Abstract
Background
Both growth hormone (GH) excess and GH deficiency are associated with body composition and biomarkers of cardiovascular risk in patients with pituitary disorders. However, the effects of developing GH deficiency after definitive treatment of acromegaly are largely unknown.
Objective
To determine whether development of GH deficiency after definitive therapy for acromegaly is associated with increased visceral adiposity and biomarkers of cardiovascular risk compared to GH sufficiency after definitive therapy for acromegaly.
Design
Cross-sectional
Patients
We studied three groups of subjects, all with a history of acromegaly (n=76): subjects with subsequent GH deficiency (GHD; n=31), subjects with subsequent GH sufficiency (GHS; n=25), and subjects with active acromegaly (AA; n=20). No study subjects were receiving somatostatin analogues, dopamine agonists or hGH.
Measurements
Body composition (by DXA), abdominal adipose tissue depots (by cross-sectional CT), total body water (by bioimpedance analysis) and carotid intima-media thickness (IMT) were measured. Fasting morning serum was collected for high-sensitivity C-reactive protein (hsCRP), lipids and lipoprotein levels. An oral glucose tolerance test was performed, and homeostasis model of assessment-insulin resistance (HOMA-IR) was calculated.
Results
Abdominal visceral adipose tissue, total adipose tissue, and total body fat were higher in subjects with GHD than GHS or AA (p < 0.05). Subcutaneous abdominal fat was higher, and fibrinogen and IMT were lower in GHD (but not GHS) than AA (p < 0.05). Patients with GHD had the highest hsCRP, followed by GHS, and hsCRP was lowest in AA (p < 0.05). Fasting glucose, 120-minute glucose, fasting insulin, HOMA-IR, and percent total body water were lower in GHD and GHS than AA (p < 0.05). Triglycerides were higher in GHS than AA (p < 0.05). Lean body mass, mean arterial pressure, total cholesterol, HDL, and LDL were comparable among groups.
Conclusions
Development of GHD after definitive treatment of acromegaly may adversely affect body composition and inflammatory biomarkers of cardiovascular risk but does not appear to adversely affect glucose homeostasis, lipids and lipoproteins, or other cardiovascular risk markers.
Keywords: Acromegaly, Growth hormone, Growth hormone deficiency
Introduction
Growth hormone (GH) excess in acromegaly confers increased morbidity and mortality 1, primarily of cardiovascular etiology, from the effects of elevated GH levels, including hypertension, type 2 diabetes, dyslipidemia, and endothelial dysfunction 2. Decreasing GH and/or insulin-like growth factor 1 (IGF-I) levels attenuates this risk, and normalization of GH and/or IGF-I levels can reduce mortality rates to that of the general population 1, 3, 4. Similarly, many of the biomarkers of cardiovascular risk that are elevated in acromegaly, such as serum lipids, improve with normalization of IGF-I levels 5–8. With advances in surgical techniques, radiotherapy and in medical therapy, biochemical control can be achieved in the majority of patients with acromegaly. However, many patients with somatotroph tumors develop GH deficiency after surgery and/or radiation therapy. In a study of the development of GH deficiency in acromegaly, 55% of patients treated with surgery and 70% of those treated with surgery and radiation subsequently developed GH deficiency (61% overall rate) 9. Despite the high prevalence of GH deficiency after definitive treatment of acromegaly, its effects on body composition and cardiovascular risk markers are largely unknown.
GH deficiency in patients with treatment-associated hypopituitarism for non-somatotroph tumors is associated with abnormalities in body composition and increased cardiovascular risk markers, including increased total and truncal fat 10, insulin resistance 11, systolic hypertension 12, carotid intima-medial thickness (IMT) 13, high-sensitivity C-reactive protein (hsCRP) 14, dyslipidemia 12 and decreased plasma fibrinolytic activity 15. Moreover, GH administration in GH-deficient patients improves body composition, with a reduction in total and visceral fat and an increase in lean body and muscle mass 16–22. Cardiovascular risk markers, including hsCRP, apoprotein B, tissue plasminogen activator, plasminogen activator inhibitor-1 and IMT also improve with replacement in GH deficiency 13, 17, 23–25.
We hypothesized that in patients with GH deficiency following definitive treatment of acromegaly, the development of GH deficiency may be associated with increased visceral adiposity, reduced lean body mass and increased cardiovascular risk markers. We therefore compared body composition and cardiovascular risk marker variables in a group of patients who had developed GH deficiency after definitive treatment of acromegaly to a group of patients who were GH-sufficient after treatment of acromegaly and to a group of patients with active acromegaly.
Materials and Methods
Study Participants
We studied three groups of subjects (n=76), all with a history of acromegaly: subjects who developed GH deficiency after definitive therapy for acromegaly (GHD; n=31), subjects who remained GH sufficient after definitive therapy for acromegaly (GHS; n=25), and subjects with active acromegaly (AA; n=20). GH deficiency was diagnosed based on a peak GH <5 μg/L in response to GH-releasing hormone-arginine stimulation, or an IGF-I level more than two standard deviations below the age-specific normal range in subjects with at least three other documented anterior pituitary hormone deficiencies 26. Subjects receiving thyroid hormone, gonadal steroid and/or cortisol replacement therapy were required to have been receiving stable replacement doses for at least three months prior to entry into the study. For the AA group, study visits were coordinated with pre-operative testing or medication teaching, and treatment of acromegaly was not delayed by study participation. Duration of growth hormone deficiency was estimated as time since most recent pituitary surgical or radiation procedure.
Exclusion criteria included use of somatostatin analogs within the past six months, growth hormone receptor antagonists or dopamine agonists, untreated adrenal or thyroid deficiency, hemoglobin <110 g/L, ALT or AST >3x upper limit of normal, creatinine level >221 μmol/L, congestive heart failure (New York Heart Association’s Class II-IV), unstable coronary artery or cerebrovascular disease, pregnancy, breastfeeding, or known acute illness. The study was conducted in the General Clinical Research Centers of the Massachusetts General Hospital and Massachusetts Institute of Technology. The study was approved by the Partners Healthcare Inc. and Massachusetts Institute of Technology Institutional Review Boards, and all subjects gave written informed consent. The trial is registered at ClinicalTrials.gov, number NCT00182091. Clinical characteristics, body composition and cardiovascular risk results on a subset of the GHD group have been previously published as baseline results of a longitudinal study 27, clinical characteristics of a subset of the GHD and GHS groups have been previously published 28, and echocardiographic measurements on a subset of the three groups has also been previously published 29; but no body composition or cardiovascular risk marker data have been previously published for the GHS or AA groups.
Biochemical Testing
Serum and plasma samples were collected and stored at −80°C. Serum IGF-I levels were measured using an Immulite 2000 automated immunoanalyzer (Diagnostic Products Corp., Inc, Los Angeles, CA), a solid-phase, enzyme-labeled chemiluminescent immunometric assay with an inter-assay coefficient of variation (cv) of 3.7–4.2%. Two-hour oral glucose tolerance testing (OGTT) was performed, and the homeostasis model assessment of insulin resistance (HOMA-IR) was calculated.
Body Composition Testing
Total body water (TBW) was assessed by bioelectric impedance analysis using Bioelectrical Analyzer model BIA 101 (RJL Systems, Michigan). Fat mass and lean body mass were measured by dual-energy x-ray absorptiometry (DXA) using a Hologic QDR-4500 densitometer (Hologic Inc., Waltham, Massachusetts). Abdominal adipose deposition -- including cross-sectional total (TAT), subcutaneous (SAT), and intra-abdominal or visceral (VAT) adipose tissue compartments -- was determined in duplicate using single-slice quantitative CT scans at the level of L4 with 10 mm thick axial images (General Electric RP High Speed Helical CT Scanner, Milwaukee, WI) and graphical analysis software (General Electric Advantage Windows Work Station Version 2.0, General Electric). Technical factors for the scanning were 80 kVp, 70mA, and a 2-second scan time.
Cardiovascular Testing
Carotid IMT was performed and analyzed by a single cardiologist, blinded to subject disease status, as follows: long-axis B-mode images were obtained from the distal one cm of the left and right common carotid arteries of each subject, using a variable frequency 5–12 MHz ultrasound transducer on a Philips HDI 5000 ultrasound system (Philips Medical Systems, Bothell, WA). After image digitization, average carotid IMT over each distal one cm segment was calculated using semi-automated edge detection software. Mean carotid IMT was averaged from each set of measurements (right and left). HsCRP was measured using a latex particle enhanced immunoturbidimetric assay on the Hitachi 917 (Equal Diagnostics, Exton, PA, USA), with an interassay cv of <5.08%. Levels greater than 95 nmol/L were excluded from analysis, as hsCRP levels in this range reflect acute disease, such as infection or inflammation.
Statistical Analysis
JMP Statistical Discoveries Software (version 9.0.1; SAS Institute, Inc., Cary, NC) was used for statistical analysis. Variables were log-transformed prior to significance testing in order to normalize the distribution of data. Fisher’s Exact testing was used to compare categorical variables. Fisher’s Least Significant Difference testing was used for analysis of continuous variables, in which a preliminary test (overall ANOVA) is followed by a pairwise comparison of the three groups. Further adjustment for multiple comparisons is not necessary when there are three groups due to the use of a preliminary test of significance 30. The overall ANOVAs are presented in Table 2, with pairwise ANOVAs presented in the text. Means were compared with analysis of variance (ANOVA). Welch ANOVA was performed when unequal variances were present. Univariate associations were determined, and Pearson coefficients are reported. Stepwise multivariate analyses were performed to control for the presence of anterior pituitary deficiencies for all variables except for hsCRP, for which the model was unstable. The following variables were entered into the models: presence of adrenal insufficiency, free thyroxine level, and presence of hypogonadism (defined as the absence of endogenous or exogenously replaced sex steroid hormones). As a secondary analysis, we reclassified subjects using weight-based GHRH-arginine stimulation testing cut-offs 31. Statistical significance was defined as a 2-tailed p value less than 0.05. Results are expressed as mean ± SEM unless otherwise noted.
Table 2.
Body Composition, Cardiovascular Risk Markers and Metabolic Variables
| GHD (n = 31) | GHS (n = 25) | AA (n = 20) | p | |
|---|---|---|---|---|
|
| ||||
| Body mass index (kg/m2) | 31.5 ± 1.2 | 28.4 ± 1.2 | 29.1 ± 1.5 | 0.14 |
| Waist/hip ratio | 0.91 ± 0.01 | 0.86 ± 0.01 | 0.89 ± 0.02 | 0.04a |
| Total body water (%) | 50.2 ± 1.3 | 50.9 ± 1.2 | 55.7 ± 1.2 | 0.01b,c |
| Total fat (kg) | 31.6 ± 1.9 | 26.3 ± 2.0 | 23.4 ± 2.3 | 0.009a,c |
| Percent fat (%) | 33.9 ± 1.3 | 31.6 ± 1.6 | 25.7 ± 1.6 | 0.001b,c |
| Total lean (kg) | 58.5 ± 2.7 | 54.1 ± 2.7 | 64.2 ± 3.8 | 0.11 |
| Extremity lean (kg) | 25.8 ± 1.3 | 23.3 ± 1.4 | 28.5 ± 1.9 | 0.08 |
| Subcutaneous abdominal adipose tissue (cm2) | 323.5 ± 22.3 | 284.0 ± 30.1 | 245.6 ± 34.8 | 0.03c |
| Visceral abdominal adipose tissue (cm2) | 165.8 ± 15.0 | 106.4 ± 10.0 | 106.9 ± 18.8 | 0.001a,c |
| Total abdominal adipose tissue (cm2) | 476.0 ± 26.4 | 390.4 ± 36.4 | 352.5 ± 45.3 | 0.006a,c |
| Mean arterial pressure (mmHg) | 90 ± 1 | 87 ± 2 | 93 ± 3 | 0.15 |
| Total cholesterol (mmol/L) | 5.06 ± 0.19 | 4.67 ± 0.30 | 4.68 ± 0.28 | 0.36 |
| LDL cholesterol (mmol/L) | 3.16 ± 0.16 | 2.82 ± 0.21 | 2.88 ± 0.28 | 0.33 |
| HDL cholesterol (mmol/L) | 1.33 ± 0.07 | 1.51 ± 0.09 | 1.16 ± 0.07 | 0.05 |
| Triglycerides (mmol/L) | 1.24 ± 0.09 | 1.08 ± 0.11 | 1.38 ± 0.08 | 0.03b |
| Carotid intima-media thickness (mm) | 0.661 ± 0.027 | 0.686 ± 0.018 | 0.771 ± 0.035 | 0.04c |
| hsCRP (nmol/L) | 27.9 ± 4.5 | 15.9 ± 4.3 | 7.5 ± 2.9 | 0.0001a,b,c |
| Fasting glucose (mmol/L) | 4.71 ± 0.09 | 4.74 ± 0.09 | 5.98 ± 0.48 | 0.01b,c |
| 120-min glucose (mmol/L) | 6.15 ± 0.43 | 5.80 ± 0.34 | 8.80 ± 0.96 | 0.02b,c |
| Fasting insulin (uIU/mL) | 9.89 ± 0.98 | 7.92 ± 0.88 | 17.43 ± 1.93 | <0.0001b,c |
| HOMA-IR | 2.17 ± 0.24 | 1.68 ± 0.20 | 3.83 ± 0.32 | <0.0001b,c |
| HbA1c (%) | 5.6 ± 0.1 | 5.5 ± 0.1 | 6.0 ± 0.3 | 0.12 |
| Resting energy expenditure (kcal/d) | 1632 ± 66 | 1513 ± 65 | 1945 ± 108 | 0.002b,c |
| REE controlling for total lean mass | 0.02b,c | |||
| Fibrinogen (μmol/L) | 9.58 ± 0.36 | 10.11 ± 0.45 | 11.65 ± 0.80 | 0.03c |
Means ± SEMs are presented. GHD = growth hormone deficiency after definitive treatment of acromegaly, GHS = growth hormone sufficiency after definitive treatment of acromegaly, AA = active acromegaly, LDL = low-density lipoprotein, HDL = high-density lipoprotein, hsCRP = high-sensitivity C-reactive protein, HOMA-IR = homeostasis model of assessment - insulin resistance, REE = resting energy expenditure.
, p < 0.05 between GHD and GHS groups;
, p < 0.05 between GHS and AA groups;
, p < 0.05 between GHD and AA groups.
Results
Clinical Characteristics
Clinical characteristics and biochemical assessments are shown in Table 1. The mean age (range 19–75 years), mean arterial pressure, body mass index (range 20.4–48.3 kg/m2), and hemoglobin A1c were comparable among the groups. As expected, mean IGF-I levels and Z-scores differed among the groups, with the lowest levels in the GHD group and the highest levels in the AA group.
Table 1.
Clinical Characteristics
| GHD (n = 31) | GHS (n = 25) | AA (n = 20) | |
|---|---|---|---|
|
| |||
| Age (yr) | 47 ± 2 | 47 ± 2 | 46 ± 3 |
| Gender (n) | 13M/18F | 7M/18F | 10M/10F |
| Acromegaly symptom onset (mo) | 81 ± 17 | ||
| Time since acromegaly diagnosis (mo) | 14 ± 7 | ||
| Treatment | |||
| Surgery (n; %) | 28 (90%) | 25 (100%) | 6 (30%) |
| Radiation therapy (n; %) | 24 (77%) | 8 (32%) | 1 (5%) |
| Surgery and radiation (n; %) | 21 (68%) | 8 (32%) | 1 (5%) |
| Anterior pituitary deficiencies | |||
| Adrenal insufficiency (n; %) | 15 (48%) | 3 (12%) | 0 (0%) |
| Hypothyroidism (n; %) | 23 (74%) | 7 (28%) | 0 (0%) |
| Hypogonadism (n; %) | 28 (90%) | 5 (20%) | 3 (15%) |
| Estimated duration of growth hormone deficiency (mo) | 139 ± 20 | ||
| Hormone replacement | |||
| Oral glucocorticoid use (n; %) | 15 (48%) | 3 (12%) | 1 (5%) |
| Inhaled/topical glucocorticoid use (n; %) | 0 (0%) | 2 (8%) | 1 (5%) |
| Thyroid hormone use (n; %) | 23 (74%) | 9 (36%) | 3 (15%) |
| Male testosterone use (n; %) | 10 (77%) | 1 (14%) | 0 (0%) |
| Female estrogen use (n; %) | 11 (61%) | 4 (22%) | 2 (20%) |
| Oral estrogen use (n; %) | 9 (50%) | 3 (17%) | 2 (20%) |
| Transdermal estrogen use (n; %) | 2 (11%) | 1 (6%) | 0 (0%) |
| Tobacco use (n; %) | 4 (13%) | 4 (16%) | 4 (20%) |
| Antihypertensive medication use (n; %) | 6 (19%) | 10 (40%) | 7 (35%) |
| Diabetes medication use (n; %) | 2 (7%) | 1 (4%) | 2 (10%) |
| Free thyroxine (pmol/L) | 14.9 ± 0.7 | 15.9 ± 0.6 | 16.5 ± 0.9 |
| Insulin-like growth factor I (μg/L) | 91.0 ± 8.9 | 137.9 ± 11.4 | 606.5 ± 49.7 |
| Insulin-like growth factor I SDS | −2.0 ± 0.1 | −1.3 ± 0.2 | 5.1 ± 0.8 |
| Fasting growth hormone (nmol/L) | 0.45 ± 0.10 | 1.46 ± 0.34 | |
| Peak growth hormone (μg/L) | 2.93 ± 0.26 | 29.2 ± 4.9 | |
Means ± SEM are presented. GHD = growth hormone deficiency after definitive treatment of acromegaly, GHS = growth hormone sufficiency after definitive treatment of acromegaly, AA = active acromegaly, SDS = standard deviation score.
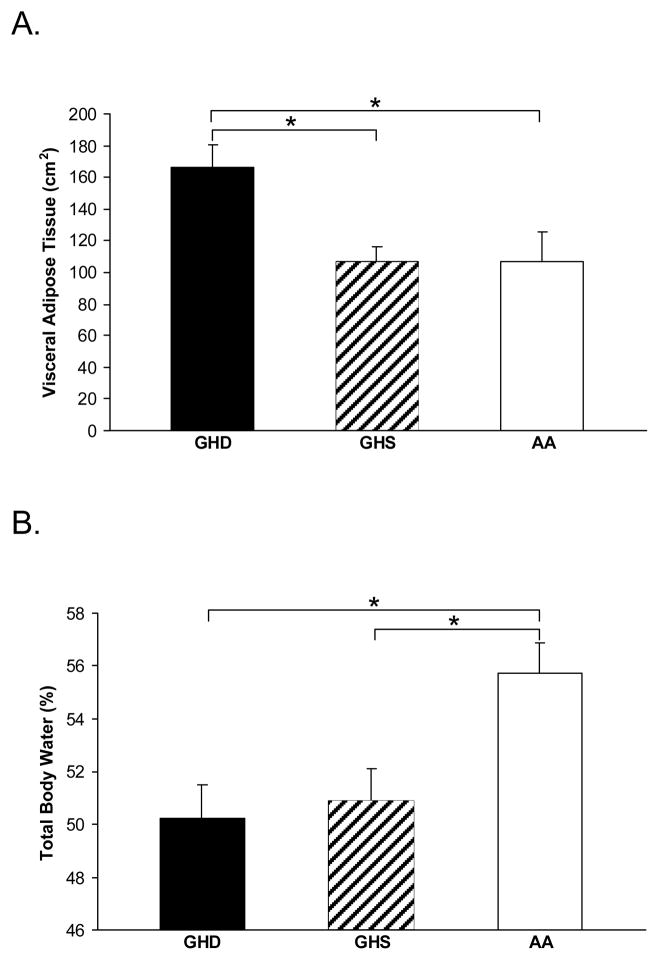
Body Composition
Body composition results are shown in Table 2. Total abdominal adipose tissue (TAT) and VAT (Figure 1A) were higher in GHD compared to both GHS and AA. SAT was higher in GHD compared to AA, but the GHD and GHS groups were not different. TBW percentage (Figure 1B) was higher and body fat percentage was lower in the AA group compared to both the GHD and GHS groups. There was a trend toward higher extremity lean mass in the AA group (p = 0.08).
Figure 1.
Mean visceral adipose tissue (panel A) was highest in the GHD group compared to both the GHS and AA group, and there was no difference between the GHS and AA groups. Mean total body water percentage (panel B) was highest in the AA group compared to both the GHD and GHS groups, and there was no difference between the GHD and GHS groups. GHD = growth hormone deficiency after definitive treatment of acromegaly, GHS = growth hormone sufficiency after definitive treatment of acromegaly, AA = active acromegaly. *, p < 0.05.
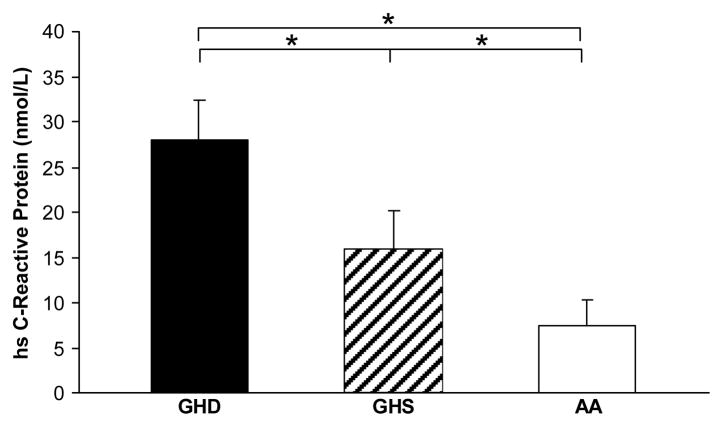
Cardiovascular Risk Biomarkers
Levels of cardiovascular risk biomarkers are shown in Table 2. HsCRP differed among all groups, with the highest levels in GHD and lowest levels in AA (Figure 2). Triglycerides were lower in GHS than AA. Mean arterial pressure, total cholesterol, HDL, and LDL levels were comparable among groups. Fibrinogen and IMT were lower in GHD than AA (p = 0.01 for both), with a trend toward lower IMT in GHS compared to AA (p = 0.05).
Figure 2.
Mean hsCRP levels were highest in the GHD group and lowest in the AA group, with significant differences between each pair of groups. GHD = growth hormone deficiency after definitive treatment of acromegaly, GHS = growth hormone sufficiency after definitive treatment of acromegaly, AA = active acromegaly, hsCRP = high-sensitive C-reactive protein. *, p < 0.05.
Metabolic Variables
Metabolic variable results are shown in Table 2. All glucose handling variables, including fasting glucose, 120-minute OGTT glucose, fasting insulin, and HOMA-IR, were higher in the AA group compared to GHD and GHS, but were not different between the GHD and GHS groups. Resting energy expenditure also followed this pattern, with higher values in the AA group, but no significant difference between the GHD and GHS groups. There was no difference in respiratory quotient or basal metabolic rate.
Associations with IGF-I
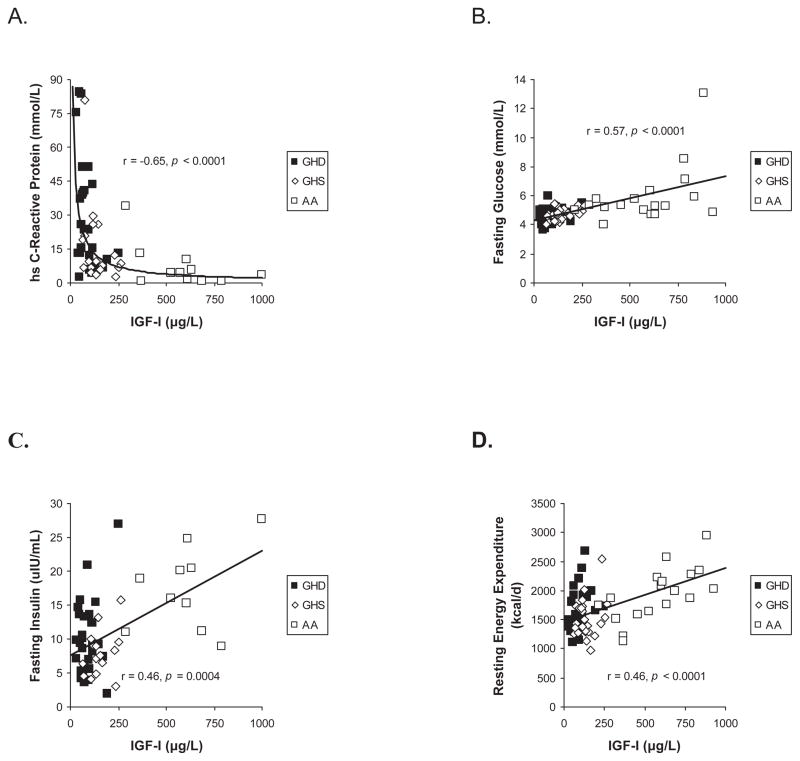
IGF-I levels in all groups combined were inversely associated with VAT (r = −0.31, p = 0.006), hsCRP (r = −0.64, p < 0.0001, Figure 3A), HDL (r = −0.30, p = 0.02) and total body fat percentage (r = −0.38, p = 0.0008). In contrast, IGF-I levels were positively associated with fasting glucose (r = 0.57, p < 0.0001, Figure 3B), 120-minute OGTT glucose (r = 0.42, p = 0.0003), HOMA-IR (r = 0.41, p = 0.002), hemoglobin A1c (r = 0.26, p = 0.03), resting energy expenditure (r = 0.46, p < 0.0001, Figure 3C), total lean mass (r = 0.29, p = 0.01), extremity lean mass (r = 0.28, p = 0.02), fibrinogen (r = 0.27, p = 0.02), and carotid IMT (r = 0.33, p = 0.004). There were no significant associations between IGF-I levels and SAT, TAT, total cholesterol, LDL, or triglyceride levels.
Figure 3.
IGF-I levels were inversely associated with hsCRP in a logarithmic fashion (panel A) and positively associated with fasting glucose (panel B), fasting insulin (panel C) and resting energy expenditure (panel D)
Association with disease duration
Visceral adipose tissue mass correlated with time since onset of acromegaly symptoms (r = 0.48, p = 0.04) among the AA group. Time elapsed since diagnosis of acromegaly was not associated with any outcome variable within the AA group. Duration of growth hormone deficiency correlated with total abdominal adipose tissue mass (r = 0.41, p = 0.03) within the GHD group.
Controlling for the presence of anterior pituitary deficiencies
The differences between the groups remained significant after controlling for the presence of adrenal insufficiency, hypogonadism, and free T4 levels, with the exception of waist to hip ratio (p = 0.07), total fat between the GHD and GHS groups (p = 0.07), subcutaneous abdominal adipose tissue (p = 0.06), and resting energy expenditure (controlled for lean body mass) between the GHD and AA groups (p = 0.05). The following variables became significantly different after controlling for adrenal insufficiency, hypogonadism and free T4 levels: total lean body mass between the GHS and AA groups (p = 0.04), extremity lean mass between the GHS and AA groups (p = 0.03), and carotid IMT between the GHS and AA groups (p = 0.02).
Secondary analysis using weight-based GHD criteria
Using weight-based GHRH-arginine stimulation test GH level cut-offs published after the study was designed (peak GH ≤ 11.5 μg/L for BMI < 25 kg/m2, peak GH ≤ 8.0 μg/L for 25 kg/m2 ≤ BMI < 30 kg/m2, and peak GH ≤ 4.2 μg/L for BMI ≥ 30 kg/m2) 31, two subjects previously classified as “GHD” were re-classified as “GHS”, and one subject previously classified as “GHS” was classified as “GHD”. After reclassification, all results remained significant with the exception of the significant difference in waist/hip ratio between the GHD and GHS groups (p = 0.06) and total fat between the GHD and GHS groups (p = 0.07). Carotid IMT was significantly lower in the GHS compared with the AA group (p = 0.04) after the reclassification.
Discussion
Both GH excess and GH deficiency are associated with increased cardiovascular morbidity and mortality. Successful reduction of elevated GH levels in acromegaly is associated with a reduction in mortality 3, but the development of subsequent GH deficiency may be a contributing factor to cardiovascular morbidity and mortality in this group of patients. In this cross-sectional study, we investigated the effects of GH deficiency on biomarkers of cardiovascular risk and body composition in men and women with GH deficiency following definitive treatment of acromegaly. Our data suggest that GH deficiency may be associated with more visceral adipose tissue, total fat, and hsCRP levels, but does not appear to be associated with other cardiovascular risk markers abnormalities.
A few studies have examined the effects of GH deficiency on body composition and cardiovascular risk markers in patients with prior acromegaly 9, 27, 29, 32–34, but none have compared these endpoints in patients who developed GHD with patients who are GH-sufficient after treatment for acromegaly. Feldt-Rasmussen et al. reported a retrospective analysis of the KIMS data and reported that mean waist circumference reduction after GH replacement was similar in patients with GH deficiency whether they had prior acromegaly or other etiologies of GH deficiency 32. Norrman et al. compared ten patients with GH deficiency and prior acromegaly with ten GH-deficient controls with non-functioning pituitary adenomas of comparable age, gender and weight, and showed no differences between the groups in body mass index or waist-hip ratio 33. However, there was a significantly higher mean low-density lipoprotein cholesterol level in the patients with prior acromegaly. We have shown that patients with GH deficiency and prior acromegaly have cardiac morphologic differences when compared to patients with GH sufficiency after definitive treatment of acromegaly 29 and experience impaired quality of life, the latter of which was recently confirmed by another group 28, 35. To our knowledge there have been no published data comparing body composition variables and cardiovascular risk markers in GHD patients with prior acromegaly compared to GH sufficient patients. In the study we describe here, we compared three groups of subjects: one group who developed GH deficiency after definitive therapy of acromegaly (GHD), a second group with GH sufficiency after definitive therapy of acromegaly (GHS), and a third group with active acromegaly (AA). Including both the GHS and AA control group allowed us to make the important comparisons between active acromegaly and definitively treated acromegaly, as well as to study the effects of development of GH deficiency after successful treatment of acromegaly.
It is known that treatment of acromegaly decreases the cardiovascular risk and metabolic effects associated with excess GH. In this cross-sectional study, we showed that development of GH deficiency after definitive therapy of acromegaly is associated with greater amounts of visceral adipose tissue and higher concentrations of hsCRP than in those who remain GH sufficient subsequent to treatment. These findings are consistent with published data on the effects of GHD in patients who have developed GHD after treatment of non-somatotroph pituitary disease, in which visceral adipose tissue mass 10 and hsCRP 14 have been shown to be increased. However, this had not previously been demonstrated in patients with acromegaly, who might or might not respond differently to GHD after a history of chronic GH excess exposure. Of note, the effects of GH on hsCRP in this study are consistent with a previous study in which the GH receptor antagonist pegvisomant was administered to subjects with acromegaly and resulted in an increase in hsCRP5. Figures 2 and 3 also make the point that the relationship of both visceral fat mass and hsCRP with GH extends to the GH excess state, i.e. that, despite the many adverse effects of active acromegaly, visceral adipose tissue mass and inflammatory markers are actually more favorable in patients with acromegaly than in GHD or even GHS. In contrast, metabolic variables reflecting glucose homeostasis, percent total body water and resting energy expenditure were comparable between GHD and GHS. This implies that the majority of the GH effect on these variables occurs with elimination of GH excess. However, we may have observed a difference between the GHD and GHS groups had we studied larger numbers because all of these variables correlated with IGF-I levels, suggesting a GH effect through the acromegaly to GHD spectrum. Both fasting glucose and fasting insulin correlated with IGF-I levels, reflecting the known effects of GH excess – long understood to result in an increased volume of distribution of glucose, hyperinsulinemia and carbohydrate intolerance, which can rapidly reverse with definitive treatment of acromegaly 36. Of interest as well, REE correlated with IGF-I level, a phenomenon that may contribute to the weight gain observed after definitive treatment for acromegaly in many patients and which may be exacerbated by the development of GHD. We did not observe a deleterious effect on lipid profiles in subjects with GHD after definitive treatment of acromegaly, though we did find that HDL levels correlated negatively with IGF-I. The lack of a stronger effect of GHD on lipids and lipoproteins is consistent with findings from some studies and not others of patients with GHD of non-somatotroph etiology, and contrasts those of a meta-analysis 21, which showed an improvement in lipids and lipoprotein profiles with GH administration. It is possible that we might have observed higher total and LDL cholesterol in patients with GHD compared with GH sufficiency should we have studied larger numbers. Interestingly, in this study, GHD was not associated with any clearly favorable effects, as might be expected if there were a linear relationship between GH levels and the endpoints studied, although carotid IMT was lower in the GHD (but not GHS) group than in the active acromegaly group. However, it is not clear whether this reflects a thinner carotid muscle layer or decreased amounts of plaque. Our data may have important clinical implications for patients who develop GH deficiency following definitive treatment of acromegaly and suggest that ideal GH targets during and after treatment of acromegaly may lie within the normal range, with a goal of avoiding the development of GHD.
Nevertheless, the published data to date have not definitively established a benefit of GH replacement therapy in patients with prior acromegaly who have developed GHD. We recently administered GH replacement or placebo to 30 patients with GH deficiency and prior acromegaly in a randomized trial and demonstrated that GH administration results in a decrease in VAT and hsCRP compared with placebo 27. This is consistent with the results in the current study in which we found that both of these variables were increased in study subjects who had developed GHD compared with those who remained GH sufficient after treatment of acromegaly. In addition, in our randomized, placebo-controlled study, GH replacement reduced total fat mass and total abdominal fat mass, and increased lean body mass when compared with placebo 27. These results suggest that GH replacement might be beneficial in such patients and that it might reverse some of body composition and cardiovascular risk marker abnormalities that may develop as a result of GH deficiency. However, open-label studies from three other groups did not demonstrate as robust results, and in one study serious cardiovascular events occurred in three study subjects who received GH 33. Van der Klaauw et al. treated 16 patients with GH deficiency and prior acromegaly with open-label GH replacement for one year and reported no improvement in body composition or cardiovascular risk markers 34. Norrman et al. reported an increase in body cell mass and high-density lipoprotein levels, with a trend towards lower total cholesterol and low-density lipoprotein cholesterol levels after two years of GH administration in a group of 10 patients with GHD and prior acromegaly. One study subject had a myocardial infarction and two study subjects experienced cerebral infarctions, in contrast to no vascular events in the non-functioning hypothalamic-pituitary disease group 33. Therefore, it is not clear whether GH replacement therapy should be routinely prescribed for patients who develop GHD after definitive treatment for acromegaly, and it should be avoided in patients with uncontrolled hypertension or serious cardiovascular disease until further safety information is available.
A limitation of our study is its cross-sectional design. A prospective study following patients with acromegaly over time, starting at the time of diagnosis through therapy and beyond would likely provide important additional data. Another limitation is the heterogeneity of the patients. Although our study population was more homogeneous than in many studies of patients with adult-onset GH deficiency in that all study subjects had a history of the same underlying pituitary disease. However, there was variability with respect to some clinical characteristics that might have important effects on our endpoints, particularly anterior pituitary deficiencies. In order to address this issue, we controlled for each anterior pituitary deficiency. It should be noted that the IGF-I SDS in the GH sufficient group was −1.3. We thought it important to use stringent cut-offs for the diagnosis of GH deficiency, but that this may have resulted in the inclusion of some study subjects in the GH-sufficient group who had partial GHD. In addition, as we used the GH-releasing hormone-arginine stimulation test for diagnosis of GH deficiency, there is a possibility that some patients who received radiation therapy were misclassified as GH sufficient. This may have resulted in an inability to detect additional differences between the GHD and GH-sufficient groups. Finally, we did not have a precise way of measuring the onset of acromegaly or GHD. The onset of acromegaly likely precedes the recognition of signs and symptoms and diagnosis in many or all patients by years. Therefore, we controlled for time since onset of symptoms of acromegaly as well as diagnosis. The onset of GHD is also difficult to determine. We used a uniform measure – time since last pituitary surgical or radiation procedure. However, we recognize that the timing of the onset of the development of GHD following these procedures is highly variable. Therefore, although the duration of disease in both acromegaly and GHD is likely a very important determinant of body composition and cardiovascular risk 37, 38, we were limited in our ability to detect these associations.
In summary, in this study, we show that the development of GH deficiency after definitive treatment of acromegaly may be associated with unfavorable body composition and cardiovascular risk marker levels in some respects, specifically accumulation of visceral adiposity and hsCRP, but not others. Further studies are warranted to explore the long-term efficacy and safety of GH replacement in this population.
Acknowledgments
This work was supported in part by an investigator-initiated research grant from Pfizer, a grant from the Guthart Family Foundation, and NIH grants MO1-RR-01066, ULI-RR-0257801, and T32-DK-007028-36.
References
- 1.Dekkers OM, Biermasz NR, Pereira AM, et al. Mortality in acromegaly: a metaanalysis. J Clin Endocrinol Metab. 2008;93:61–67. doi: 10.1210/jc.2007-1191. [DOI] [PubMed] [Google Scholar]
- 2.Clayton RN. Cardiovascular function in acromegaly. Endocr Rev. 2003;24:272–277. doi: 10.1210/er.2003-0009. [DOI] [PubMed] [Google Scholar]
- 3.Swearingen B, Barker FG, 2nd, Katznelson L, et al. Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J Clin Endocrinol Metab. 1998;83:3419–3426. doi: 10.1210/jcem.83.10.5222. [DOI] [PubMed] [Google Scholar]
- 4.Holdaway IM, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol. 2008;159:89–95. doi: 10.1530/EJE-08-0267. [DOI] [PubMed] [Google Scholar]
- 5.Sesmilo G, Fairfield WP, Katznelson L, et al. Cardiovascular risk factors in acromegaly before and after normalization of serum IGF-I levels with the GH antagonist pegvisomant. J Clin Endocrinol Metab. 2002;87:1692–1699. doi: 10.1210/jcem.87.4.8364. [DOI] [PubMed] [Google Scholar]
- 6.Cohen R, Chanson P, Bruckert E, et al. Effects of octreotide on lipid metabolism in acromegaly. Horm Metab Res. 1992;24:397–400. doi: 10.1055/s-2007-1003343. [DOI] [PubMed] [Google Scholar]
- 7.Colao A, Marzullo P, Lombardi G. Effect of a six-month treatment with lanreotide on cardiovascular risk factors and arterial intima-media thickness in patients with acromegaly. Eur J Endocrinol. 2002;146:303–309. doi: 10.1530/eje.0.1460303. [DOI] [PubMed] [Google Scholar]
- 8.Oscarsson J, Wiklund O, Jakobsson KE, et al. Serum lipoproteins in acromegaly before and 6–15 months after transsphenoidal adenomectomy. Clin Endocrinol (Oxf) 1994;41:603–608. doi: 10.1111/j.1365-2265.1994.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 9.Ronchi CL, Giavoli C, Ferrante E, et al. Prevalence of GH deficiency in cured acromegalic patients: impact of different previous treatments. Eur J Endocrinol. 2009;161:37–42. doi: 10.1530/EJE-09-0222. [DOI] [PubMed] [Google Scholar]
- 10.Weaver JU, Monson JP, Noonan K, et al. The effect of low dose recombinant human growth hormone replacement on regional fat distribution, insulin sensitivity, and cardiovascular risk factors in hypopituitary adults. J Clin Endocrinol Metab. 1995;80:153–159. doi: 10.1210/jcem.80.1.7829604. [DOI] [PubMed] [Google Scholar]
- 11.Johansson JO, Fowelin J, Landin K, et al. Growth hormone-deficient adults are insulin-resistant. Metabolism. 1995;44:1126–1129. doi: 10.1016/0026-0495(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 12.Rosen T, Eden S, Larson G, et al. Cardiovascular risk factors in adult patients with growth hormone deficiency. Acta Endocrinol (Copenh) 1993;129:195–200. doi: 10.1530/acta.0.1290195. [DOI] [PubMed] [Google Scholar]
- 13.Pfeifer M, Verhovec R, Zizek B, et al. Growth hormone (GH) treatment reverses early atherosclerotic changes in GH-deficient adults. J Clin Endocrinol Metab. 1999;84:453–457. doi: 10.1210/jcem.84.2.5456. [DOI] [PubMed] [Google Scholar]
- 14.Sesmilo G, Miller KK, Hayden D, et al. Inflammatory cardiovascular risk markers in women with hypopituitarism. J Clin Endocrinol Metab. 2001;86:5774–5781. doi: 10.1210/jcem.86.12.8087. [DOI] [PubMed] [Google Scholar]
- 15.Devin JK, Blevins LS, Jr, Verity DK, et al. Markedly impaired fibrinolytic balance contributes to cardiovascular risk in adults with growth hormone deficiency. J Clin Endocrinol Metab. 2007;92:3633–3639. doi: 10.1210/jc.2007-0609. [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen JO, Pedersen SA, Thuesen L, et al. Beneficial effects of growth hormone treatment in GH-deficient adults. Lancet. 1989;1:1221–1225. doi: 10.1016/s0140-6736(89)92328-3. [DOI] [PubMed] [Google Scholar]
- 17.Beauregard C, Utz AL, Schaub AE, et al. Growth hormone decreases visceral fat and improves cardiovascular risk markers in women with hypopituitarism: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:2063–2071. doi: 10.1210/jc.2007-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuneo RC, Judd S, Wallace JD, et al. The Australian Multicenter Trial of Growth Hormone (GH) Treatment in GH-Deficient Adults. J Clin Endocrinol Metab. 1998;83:107–116. doi: 10.1210/jcem.83.1.4482. [DOI] [PubMed] [Google Scholar]
- 19.Bengtsson BA, Eden S, Lonn L, et al. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab. 1993;76:309–317. doi: 10.1210/jcem.76.2.8432773. [DOI] [PubMed] [Google Scholar]
- 20.Baum HB, Biller BM, Finkelstein JS, et al. Effects of physiologic growth hormone therapy on bone density and body composition in patients with adult-onset growth hormone deficiency. A randomized, placebo-controlled trial. Ann Intern Med. 1996;125:883–890. doi: 10.7326/0003-4819-125-11-199612010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Maison P, Griffin S, Nicoue-Beglah M, et al. Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: a Metaanalysis of Blinded, Randomized, Placebo-Controlled Trials. J Clin Endocrinol Metab. 2004;89:2192–2199. doi: 10.1210/jc.2003-030840. [DOI] [PubMed] [Google Scholar]
- 22.Salomon F, Cuneo RC, Hesp R, et al. The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med. 1989;321:1797–1803. doi: 10.1056/NEJM198912283212605. [DOI] [PubMed] [Google Scholar]
- 23.Sesmilo G, Biller BM, Llevadot J, et al. Effects of growth hormone administration on inflammatory and other cardiovascular risk markers in men with growth hormone deficiency. A randomized, controlled clinical trial. Ann Intern Med. 2000;133:111–122. doi: 10.7326/0003-4819-133-2-200007180-00010. [DOI] [PubMed] [Google Scholar]
- 24.Bollerslev J, Ueland T, Jorgensen AP, et al. Positive effects of a physiological dose of GH on markers of atherogenesis: a placebo-controlled study in patients with adult-onset GH deficiency. Eur J Endocrinol. 2006;154:537–543. doi: 10.1530/eje.1.02125. [DOI] [PubMed] [Google Scholar]
- 25.Johansson JO, Landin K, Johannsson G, et al. Long-term treatment with growth hormone decreases plasminogen activator inhibitor-1 and tissue plasminogen activator in growth hormone-deficient adults. Thromb Haemost. 1996;76:422–428. [PubMed] [Google Scholar]
- 26.Hartman ML, Crowe BJ, Biller BM, et al. Which patients do not require a GH stimulation test for the diagnosis of adult GH deficiency? J Clin Endocrinol Metab. 2002;87:477–485. doi: 10.1210/jcem.87.2.8216. [DOI] [PubMed] [Google Scholar]
- 27.Miller KK, Wexler T, Fazeli P, et al. Growth hormone deficiency after treatment of acromegaly: a randomized, placebo-controlled study of growth hormone replacement. J Clin Endocrinol Metab. 2010;95:567–577. doi: 10.1210/jc.2009-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wexler T, Gunnell L, Omer Z, et al. Growth hormone deficiency is associated with decreased quality of life in patients with prior acromegaly. J Clin Endocrinol Metab. 2009;94:2471–2477. doi: 10.1210/jc.2008-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wexler TL, Durst R, McCarty D, et al. Growth hormone status predicts left ventricular mass in patients after cure of acromegaly. Growth Horm IGF Res. 2010;20:333–337. doi: 10.1016/j.ghir.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayter A. The Maximum Familywise Error Rate of Fisher’s Least Significant Difference Test. Journal of the American Statistical Association. 1986;81:1000–1004. [Google Scholar]
- 31.Corneli G, Di Somma C, Baldelli R, et al. The cut-off limits of the GH response to GH-releasing hormone-arginine test related to body mass index. Eur J Endocrinol. 2005;153:257–264. doi: 10.1530/eje.1.01967. [DOI] [PubMed] [Google Scholar]
- 32.Feldt-Rasmussen U, Abs R, Bengtsson BA, et al. Growth hormone deficiency and replacement in hypopituitary patients previously treated for acromegaly or Cushing’s disease. Eur J Endocrinol. 2002;146:67–74. doi: 10.1530/eje.0.1460067. [DOI] [PubMed] [Google Scholar]
- 33.Norrman LL, Johannsson G, Sunnerhagen KS, et al. Baseline characteristics and the effects of two years of growth hormone (GH) replacement therapy in adults with GH deficiency previously treated for acromegaly. J Clin Endocrinol Metab. 2008;93:2531–2538. doi: 10.1210/jc.2007-2673. [DOI] [PubMed] [Google Scholar]
- 34.van der Klaauw AA, Bax JJ, Roelfsema F, et al. Limited effects of growth hormone replacement in patients with GH deficiency during long-term cure of acromegaly. Pituitary. 2009;12:339–346. doi: 10.1007/s11102-009-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada S, Fukuhara N, Nishioka H, et al. Growth hormone deficiency in patients after cure of acromegaly by surgery alone. Eur J Endocrinol. 2011 doi: 10.1530/EJE-11-0657. [DOI] [PubMed] [Google Scholar]
- 36.Sonksen PH, Greenwood FC, Ellis JP, et al. Changes of carbohydrate tolerance in acromegaly with progress of the disease and in response to treatment. J Clin Endocrinol Metab. 1967;27:1418–1430. doi: 10.1210/jcem-27-10-1418. [DOI] [PubMed] [Google Scholar]
- 37.Hew FL, Koschmann M, Christopher M, et al. Insulin resistance in growth hormone-deficient adults: defects in glucose utilization and glycogen synthase activity. J Clin Endocrinol Metab. 1996;81:555–564. doi: 10.1210/jcem.81.2.8636267. [DOI] [PubMed] [Google Scholar]
- 38.Holdaway IM, Rajasoorya RC, Gamble GD. Factors influencing mortality in acromegaly. J Clin Endocrinol Metab. 2004;89:667–674. doi: 10.1210/jc.2003-031199. [DOI] [PubMed] [Google Scholar]