Abstract
Objectives
To develop a dual laser-assisted lamellar anterior keratoplasty (LALAK) technique, using excimer and femtosecond lasers to perform surgery on eye-bank eyes.
Methods
First we compared corneal stromal surfaces produced by (1) deep excimer ablation, (2) femtosecond lamellar cuts, and (3) manual dissection, and evaluated the effect of excimer laser smoothing with fluid masking on each surface. Masked observers graded scanning electron microscopy (SEM) images on a 5-point roughness scale.
Then we performed a 6-mm diameter excimer laser phototherapeutic keratectomy (PTK) ablation to a residual bed thickness of 200μm, followed by laser smoothing. We used the femtosecond laser to cut donors in a modified top-hat design with a thin tapered brim, which fitted into a manually dissected circumferential pocket at the base of the recipient bed. Fourier-domain optical coherence tomography (OCT) was used to measure corneal pachymetry and evaluate graft fit.
Results
Deep excimer ablation with smoothing (n=4) produced a significantly (p<0.05) smoother surface (grade=3.5) than deep excimer alone (n=4, grade=3.8) or manual dissection with (n=1, grade=3.8) and without smoothing (n=1, grade=4.8). Deep femtosecond cuts (n=2) produced macroscopic concentric ridges on the stromal surface. Experimental LALAK was performed on 4 recipients prepared by deep excimer ablation and 4 donors cut with the femtosecond laser. After suturing good peripheral graft-host match was observed on FD-OCT imaging.
Conclusion
These preliminary studies show that the LALAK technique permits improved interface smoothness and graft edge matching. Clinical trials are needed to determine whether these improvements can translate to better vision.
Introduction
Although anterior lamellar keratoplasty offers the potential of increased graft lifespan and reduced rejection risk by comparison with penetrating keratoplasty (PKP), this corneal transplantation technique has failed to gain widespread popularity among corneal surgeons. This is because current manual anterior lamellar keratoplasty dissection techniques are time-consuming and technically difficult, and carry a risk of intraoperative perforation of Descemet's membrane requiring conversion to PKP. Moreover, even in skilled hands the visual results are generally inferior to PKP, due to interface haze and opacity.1-5 New approaches to improve the practicability and outcomes of anterior lamellar keratoplasty surgery are required.
Excimer and femtosecond laser technology have provided corneal surgeons with the capability to perform precise and reliable dissection and reshaping of the cornea, but are not widely used as yet for anterior lamellar keratoplasty surgery. Femtosecond lasers have been used in penetrating keratoplasty, and can be programmed to create customized incisions which facilitate precise matching of donor and recipient wound edges, thereby enhancing wound stability and strength.7-9 However a potential drawback of femtosecond lasers in anterior lamellar keratoplasty is that deep lamellar femtosecond cuts have been reported to produce irregular dissections.10 In contrast, deep excimer ablation has been used as a means of producing smooth, even stromal beds, but the wound edge is similar to the vertical edge produced by a trephine cut,11 and a trephine-cut donor button placed in this recipient bed tends to protrude anteriorly beyond the recipient corneal rim (Figure 1). The ideal anterior lamellar keratoplasty technique (one that reliably produces a smooth, even stromal bed, combined with precise wound edge matching, and negligible risk of perforation) has yet to be developed with either the excimer or femtosecond laser alone.
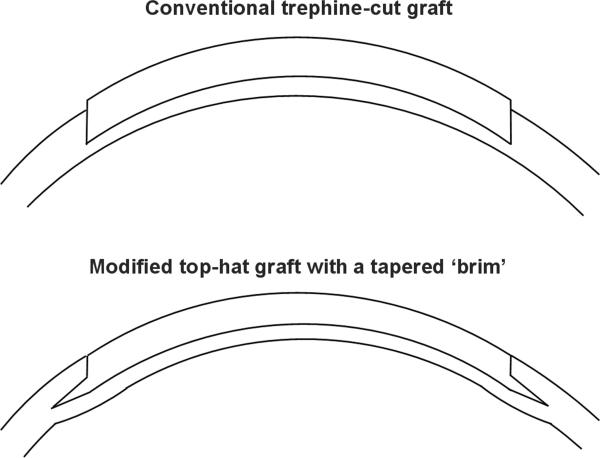
Figure 1.
Diagrams illustrating the design of the donor and recipient.
(A) A donor button prepared using a conventional trephine tends to protrude anteriorly from the host bed.
(B) The narrow tapered brim of the modified tophat donor fits into the recipient side pocket, holding the graft into the recipient bed, and providing a smooth anterior contour with even matching of the donor and recipient edges.
In this study we developed a dual laser anterior lamellar keratoplasty technique in an attempt to combine the advantages of both laser systems. The goals of this surgical technique were to achieve a smooth graft- host interface, combined with closely matched graft-host dimensions and a tongue-in-groove wound edge configuration to provide increased wound strength and a smooth transition at the anterior wound edge (Figure 1). We called this technique ‘Dual Laser-Assisted Lamellar Anterior Keratoplasty’ (LALAK).
Materials and Methods
Preparation of eye-bank eyes
Eye bank corneoscleral buttons unsuitable for human transplantation, with no prior history of refractive surgery or central corneal opacity, were used for this study. They were obtained from the San Diego Eye Bank and were preserved at 4° C in tissue storage medium (Optisol-GS, Bausch & Lomb, Irvine, CA). The corneas were mounted on Barron artificial anterior chambers (Katena Products, Inc., Denville, NJ) which were filled with Optisol-GS through a side port. Saline bottles on an IV pole were used to maintain intraocular pressure (IOP). The corneal epithelium was removed from all donors and recipients using a Merocel sponge.
Comparison of Stromal Bed Surfaces Produced by Different Dissection Techniques
(1) Manual Dissection
Femtosecond side cuts and a ring lamellar cut were performed, aiming to leave a residual stromal bed of 100μm. White and blue Melles DALK spatulas (DALK Spatula Dissector, Dutch Ophthalmic USA, Kingston, NH) were used to perform deep lamellar manual dissection starting from the deepest point on the ring lamellar cut.
(2) Deep Excimer Laser Ablation
Deep excimer ablation was performed using phototherapeutic keratectomy (PTK) settings until a residual stromal bed thickness of 200 μm was reached.
(3) Femtosecond laser deep lamellar dissection
Femtosecond side cuts (energy setting 2.4 μJ) and a full lamellar cut at 350μm depth (using energy settings of 1.0μJ and spot/line separation of 7 ×7μm) were performed.
Excimer Laser Smoothing
Four passes of excimer laser smoothing were performed on all recipient stromal surfaces prepared using each of the above techniques. The following PTK settings were used for the smoothing passes: 10μm deep at 6.5/0mm optical zone/transitional zone diameters. During smoothing, one half of the cornea was masked with a sponge to serve as a control during grading of surface roughness. Optisol-GS was used as a masking agent, and reapplied every 10μm using a Merocel sponge.
Scanning Electron Microscopy
The recipient corneas were removed from the artificial anterior chambers and immediately immersed in half-strength Karnovsky's fixative (2% paraformaldehyde, 2.5% glutaraldehyde, and 0.025% calcium chloride and 0.1 M cacodylate buffer) and fixed at 4°C overnight. The specimens were rinsed in 0.1 M cacodylate buffer, and dehydrated by immersion in a graded series of ethyl alcohol solutions. They were treated with hexamethyldisilazane and air-dried. They were then mounted on aluminum stubs using colloidal silver liquid, and sputter coated with a thin film of gold/palladium. The specimens were viewed on a Scanning Electron Microscopy (SEM) imaging system (JEOL JSM-6390LV, JEOL Technics Ltd, Japan).
Surface Roughness Grading
A subjective 5-point grading scale12 was used to compare the roughness on SEM of excimer deep ablation alone, excimer deep ablation with smoothing, and manual dissection with and without smoothing. Photographs at 100X magnification taken at each quadrant of each specimen were graded. As a control group, SEM images of the stromal bed following peeling of Descemet's membrane were included.
The SEM images were randomized and graded by 2 masked observers who were asked to rate the overall smoothness or roughness of the stromal bed. Photographs were labeled by number without disclosing the group assignment, and were presented in a random order to the masked observers. Each photograph was graded as follows: 1 = smoothest surface among all; 2 = next smoothest; 3 = intermediate group; 4 = rough, but not worst; 5 = roughest surface among all. The scores for individual specimens were averaged and the mean scores for the groups were compared using a Mann-Whitney non-parametric test and the Kruskal-Wallis test.
Dual Laser-Assisted Lamellar Anterior Keratoplasty Technique
Donor-Recipient Design
The donor design comprised a modified top-hat shape with a narrow tapered ‘brim’ intended to fit into a manually dissected circumferential pocket around the recipient bed (Figure 1). Our goal was to achieve good graft-host apposition and a smooth contour at anterior graft host junction, while simultaneously blending the recipient periphery with the tapered brim of the graft. Without the brim and side pocket the graft tends to protrude from the excimer ablated bed (Figure 1). By using this donor-recipient design we also hoped to improve the anterior surface curvature of the graft, and eliminate anterior wound gape. Recipient ablation depth and donor brim depth (ring lamellar cut) were both set to (Recipient minimum corneal thickness on OCT pachymetry - 200) μm.
Donor Preparation
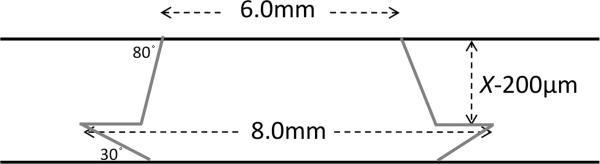
Donors were prepared using the femtosecond laser (Figure 2). The laser settings were programmed on the Intralase FS 60 kHz femtosecond laser (Abbot Medical Optics, Inc., Santa Ana, CA). Femtosecond laser alignment incisions measuring 1mm long and 20 μm wide were placed on the donor. Energy of 2.0μJ and spot separation of 5×5 μm were used for the ring lamellar cuts. Posterior side cut depth was 1100 μm and posterior side cut angle was 30°. The anterior side cut angle was 100°. The total duration of the laser ablation was 149 seconds. The cornea was applanated under the femtosecond laser system at an IOP of 65 mmHg. The donor button was separated from the rim by blunt dissection using a Sinsky hook. The endothelium was removed by staining it for one minute with trypan blue 0.06% (Visionblue, Dutch Ophthalmic USA, Kingston, NH), and peeling it off with forceps.
Figure 2.
Diagram illustrating the design of the modified tophat donor. X= recipient minimum corneal thickness by OCT pachymetry.
Recipient Preparation by Deep Excimer Ablation
Two concentric ring marks at 6 and 8mm diameter together with an 8-bite radial mark were placed on the recipient cornea. A -12-diopter soft contact lens (Flexlens, Walman Optical, Minneapolis, MN) was trephined to give a 6mm diameter opening, and was used to mask the peripheral cornea to obtain regular, vertical edges. We observed that for PTK ablation, the VisX excimer laser ablates 20% deeper than the nominal setting (data to be published separately). Therefore for every 100 μm planned ablation depth, 80μm of PTK ablation was programmed with a 6.5/0mm oz/tz (optical zone/transitional zone) diameter. To correct the central island effect which occurs with deep PTK ablation , we tested 4 different anti-central island settings of 5%, 10%, 12% and 20% of nominal ablation depth with a 6.5/0mm oz/tz. Following PTK ablation to a depth of 200 μm, a new contact lens was applied. Thereafter ablation was continued in two further 50μm steps until a residual stromal bed thickness of 200 μm was reached. Ultrasound pachymetry was used to measure stromal bed thickness after each ablation cycle. When ablation was complete, a circumferential recipient pocket at the level of the stromal bed was dissected out to the 8mm ring mark using a circular motion of a crescent blade .
Wound Closure Technique
Four cardinal 10/0 nylon sutures were placed, then the donor skirt was tucked into the recipient pocket using a Sinsky hook. The donor button was then secured with 8 interrupted and one eight-bite continuous 10/0 nylon sutures (Figure 2).
Optical Coherence Tomography
A Fourier-domain optical coherence tomography (OCT) system operating at 830 nm wavelength (RTVue with corneal adaptor module by Optovue Inc., Fremont, CA) was used to obtain high-resolution cross-sectional images. The system had a transverse scan width of 6 mm, an axial resolution of 5 μm, and a speed of 26,000 axial scans per second. After removal of the epithelium, corneal pachymetry scans were performed on all donor and recipient corneas, and the thinnest area within the central 6mm of the recipient cornea was identified and measured. High resolution line scans were taken after deep stromal ablation, before and after smoothing, and after donor-recipient suturing. OCT pachymetry was also used to identify the deepest point on the lamellar ring at which to start manual dissection.
Results
One recipient was prepared by manual dissection, and two were prepared by femtosecond dissection. Four recipient beds were prepared by deep excimer ablation, these were also used in the lamellar keratoplasty experiment. Four corneas were used as donors.
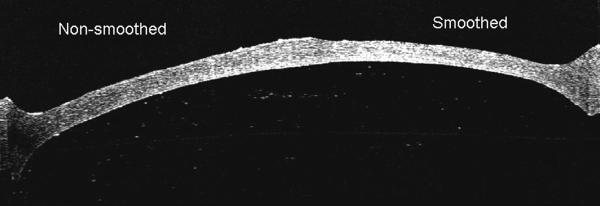
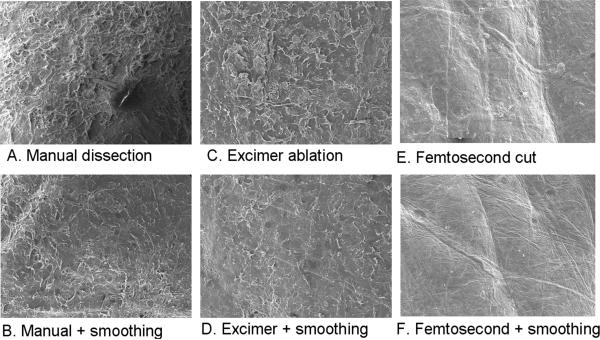
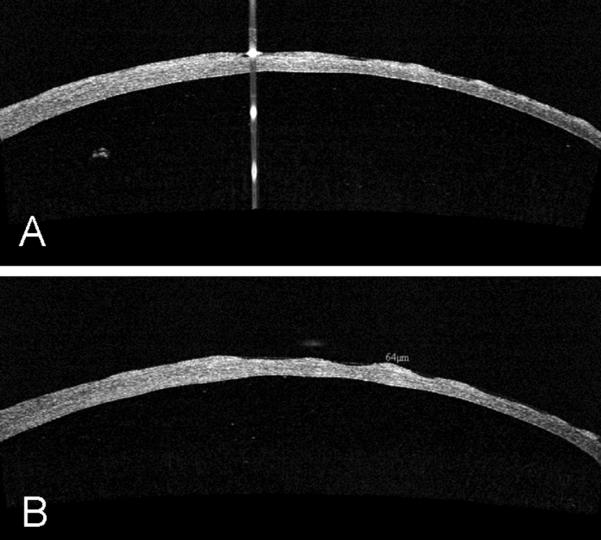
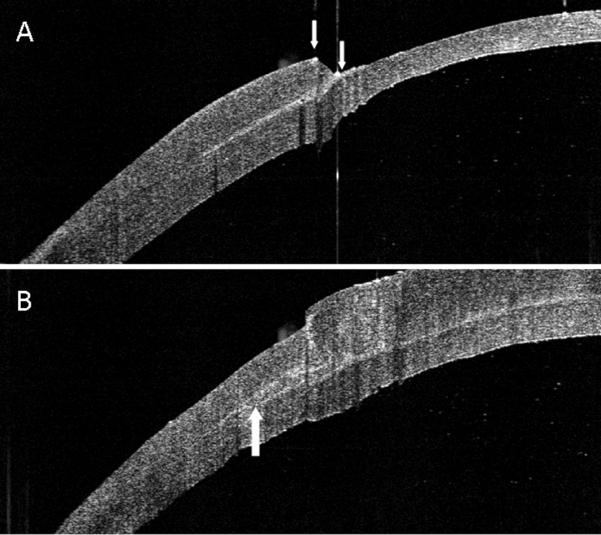
There was a statistically significant difference in the surface smoothness scores of the different stromal bed surfaces (p<0.0001). The effect of excimer smoothing was seen in the transition from smoothed to non-smoothed areas visible on both OCT and SEM images (Figures 3, 4). Deep excimer ablation with smoothing passes reliably produced a significantly (p<0.05) smoother surface (mean roughness grade=3.5) than either deep excimer alone (mean grade =3.83) or manual dissection with (mean grade =3.83) and without smoothing (mean grade=4.75). There was no difference (p=0.11) between manual dissection and deep excimer ablation (Figure 4). Although on a microscopic level femtosecond cuts were smoother than deep excimer ablation with smoothing passes, on a macroscopic level large concentric ridges were created by deep lamellar femtosecond cuts which persisted even after excimer smoothing with fluid masking (Figure 6). The femtosecond laser also cut 18% deeper than the programmed settings in the first cornea and 17% deeper in the second cornea.
Figure 3.
OCT image showing the effect of excimer stromal bed smoothing with optisol fluid masking. The transition is visible from the non-smoothed area on the left side to the smoothed area on the right.
Figure 4.
A-F. Scanning electron microscopy images at 100x magnification of the stromal beds following (A) manual dissection with smoothing, (B) manual dissection without smoothing, (C) deep excimer ablation without smoothing, (D) deep excimer ablation with smoothing, (E) deep femtosecond lamellar cut without smoothing, (F) deep femtosecond lamellar cut with smoothing.
Figure 6.
(A) OCT image showing irregular concentric ridges produced by deep femtosecond lamellar cuts. (B) The ridges are seen to persist following excimer smoothing with fluid masking.
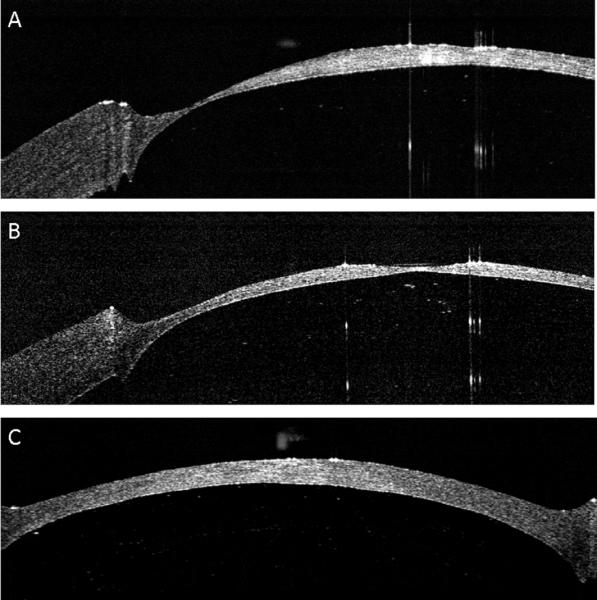
Deep excimer ablation of the 4 recipient beds was performed successfully, with no perforations occurring in any of the eyes. The contact lens was effective in masking the peripheral cornea and producing a sharp ablation edge (Figure 7A). We found an anti-central island correction of 12 % of the nominal PTK ablation to be optimal. Ten percent was too little, leaving a residual central island, and 20% was too much, creating a depression in the central cornea (Figure 8). The femtosecond laser successfully cut the custom-shaped donors which separated easily from the rim in all cases. Duration of deep excimer ablation for recipient bed preparation was 422 seconds in comparison to 180 seconds for deep femtosecond lamellar cuts with alignment incisions. The tongue-in-groove donor-recipient design produced good peripheral graft – host matching with a mild posterior bulge which was seen on OCT (Figure 7B). A small amount of vertical anterior misalignment was seen on OCT in some corneas.
Figure 7.
OCT images of the donor-recipient junction. (A). OCT image of the pocket dissected around the base of the recipient bed. Arrows indicate the vertical side wall produced by contact lens masking, and the manually dissected recipient pocket. (B) OCT image of the graft-host junction demonstrating the skirt of the donor fitting into the pocket in the recipient bed (arrow).
Figure 8.
High resolution anterior segment OCT images showing: (A) Central island formation with deep PTK ablation (B) The overcorrecting effect of a 20% anti-central island ablation causing a central dip in the stromal bed; (C) The effect of 12 % anti-central island correction, which creates an even stromal bed contour.
Discussion
Excimer phototherapeutic keratectomy and photorefractive keratectomy are well-established methods of treating corneal surface irregularity and refractive error following penetrating keratoplasty.13 However, to our knowledge, ours is the first study in which excimer laser smoothing has been used to improve the smoothness of the graft-host interface surfaces in anterior lamellar keratoplasty. Clinical studies will be necessary to determine whether this smoothing technique translates into reduced postoperative scarring and haze formation, and improved interface clarity and visual outcomes in vivo.
Microkeratome cuts have been proposed as a more reliable alternative to manual dissection for lamellar keratoplasty surgery, and can produce smooth interface surfaces. However, cutting the donor button can be technically difficult and graft-host size mismatch may occur, necessitating that backup donor tissue be always available.6
Several studies have reported on the results of femtosecond laser corneal dissection for lamellar keratoplasty surgery.7-9 Encouraging visual results have been reported with shallow femtosecond-assisted sutureless anterior lamellar keratoplasty, for the treatment of superficial stromal corneal scarring, which involves shallow cuts in the anterior stroma at 200 microns depth.14 However, two significant limitations have been described with femtosecond lamellar cuts in the deep corneal stroma. In this study we confirmed reports by others that deep femtosecond lamellar cuts may be substantially deeper than the programmed settings, increasing the risk of perforation.10 We also confirmed that deep lamellar cuts produce macroscopic irregular concentric ridges on the stromal bed surface.10, 15 Such irregular cuts may be partially due to increased scatter and attenuation of laser energy in the deeper stromal layers of the cornea, and have been reported with both flat and curved applanation lenses. We had initially planned to perform anterior lamellar keratoplasty using the femtosecond laser, however the ridges persisted even after excimer smoothing and were incompatible with our goal of a smooth graft-host interface. This led us to set aside the femtosecond approach for stromal bed preparation and instead we focused on developing a technique using deep excimer ablation. However we acknowledge that since the femtosecond lamellar cut, combined with excimer smoothing, produced the smoothest surface on SEM, it is possible that this method might produce the best optical clarity in vivo regardless of the macroscopic ridges. Until human studies are done with these two different laser techniques for recipient bed preparation, neither one can be completely be abandoned or ruled out, since at this time we don't know which will ultimately translate into better visual results. It may be that deep excimer laser ablation will produce postoperative haze and prove ultimately the inferior of the two lasers. The ultimate test of these two techniques will lie in in-vivo studies correlating to visual outcomes.
By studying deep excimer ablation in cadaver eyes we found that deep ablation followed by smoothing passes with fluid masking could reliably produce smooth, even recipient stromal beds with no perforations. The duration of the procedure, although longer than full lamellar femtosecond cuts, was shorter than manual lamellar dissection. Several other studies have also reported favourable outcomes using excimer laser for anterior lamellar keratoplasty. Two studies reported good visual outcomes with no loss of endothelial cells more than 2 years following deep excimer ablation for keratoconus, where similarly to our study, the target residual stromal bed thickness was 200 microns.11, 17 Concerns exist among cornea specialists that deep excimer ablation of large amounts of corneal tissue, which involves high laser energy levels for several minutes, may result in endothelial damage. However, excimer treatments in humans as deep as 100 microns over Descemet's membrane have been performed with no observed change in endothelial cell count or morphology,18 and in another study of 3 patients who had deep excimer keratoplasty at depths ranging from 320 to 415 microns for the treatment of circumscribed corneal opacities, no interface opacities occurred, and clinical results superior to conventional dissection methods were reported.19 Another potential limitation of our LALAK technique is haze formation, a well known occurrence with deep excimer laser ablations. We hope that by performing smoothing passes after deep excimer ablation, we can minimize this problem. However to more definitively answer this question, a further set of in vivo experiments in live animal corneas could be performed, using confocal microscopy to evaluate haze formation.
The femtosecond laser provides many options for customizing the donor configuration in anterior lamellar keratoplasty. In this study we chose a tongue-in-groove design of donor and recipient, where the narrow tapered ‘brim’ of the modified top-hat donor tucked into a manually dissected recipient pocket. We hypothesized that by using this design, we could achieve a smooth anterior corneal contour and avoid anterior gaping, while the donor ‘brim’ blended with the recipient rim and created a mild circumferential bulge on the peripheral posterior cornea away from the visual axis, which is unlikely to adversely affect corneal optics. An important difference between clinical intralase enabled keratoplasty (IEK), where good donor to host alignment in the classic zig-zag shape can be seen as early as the first postoperative day, and our experimental lamellar keratoplasty technique, is that IEK is full thickness. Hence any swelling of the donor following IEK manifests as mismatch of the posterior corneal boundary because the anterior boundary is held flush by the suture. In lamellar keratoplasty the posterior boundary is held in place by the host bed so the anterior boundary will be flush only after edema is resolved.
Using our technique, OCT imaging of the peripheral graft-host junction shows a small but definite anterior vertical misalignment of the donor button (Figure 7B), due to marked swelling of the donor graft tissue which measured 725μm in thickness on OCT. This significant swelling which would not happen in a live postoperative eye with working endothelium, occurred because the experiments were done using non-transplant grade corneas which were processed as low priority, and the tissue was frequently old with poor endothelial function. The tissue of the recipient model was somewhat deturgesced by the tension from the pressure inside the artificial anterior chamber, but there was no functioning endothelial pump to deturgesce the graft as there would be in a normal living eye. For this reason, the smooth anterior fit can only be demonstrated in actual live surgery.
We acknowledge the limitations of this preliminary laboratory study. Due to the scarcity and expense of eye bank eyes, and because the main purpose of our study was to assess the feasibility of LALAK, we performed only one manual dissection and 2 femtosecond lamellar dissections as smoothness controls. We used subjective grading by masked observers to assess surface roughness rather than image processing software which in our experience can be influenced by image brightness and artifact induced during tissue processing.
Some limitations of the dual LALAK technique include the expense and availability of excimer and femtosecond lasers, and the logistics of the surgery, since these lasers are not usually located in an operating room setting. For this procedure, it may be necessary to have the excimer laser in the operating room, however an on-site femtosecond laser is not a requirement, as donor buttons can be ordered pre-cut from the eye bank. Another limiting factor is the small recipient bed diameter of 6mm which is intrinsic to the VisX broad beam laser, and limits the applicability of this technique to adult patients. For adults, a larger ablation zone diameter of 8mm is necessary to gain a good optical result, which is possible with some flying spot lasers. A 6mm diameter ablation zone may be more useful for pediatric corneal transplantation, where sutures are removed earlier. In cases where the corneal thickness is extremely irregular, such as focal thinning due to ulceration, the LALAK technique may not be suitable. We acknowledge that OCT images in a small number of cadaver eyes do not necessarily translate into good optical outcomes in humans, and further studies are needed.
Some potential advantages of this dual laser technique over manual techniques might include a more reliable dissection, stronger wound healing, and better visual outcomes due to improved interface quality. Obtaining donor material could be easier because the endothelial quality of the donor cornea is not as crucial when the perforation risk is low. This technique might also potentially be useful for children with stromal corneal scarring because the surgery is extraocular, and hazards of penetrating keratoplasty in children including positive vitreous pressure, endophthalmitis, peripheral anterior synechiae and secondary glaucoma could be avoided.
Conclusion
This study demonstrates that the precision of both excimer and femtosecond lasers can be combined to perform anterior lamellar keratoplasty in a controlled, precise fashion, reducing the risk of perforation, and reliably improving stromal bed smoothness. Further studies are necessary to determine the role of the dual LALAK method as a potential alternative to current anterior lamellar keratoplasty techniques.
Figure 5.
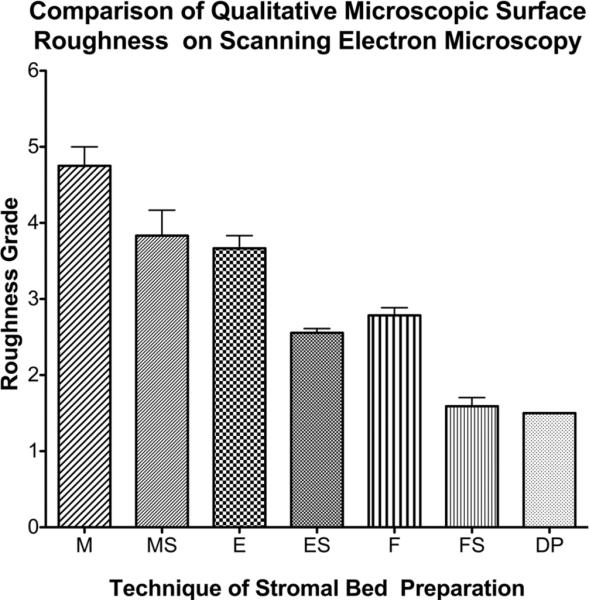
Graph comparing the stromal bed smoothness grade of the scanning electron microscopy images with different preparation techniques. The mean smoothness grade of the stromal surfaces was significantly different (p<0.0001). Deep excimer ablation with smoothing was significantly smoother than deep excimer alone or manual dissection with smoothing (p<0.05). DP=Stromal bed following peeling of Descemet's membrane; ES= Excimer ablation with smoothing; E= Excimer ablation alone; F= Femtosecond lamellar cut; FS = femtosecond cut with excimer smoothing; MS=Manual dissection with smoothing; M= Manual dissection alone.
Acknowledgments
□ Proprietary Interests: Maolong Tang and David Huang received grant support from Optovue Inc.; David Huang received travel support, patent royalty, and stock options from Optovue, Inc.; David Huang receives royalty from a patent on optical coherence tomography licensed to Carl Zeiss Meditec, Inc.
Financial Support: This study was supported by NIH grants RO1 EY017723 and RO1 EY018184; a grant from Optovue Inc., and endowment funding from the Charles C. Manger III, MD Chair in Corneal Laser Surgery, and the Skilling Foundation.
Dr. Catherine Cleary is supported by a grant from the Irish College of Ophthalmologists.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anwar M, Teichmann KD. Big-bubble technique to bare Descemet's membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002 Mar;28(3):398–403. doi: 10.1016/s0886-3350(01)01181-6. [DOI] [PubMed] [Google Scholar]
- 2.Melles GR, Lander F, Rietveld FJ, Remeijer L, Beekhuis WH, Binder PS. A new surgical technique for deep stromal, anterior lamellar keratoplasty. Br J Ophthalmol. 1999 Mar;83(3):327–333. doi: 10.1136/bjo.83.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melles GR, Remeijer L, Geerards AJ, Beekhuis WH. A quick surgical technique for deep, anterior lamellar keratoplasty using visco-dissection. Cornea. 2000 Jul;19(4):427–432. doi: 10.1097/00003226-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Fontana L, Parente G, Tassinari G. Clinical outcomes after deep anterior lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2007 Jan;143(1):117–124. doi: 10.1016/j.ajo.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Watson SL, Ramsay A, Dart JK, Bunce C, Craig E. Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. Ophthalmology. 2004 Sep;111(9):1676–1682. doi: 10.1016/j.ophtha.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Busin M, Zambianchi L, Arffa RC. Microkeratome-assisted lamellar keratoplasty for the surgical treatment of keratoconus. Ophthalmology. 2005 Jun;112(6):987–997. doi: 10.1016/j.ophtha.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Farid M, Steinert RF. Deep anterior lamellar keratoplasty performed with the femtosecond laser zigzag incision for the treatment of stromal corneal pathology and ectatic disease. J Cataract Refract Surg. 2009 May;35(5):809–813. doi: 10.1016/j.jcrs.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Chan CC, Ritenour RJ, Kumar NL, Sansanayudh W, Rootman DS. Femtosecond laser-assisted mushroom configuration deep anterior lamellar keratoplasty. Cornea. Mar;29(3):290–295. doi: 10.1097/ICO.0b013e3181b77873. [DOI] [PubMed] [Google Scholar]
- 9.Price FW, Jr., Price MO, Grandin JC, Kwon R. Deep anterior lamellar keratoplasty with femtosecond-laser zigzag incisions. J Cataract Refract Surg. 2009 May;35(5):804–808. doi: 10.1016/j.jcrs.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Soong HK, Mian S, Abbasi O, Juhasz T. Femtosecond laser-assisted posterior lamellar keratoplasty: initial studies of surgical technique in eye bank eyes. Ophthalmology. 2005 Jan;112(1):44–49. doi: 10.1016/j.ophtha.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 11.Spadea L, Giammaria D, Fiasca A, Verrecchia V. Excimer laser-assisted lamellar keratoplasty for the surgical treatment of keratoconus. J Cataract Refract Surg. 2009 Jan;35(1):105–112. doi: 10.1016/j.jcrs.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Sarayba MA, Ignacio TS, Binder PS, Tran DB. Comparative study of stromal bed quality by using mechanical, IntraLase femtosecond laser 15- and 30-kHz microkeratomes. Cornea. 2007 May;26(4):446–451. doi: 10.1097/ICO.0b013e318033e7cc. [DOI] [PubMed] [Google Scholar]
- 13.Kornmehl EW, Steinert RF, Puliafito CA. A comparative study of masking fluids for excimer laser phototherapeutic keratectomy. Arch Ophthalmol. 1991 Jun;109(6):860–863. doi: 10.1001/archopht.1991.01080060124039. [DOI] [PubMed] [Google Scholar]
- 14.Yoo SH, Kymionis GD, Koreishi A, et al. Femtosecond laser-assisted sutureless anterior lamellar keratoplasty. Ophthalmology. 2008 Aug;115(8):1303–1307. e1301. doi: 10.1016/j.ophtha.2007.10.037. 1307. [DOI] [PubMed] [Google Scholar]
- 15.Mian SI, Soong HK, Patel SV, Ignacio T, Juhasz T. In vivo femtosecond laser-assisted posterior lamellar keratoplasty in rabbits. Cornea. 2006 Dec;25(10):1205–1209. doi: 10.1097/01.ico.0000231491.95377.0b. [DOI] [PubMed] [Google Scholar]
- 16.Mehta JS, Parthasarthy A, Por YM, Cajucom-Uy H, Beuerman RW, Tan D. Femtosecond laser-assisted endothelial keratoplasty: a laboratory model. Cornea. 2008 Jul;27(6):706–712. doi: 10.1097/QAI.0b013e31815ee267. [DOI] [PubMed] [Google Scholar]
- 17.Bilgihan K, Ozdek SC, Sari A, Hasanreisoglu B. Excimer laser-assisted anterior lamellar keratoplasty for keratoconus, corneal problems after laser in situ keratomileusis, and corneal stromal opacities. J Cataract Refract Surg. 2006 Aug;32(8):1264–1269. doi: 10.1016/j.jcrs.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 18.Krumeich JH, Schoner P, Lubatschowski H, Gerten G, Kermani O. [Excimer laser treatment in deep lamellar keratoplasty 100 micrometer over Descemet's membrane]. Ophthalmologe. 2002 Dec;99(12):946–948. doi: 10.1007/s00347-002-0670-5. [DOI] [PubMed] [Google Scholar]
- 19.Eckhardt HB, Hutz WW, Heinrich AW, Kaiser WE. [Lamellar keratoplasty with the excimer laser. Initial clinical results]. Ophthalmologe. 1996 Jun;93(3):242–246. [PubMed] [Google Scholar]