes in AEA content, there was also significant effects of swim stress exposure on the tissue contents of both PEA [F (3, 30) = 3.58, p < 0.03; Table 1] and OEA [F (3, 30) = 4.17, p < 0.02; Table 1] within the mPFC, such that both PEA and OEA were significantly reduced on both D1 and D2 of swim stress exposure, relative to basal values (all p's < 0.05). These data indicate that stress increased metabolism of all fatty acid ethanolamides, consistent with a rapid increase in FAAH activity. There was no effect of swim stress on 2-AG content at any time point [F (3, 30) = 0.90, p > 0.05; Fig. 2b].
Figure 2.
The effect of forced swim stress on medial prefrontal endocannabinoid content. (A) Forced swim stress produced a rapid decline in medial prefrontal anandamide (AEA) content immediately following both day 1 (D1 FST) and day 2 (D2 FST) test sessions, relative to stress-naïve rats (BASAL). Moreover, AEA content was also significantly decreased following forced swim exposure on day 2 (D2 FST) compared to animals sacrificed just prior to the day 2 test session (D2 BASAL). (B) Forced swim stress did not significantly alter 2-arachidonoylglycerol (2-AG) levels in the medial prefrontal cortex at any time point relative to stress-naïve rats. Values are expressed as mean tissue levels ± SEM (n = 7-8 / treatment condition). * denotes significant differences at p < 0.05.
Table 1.
The effects of forced swim stress on the tissue content of the fatty acid ethanolamide molecules palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) in the medial prefrontal cortex.
| D1 Basal | D1 FST | D2 Basal | D2 FST | |
|---|---|---|---|---|
| PEA Content (pmol/g tissue) | 129.2 +/- 10.6 | 102.5+/- 4.9* | 111.9 +/- 6.6 | 100.8 +/- 3.0* |
| OEA Content (pmol/g tissue) | 60.1 +/- 5.8 | 42.2 +/- 2.4* | 57.8 +/- 5.8 | 44.8 +/- 0.8* |
Forced swim stress evoked a rapid decline in the tissue content of both PEA and OEA within the mPFC on both the first and second days of exposure to swim stress. Data are presented as mean values +/- SEM (n = 7-8 / condition).
denotes significant differences relative to basal (D1 basal) animals.
To determine whether the stress-induced decrease in AEA content contributed to coping strategies during swim exposure, we examined the effect of intra-vmPFC administration of the FAAH inhibitor URB597 on passive (immobility) and active (swimming and struggling) coping behaviors. Two additional cohorts of rats were also included to determine whether the behavioral effects of intra-vmPFC URB597 administration were CB1 receptor-dependent and/or 5-HT-mediated. A one-way ANOVA revealed that there was no significant difference between the control groups in each of these studies with respect to immobility [F (2,16) = 0.875, p > 0.05], swimming [F (2,16) = 0.344, p > 0.05], or struggling [F (2,16) = 0.085, p > 0.05]. Thus, for all further behavioral analyses, these control groups were pooled into a single value and compared to all other treatment conditions.
A one-way ANOVA revealed a significant main effect of treatment on immobility [F (5,43) = 6.02, p < 0.001]. Post-hoc analyses further showed that rats receiving intra-vmPFC injections of URB597 exhibited significantly reduced immobility levels compared to vehicle-treated rats, regardless of whether they received the infusion prior to day 1, prior to day 2, or prior to both days (all p's < 0.005). Rats receiving the URB597/AM251 cocktail did not differ from vehicle-treated rats, nor did rats pre-treated with the 5-HT depletor pCPA (both p's > 0.05; Fig. 3a). A one-way ANOVA also revealed a main effect of treatment on swimming behavior [F (5,43) = 10.59, p < 0.001]. Post-hoc analyses demonstrated that intra-vmPFC administration of URB597 significantly increased swimming, regardless of whether the infusion was given prior to day 1, prior to day 2, or prior to both days (all p's < 0.001). Accordingly, rats receiving the URB597/AM251 cocktail did not differ from vehicle-treated rats, nor did rats pre-treated with pCPA (both p's > 0.05; Fig. 3b). When struggling behavior was analyzed, a one-way ANOVA revealed no main effect of intra-vmPFC treatment on this coping strategy [F (5,43) = 1.79, p > 0.05; Fig. 3c]. Thus, intra-vmPFC administration of URB597 induces a CB1-receptor-dependent reduction in passive coping strategies (i.e., immobility) with a concomitant increase in active, escape-directed responses (i.e., swimming), which is dependent on the integrity of the 5-HT system.
Figure 3.
The effect of local medial prefrontocortical microinjection of the FAAH inhibitor URB597 (0.01 μg), alone or in conjunction with the CB1 receptor antagonist AM251 (0.28 ng) or the 5-HT depletor pCPA, on time spent in immobility (A), swimming (B), and struggling (C) in the forced swim test. Data are presented as mean time (s) +/- SEM (n = 6-7 / treatment condition). * denotes significant differences from vehicle at p < 0.05.
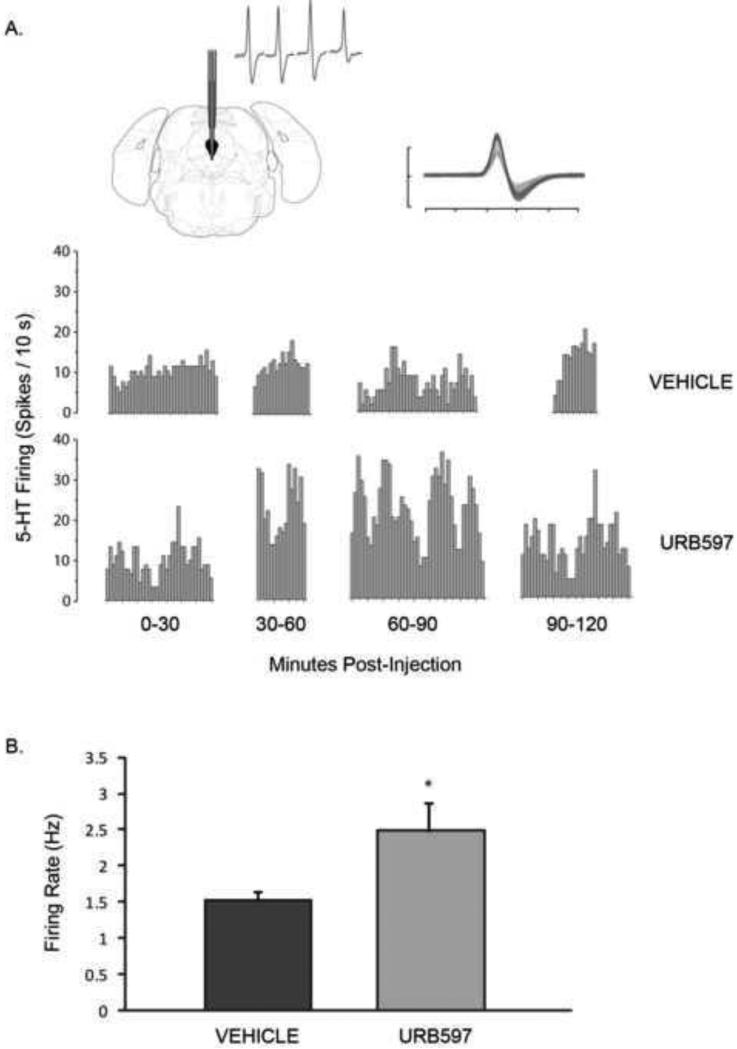
Because the behavioral effects of intra-vmPFC URB597 administration were dependent on 5-HT signaling, we next determined if local inhibition of FAAH within the vmPFC would alter the firing activity of 5-HT neurons in the dorsal raphe. For in vivo electrophysiological recordings, a total of 79 5-HT neurons were recorded from the dorsal raphe (vehicle, n = 41; URB597, n = 38; 4-5 rats/group). Figure 4 shows a representative integrated firing rate histogram of dorsal raphe 5-HT neurons recorded from rats receiving an intra-vmPFC infusion of URB597 or vehicle, as well as a typical waveform of a recorded 5-HT neuron. An independent t-test revealed that rats receiving an intra-vmPFC injection of URB597 displayed a significantly elevated 5-HT firing rate relative to vehicle-infused rats [t (77) = 2.41, p < 0.025; Fig. 4]. These data are in agreement with the behavioral studies above, suggesting that inhibition of AEA metabolism within the vmPFC coordinates behavioral coping responses to stress by modulating 5-HT neuronal output from the dorsal raphe.
Figure 4.
The effect of intra-medial-prefrontocortical administration of URB597 (0.01 μg) on the firing rate of dorsal raphe 5-HT neurons. (A) Representative integrated firing rate histograms of 5-HT neuronal activity recorded from a rat that received an intra-vmPFC infusion of vehicle (top) or URB597 (bottom). Abscissa represents time after infusion. Upper left inset: region of electrode contact within the dorsal raphe nucleus. Upper right inset: typical waveform characteristic of presumed 5-HT neurons encountered during electrophysiological recordings. Ordinate scale unit = 1 mV; abscissa scale unit = 1 ms. (B) Local microinjection of URB597 into the medial prefrontal cortex significantly enhanced the firing rate of dorsal raphe 5-HT neurons relative to vehicle-treated rats (vehicle, n = 41 neurons; URB597, n = 38 neurons). Data are presented as mean firing rate (Hz) +/- SEM (n = 4-5 rats / treatment condition). * denotes significant differences at p < 0.05.
4. Discussion
The present study demonstrated that exposure to swim stress rapidly suppressed AEA content within the medial PFC. In parallel, facilitation of AEA signaling within the vmPFC, through local FAAH inhibition, resulted in an increase in active behavioral coping responses to swim stress through a CB1 receptor dependent pathway. This increase in active coping responses following FAAH inhibition appeared to be mediated by an increase in serotonergic activity, as local FAAH inhibition within the mPFC increased dorsal raphe neuron firing and depletion of 5-HT prevented the effects of intra-PFC FAAH inhibition on active coping responses to stress. Collectively, these data demonstrate that prefrontal cortical AEA signaling may function to couple stress induction to changes in emotional behavior.
The role of AEA within the vmPFC in the coupling of stress induction to behavior parallels recent findings that stress exposure produces a rapid FAAH-mediated reduction in AEA signaling within the basolateral amygdala, which promotes activation of the HPA axis (Hill et al., 2009b). In accordance with the present results, local inhibition of FAAH within the basolateral amygdala attenuated stress-induced activation of the HPA axis (Hill et al., 2009b). Taken together, these data suggest that stress induces a widespread decrease in corticolimbic AEA signaling which contributes to changes in both neuroendocrine secretion and behavioral coping strategies.
The current evidence also suggests that changes in serotonergic transmission account for the ability of AEA signaling within the vmPFC to regulate stress coping behavior. In support of this hypothesis, the increase in active coping behaviors following FAAH inhibition is restricted to an increase in swimming behavior, but not struggling, which is the pattern observed for a 5-HT-mediated behavioral response (Cryan et al., 2005). Consistent with this, depletion of 5-HT prevented the ability of local FAAH inhibition in the mPFC to promote active coping responses, indicating that 5-HT signaling is required for this response. Furthermore, local inhibition of FAAH within the mPFC increased the firing rate of serotonergic neurons in the dorsal raphe. This finding is consistent with previous studies demonstrating that local administration of a CB1 receptor agonist into the medial PFC increases limbic serotonergic transmission and firing activity of dorsal raphe 5-HT neurons (Bambico et al. 2007), and that mice deficient in FAAH exhibit an increase in the firing activity of dorsal raphe neurons as well as corticolimbic 5-HT release (Bambico et al., 2010; Cassano et al., 2011). Further, trans-section of prefrontal cortical afferents or depletion of 5-HT can mitigate the ability of local CB1 receptor activation to promote active coping behaviors (Bambico et al., 2007). Collectively, these data indicate that facilitation of CB1 receptor signaling within the medial PFC increases the excitability and output of prefrontal cortical projection neurons, ultimately resulting in augmented dorsal raphe neuronal firing and limbic 5-HT release. This facilitation of 5-HT neurotransmission provides a mechanism of action by which CB1 receptor activation promotes active coping responses to stress; however, it should be noted that the serotonergic receptor subtypes involved in these behavioral changes have yet to be identified. The current data extends these findings by demonstrating that local AEA signaling within the vmPFC regulates the activation of the dorsal raphe by prefrontal cortical outputs, and that this AEA signal is both sensitive to stress exposure and modulates behavioral coping responses to stress.
We have recently demonstrated that CB1 receptors are predominantly localized to GABAergic terminals impinging upon pyramidal output neurons within layer V of the prelimbic region of the medial PFC (Hill et al., 2011), which is the origin of most prefrontal cortical projections to the dorsal raphe (Celada et al., 2002). As such, the mechanism by which AEA/CB1 receptor signaling within the medial PFC increases the activity of projection neurons is likely driven by an inhibition of GABA release onto projection neurons. Based on these data, we propose the working model that AEA/CB1 receptor signaling in the vmPFC tonically regulates the output of midbrain serotonergic nuclei. Exposure to an aversive stimulus would elicit a FAAH-mediated reduction in AEA content, resulting in a disinhibition of GABAergic release onto pyramidal neurons in the medial PFC, thereby decreasing vmPFC-mediated activation of dorsal raphe projections. Preventing the stress-induced decline in AEA signaling (via local administration of a FAAH inhibitor) could increase local activation of CB1 receptors on GABAergic neurons in the medial PFC, thereby reducing GABA-mediated inhibition of pyramidal neurons and allowing for enhanced dorsal raphe 5-HT transmission. As mentioned previously, this increase in 5-HT transmission enables active coping strategies to stressful stimuli (Kirby et al., 2007).
It should be noted that the ability of AEA facilitation in the vmPFC to promote excitability of dorsal raphe 5-HT firing might also be due to other mechanisms. For instance, not only do CB1 receptors exist on GABAergic interneurons in the vmPFC, they are also present to a lesser degree on glutamatergic pyramidal neurons in this region (Fortin and Levine, 2007). Medial PFC pyramidal output is known to exert both excitatory and inhibitory effects on dorsal raphe 5-HT activity via activation of GABAergic/5-HT1A receptors and AMPA/NMDA receptors, respectively (Hajos et al., 1998). Thus, it is possible that facilitation of AEA/CB1 receptor signaling on glutamatergic vmPFC pyramidal neurons acts to directly inhibit excitatory inputs to the dorsal raphe that synapse onto inhibitory GABAergic and/or 5-HT1A neurons in this region, thereby promoting disinhibition of 5-HT firing locally within the dorsal raphe and enabling active stress coping. However, arguing against this theory is evidence suggesting that electrical stimulation of medial PFC projection neurons represents an effective antidepressant strategy and promotes proactive stress coping responses in humans and rodents, respectively. For instance, deep brain stimulation of the subgenual PFC in humans (which is functionally homologous to the vmPFC in rodents) has emerged clinically as a promising novel therapeutic strategy for treatment-resistant cases of major depression (Price and Drevets, 2009). Likewise, deep brain stimulation of the rodent medial PFC promotes anxiolysis as well as a robust antidepressant-like response in the forced swim test in a 5-HT-dependent manner (Hamani et al., 2009). Moreover, electrical stimulation of the medial (but not lateral) PFC elicits substantial increases in limbic 5-HT output that likely contributes to the rapidly induced antidepressant effects of deep brain stimulation and electroconvulsive therapy (Juckel et al. 1999). Collectively, these data argue that the antidepressant profile obtained from intra-vmPFC administration of URB597 is likely due to CB1 receptor-mediated disinhibition of vmPFC pyramidal neurons, thereby allowing for increased dorsal raphe 5-HT output to corticolimbic brain regions that are functionally implicated in stress coping and emotionality.
The endocannabinoid system is now a prominent target for the development of novel antidepressants, particularly in the form of FAAH inhibition rather than direct CB1 receptor agonism (Gobbi et al., 2005; Hill et al., 2009a). The present data demonstrate that facilitation of endocannabinoid signaling within the vmPFC is likely an important neural substrate for the ability of endocannabinoids to modulate stress-regulated emotional behaviors. In line with this, a recent report has demonstrated that FAAH inhibition within the vmPFC produces anxiolytic effects, while local lentivirus-mediated overexpression of FAAH exerts the opposite behavioral effect (Rubino et al., 2008). These data suggest that stress-induced regulation of AEA signaling within the vmPFC may be important for the coordination and regulation of multiple facets of emotional behavior in response to stress. Together, this body of evidence suggests that AEA/CB1 receptor signaling in the vmPFC is tuned by environmental stimuli and through its ability to regulate serotonergic neurotransmission, could serve as both an important regulator of emotional responding and a determining factor in the nature of the coping response engaged in response to stressful stimuli.
Acknowledgements
We kindly thank Anna Morrish, Caitlin Riebe, and Silvain Dang for their care and maintenance of animals during the study, and for assisting in the behavioral testing and data collection.
Role of Funding Source
This work was supported by operating grants from the Canadian Institutes of Health Research (CIHR) to BBG, the Canadian Foundation for Innovation (CFI) and the Fonds de la Recherche en Santé du Québec (FRSQ) to GG, and the National Institutes of Health (DA09155) to CJH. RJM is a recipient of a CIHR Canadian Graduate Student Doctoral Research Award. MNH is a recipient of a postdoctoral fellowship from CIHR. FRB is a recipient of a graduate fellowship from the McGill University Health Center (MUHC) and FRSQ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
RJM designed the study, wrote the protocol and manuscript, and performed all of the lipid extractions, brain removals, surgical cannulations, microinfusions, forced swim testing, and statistical analyses. MNH assisted in the study design, scored the behavioral data, and performed all tissue dissections. FRB and GG performed all of the electrophysiology experiments. KLS and CJH conducted the liquid chromatography/mass spectrometry analyses. BBG assisted in the study design and proofread the manuscript. All authors contributed to and have approved the final manuscript.
References
- Ahn K, McKinney MK, Cravatt BF. Enzymaytic pathways that regulate endocannabinoid signaling in the nervous system. Chem. Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122:193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Katz N, Debonnel G, Gobbi G. Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J. Neurosci. 2007;27:11700–11711. doi: 10.1523/JNEUROSCI.1636-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Cassano T, Dominguez-Lopez S, Katz N, Walker CD, Piomelli D, Gobbi G. Genetic deletion of fatty acid amide hydrolase alters emotional behavior and serotonergic transmission in the dorsal raphe, prefrontal cortex, and hippocampus. Neuropsychopharmacology. 2010;35:2083–2100. doi: 10.1038/npp.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban JM, Aghajanian GK. Suppression of serotonergic neuronal firing by alpha-adrenoceptor antagonists: evidence against GABA mediation. Eur. J. Pharmacol. 1980;66:287–294. doi: 10.1016/0014-2999(80)90461-6. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol. Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Cassano T, Gaetani S, Macheda T, Laconca L, Romano A, Morgese MG, Cimmino CS, Chiarotti F, Bambico FR, Gobbi G, Cuomo V, Piomelli D. Evaluation of the emotional phenotype and serotonergic neurotransmission of fatty acid amide hydrolase-deficient mice. Psychopharmacology. 2011;214:465–476. doi: 10.1007/s00213-010-2051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Martín-Ruiz R, Casanovas JM, Artigas F. Control of the serotonergic system by the medial prefrontal cortex: potential role in the etiology of PTSD and depressive disorders. Neurotox. Res. 2002;4:409–419. doi: 10.1080/10298420290030550. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Garcia S, Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J. Comp. Neurol. 1982;207:239–254. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- Díaz-Mataix L, Artigas F, Celada P. Activation of pyramidal cells in rat medial prefrontal cortex projecting to ventral tegmental area by a 5-HT1A receptor agonist. Eur. Neuropsychopharmacol. 2006;16:288–296. doi: 10.1016/j.euroneuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Finn DP. Endocannabinoid-mediated modulation of stress responses: physiological and pathophysiological significance. Immunobiology. 2010;215:629–646. doi: 10.1016/j.imbio.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Fortin DA, Levine ES. Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cereb. Cortex. 2007;17:163–174. doi: 10.1093/cercor/bhj133. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc. Natl. Acad. Sci. U.S.A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Merali Z, Blier P. Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. Int. J. Neuropsychopharmacol. 2008;11:625–639. doi: 10.1017/S1461145707008383. [DOI] [PubMed] [Google Scholar]
- Hajos M, Richards CD, Szekely AD, Sharp T. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- Haller J, Barna I, Barsvari B, Gyimesi Pelczer K, Yasar S, Panlilio LV, Goldberg S. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology. 2009;204:607–616. doi: 10.1007/s00213-009-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Diwan M, Macedo CE, Brandão ML, Shumake J, Gonzalez-Lima F, Raymond R, Lozano AM, Fletcher PJ, Nobrega JN. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol. Psychiatry. 2009;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, Bambico FR, Patel S, Gorzalka BB, Gobbi G. The therapeutic potential of the endocannabinoid system for the development of a novel class of antidepressants. Trends. Pharmacol. Sci. 2009a;30:484–493. doi: 10.1016/j.tips.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, Gorzalka BB. Suppression of amygdalar endocannabinoid signaling by stress contribute to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009b;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, Gray JM, Hillard CJ, Gorzalka BB, Viau V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc. Natl. Acad. Sci. U.S.A. 2010a;107:9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, Bains JS. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J. Neurosci. 2010b;30:14980–14986. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Pan B, Fitzgerald M, Roberts CJ, Lee TT, Karatsoreos IN, Mackie K, Viau V, Pickel VM, McEwen BS, Liu QS, Gorzalka BB, Hillard CJ. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J. Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodo E, Chiang C, Aston-Jones G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience. 1998;83:63–79. doi: 10.1016/s0306-4522(97)00372-2. [DOI] [PubMed] [Google Scholar]
- Juckel G, Mendlin A, Jacobs BL. Electrical stimulation of rat medial prefrontal cortex enhances forebrain serotonin output: Implications for electroconvulsive therapy and transcranial magnetic stimulation in depression. Neuropsychopharmacology. 1999;21:391–398. doi: 10.1016/S0893-133X(98)00097-9. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pan YZ, Freeman-Daniels E, Rani S, Nunan JD, Akanwa A, Beck SG. Cellular effects of swim stress in the dorsal raphe nucleus. Psychoneuroendocrinology. 2007;32:712–723. doi: 10.1016/j.psyneuen.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, Morrish AC, Gorzalka BB. Local enhancement of cannabinoid CB1 receptor signaling in the dorsal hippocampus elicits an antidepressant-like effect. Behav. Pharmacol. 2007;18:431–438. doi: 10.1097/FBP.0b013e3282ee7b44. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signaling. Eur. J. Neurosci. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J. Pharmacol. Exp. Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Adaptations in endocannabinoid signaling in response to repeated homotypic stress: a novel mechanism for stress habituation. Eur. J. Neurosci. 2008;27:2821–2829. doi: 10.1111/j.1460-9568.2008.06266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. fourth ed. Academic Press; San Diego: 1998. [Google Scholar]
- Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. 2001;8:10A. doi: 10.1002/0471142301.ns0810as14. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2009;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebe CJ, Wotjak CT. Endocannabinoids and stress. Stress. 2011;14:384–397. doi: 10.3109/10253890.2011.586753. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Castiglioni C, Guidali C, Viganó D, Marras E, Petrosino S, Perletti G, Maccarrone M, Di Marzo V, Parolaro D. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb. Cortex. 2008;18:1292–1301. doi: 10.1093/cercor/bhm161. [DOI] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Stimulation of the prefrontal cortex in the rat induces patterns of activity in midbrain dopaminergic neurons which resemble natural burst events. Synapse. 1996;22:195–208. doi: 10.1002/(SICI)1098-2396(199603)22:3<195::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Zanettini C, Panlilio LV, Alicki M, Goldberg SR, Haller J, Yasar S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front. Behav. Neurosci. 2011;5:1–21. doi: 10.3389/fnbeh.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]