Abstract
Food intake is mediated, in part, through brain pathways for motivation and reinforcement. Dysregulation of these pathways may underlay some of the behaviors exhibited by patients with eating disorders. Research using animal models of eating disorders has greatly contributed to the detailed study of potential brain mechanisms that many underlie the causes or consequences of aberrant eating behaviors. This review focuses on neurochemical evidence of reward-related brain dysfunctions obtained through animal models of binge eating, bulimia nervosa, or anorexia nervosa. The findings suggest that alterations in dopamine (DA), acetylcholine (ACh) and opioid systems in reward-related brain areas occur in response to binge eating of palatable foods. Moreover, animal models of bulimia nervosa suggest that while bingeing on palatable food releases DA, purging attenuates the release of ACh that might otherwise signal satiety. Animal models of anorexia nervosa suggest that restricted access to food enhances the reinforcing effects of DA when the animal does eat. The activity-based anorexia model suggests alterations in mesolimbic DA and serotonin occur as a result of starvation coupled with excessive wheel running. These findings with animal models complement data obtained through neuroimaging and pharmacotherapy studies of clinical populations. Finally, information on the neurochemical consequences of the behaviors associated with these eating disorders will be useful in understanding these complex disorders and may inform future therapeutic approaches, as discussed here.
Keywords: acetylcholine, binge eating, clinical studies, dopamine, eating disorders, opioids, rat, serotonin
1. Introduction
The treatment of eating disorders, such as binge eating disorder, bulimia nervosa or anorexia nervosa, poses a unique challenge for clinicians. These disorders are complex, of unknown etiology, and particularly in the case of anorexia nervosa and bulimia nervosa, sometimes theorized as being caused or perpetuated by cultural pressures on women to be thin (Strober, 1995). Indeed, while culture and society may be an influence, it is now widely recognized that there exists a biological basis to these disorders (Bulik, 2004). Understanding the biology of aberrant eating is an important step in the development of appropriate pharmacotherapies. Several neurotransmitters systems have been proposed to be perturbed in these disorders (Avena et al., 2009a; Fava et al., 1989; Kaye, 2008; Kaye et al., 2009; Nathan and Bullmore, 2009), with much attention given to the role of monoamine systems, particularly dopamine (DA) and serotonin (5-HT). This review describes findings focused on these and other systems obtained with animal models of eating disorders. While different in their overt phenotype, these disorders do share some characteristics and are believed to have commonalities of dysfunction in brain areas associated with reward.
There are data emerging on a variety of neurotransmitters and hormones that may be involved in eating disorders (Barbarich et al., 2003; Casper et al., 2008; Gaetani et al., 2008; Kaye et al., 2009). This review will focus on a select few that are particularly relevant to preclinical laboratory animal models and specificity to areas of the brain that process reward and suggest pharmacological interventions based on the present laboratory animal research.
2. Feeding Behavior and Reward
Eating is regulated, in part, by areas of the brain that reinforce behavior. The neurocircuitry underlying food intake and reward is complex, and a thorough review of this topic is beyond the scope of this paper. However, detailed reviews of the multiple brain systems underlying food and reward, and how they interact, can be found elsewhere (Kelley et al., 2005; Wise, 2006). In brief, taste-sensory input from the mouth is relayed to cortical areas that must receive satiety signals from the gut via subcortical mechanisms (Rolls, 2006, 2007). Limbic areas play a crucial role in this process; notably the thalamus relays information from energy control systems in the hypothalamus (Kelley et al., 2005). The hypothalamus is also a place where many feeding-related neuropeptides exert their effects on macronutrient selection (Leibowitz and Wortley, 2004), under the control of hormones that signal nutrient stores in the periphery (Leibowitz and Hoebel, 2004; Strader and Woods, 2005).
The hypothalamus has projections directly to the nucleus accumbens (NAc) (Yoshida et al., 2006). The NAc is of interest because it is indicated in the reward of natural behaviors, such as exercise, sex and feeding. Figure 1 illustrates some of the specific neural pathways involved in the eating disorders discussed in this chapter. Specifically, the NAc is a terminal field of the mesolimbic dopaminergic system involved in hedonic and motivational aspects of feeding (Wise, 2006), as well as a location in which endogenous opioids act to both modulate DA release (Kalivas et al., 1993) and affect hedonic processes associated with food evaluation, consumption and orosensory reward processes (Bakshi and Kelley, 1993; Baldo and Kelley, 2007; Bodnar et al., 2005; Evans and Vaccarino, 1990; Pecina and Berridge, 2005). There is also a role for 5-HT in the hypothalamus and other limbic areas in feeding behavior (Halford and Blundell, 2000b; Leibowitz et al., 1988). Specifically, 5-HT has been linked to satiety. Serotonergic drugs are appetite suppressants and can induce weight loss in both laboratory animals and humans (Blundell, 1977; Halford and Blundell, 2000a), and these effects have been strongly linked to the consumption of fat (Blundell et al., 1995). Likewise, ACh from interneurons in the NAc has been associated with ingestive behavior (Hoebel et al., 2007; Pratt and Blackstone, 2009; Pratt and Kelley, 2005), and it is believed to cause satiety based on findings that show extracellular increases in ACh levels to be associated with the cessation of feeding (Mark et al., 1991; Mark et al., 1992; Mark et al., 1995; Rada and Hoebel, 2000). The effects of activation of specific cholinergic receptors on feeding behavior are still being explored (Perry et al., 2009; Pratt and Blackstone, 2009). Based on this behavioral neuroscience, it is not surprising that a dysregulation in these feeding-reward-related neurotransmitters and brain areas is seen in studies of eating disorders.
Figure 1.
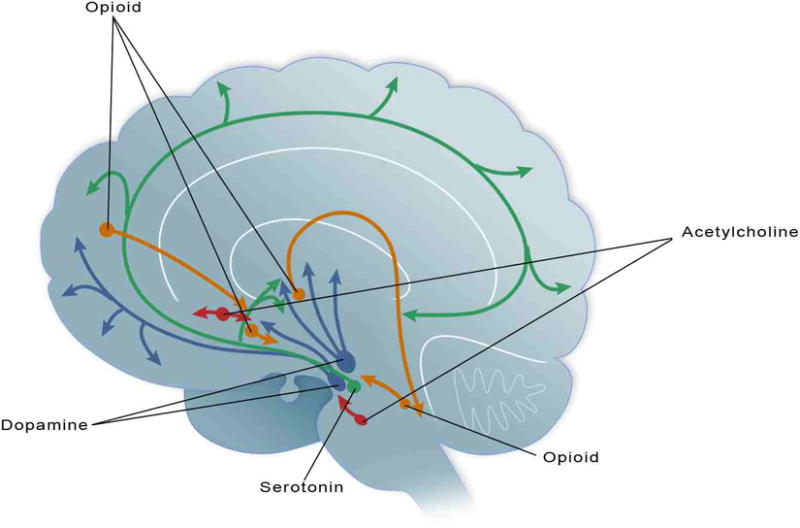
Dopamine (blue), serotonin (green), acetylcholine (red) and the opioids (orange) have each been indicated in disordered eating. This schematic illustrates some of the neuronal projections that research indicates are of particular interest in the regulation and deregulation of food intake as revealed through preclinical and clinical studies of eating disorders.
3. Utility of Animal Models in Eating Disorders Research
While today it is believed that there is a biological basis for eating disorders (Bulik, 2004), it is acknowledged that social, cultural and psychological factors can influence their development and progression, perhaps more so than in any other psychiatric disorder. These contributing factors pose a problem for clinicians developing treatment approaches. Moreover, researchers are often limited by the fact laboratory animal models cannot fully recapitulate the complexity of the human experience. Nonetheless, animal models of eating disorders are important to understanding the biochemical basis of the behaviors associated with eating disorders, as they allow insight into specific neurotransmitter systems that are involved with the behaviors associated with the disorders, with social and cultural variables removed. Thus, animal models allow for an isomorphic assessment of eating disorders in that they can recreate conditions associated with these disorders (Smith, 1989).
4.1 Binge Eating
Binge eating is most often thought of as a maladaptive behavior associated with bulimia nervosa. However, binge eating is also noted in patients with obesity, and itself is a disorder (i.e., binge eating disorder) (American Psychiatric Association, 2000; Stunkard, 1959). Recent statistics suggest that binge eating is more common than other eating disorders, affecting approximately 6% of the non-clinical population, as well as the 2% of the population that suffers from bulimia nervosa (Hudson et al., 2007). Moreover, binge eating has been linked to obesity (Hudson et al., 2007; Stunkard, 1959), which presently affects 33% of the population in the USA (Ogden et al., 2007). Binge eating is characterized by consuming an unusually large amount of food in a discrete period of time (American Psychiatric Association, 2000). Foods that are consumed during a binge episode are typically high in calories, fat, and/or sugar, and are normally considered as foods meant to be consumed in moderation (Guertin and Conger, 1999; Hadigan et al., 1989; Kales, 1990).
4.2 Animal Models of Binge Eating
Several paradigms have been developed for the study of binge-eating behavior (see (Corwin and Buda-Levin, 2004) for review, and (Avena et al., 2006a; Boggiano and Chandler, 2006; Corwin and Wojnicki, 2006; for specific details on some of these paradigms). Some models encourage voluntary binge eating in sated animals by offering limited access (e.g., 2-h/day) to a palatable food, such as one high in fat or sugar (Berner et al., 2008; Dimitriou et al., 2000), in addition to ad libitum access to standard rodent chow, for several weeks. Other models incorporate cyclic periods of food deprivation and feeding in order to stimulate animals to binge eat when a palatable food is presented (Avena et al., 2008b). Stressors, such as footshock, can also precipitate binge eating in rats (Boggiano et al., 2005).
Animal models of binge eating appear to have clinical validity. Binge eating in humans can occur in sated and hungry individuals (Marcus and Kalarchian, 2003). Periods of food restriction and bingeing have been shown in laboratory animal models to impact food consumption long after restriction has ceased (Hagan and Moss, 1997), a finding that models reports in humans (Polivy, 1996). There is also a connection between stress and binge-type eating in humans (Cattanach et al., 1988) similar to that observed in animal models. Anxiety is comorbid with binge eating in humans, and signs of anxiety-like behavior have been observed in animal models of binge eating using somatic indications and behavioral tests such as the elevated plus-maze (Avena et al., 2008a; Chandler-Laney et al., 2007; Colantuoni et al., 2002; Galic and Persinger, 2002; Wideman et al., 2005).
4.3 Animal Models of Binge Eating: DA
Data generated from laboratory animal models of binge eating have yielded important insights pertaining to physiological and neurochemical alterations that may be the cause, or consequence, of binge eating. Daily binge eating of a palatable sucrose solution has been shown to affect multiple neurotransmitter systems in reward-related brain areas. In a model developed in Bart Hoebel's laboratory, rats are food deprived for 12 h and then offered 12-h access to a 10% sucrose solution, along with standard rodent chow, for approximately 1 month (Avena et al., 2006a). This feeding schedule precipitates binge-eating behavior. Binge eating a 10% sucrose solution causes the repeated release of DA in the NAc (Avena et al., 2008b), whereas in the case of normal food intake DA release attenuates with repeated access to a palatable food (Bassareo and Di Chiara, 1997). Other laboratories have shown similar, aberrant DA functioning when binge eating high-fat diets (Liang et al., 2006). Further, the effect of binge eating on DA release in the NAc is enhanced when rats are binge-eating sucrose while at a reduced body weight (Avena et al., 2008c) (Figure 2). Further dysregulation of the mesolimbic DA system is seen when rats that are bingeing on sucrose show an increase in D1-receptor binding in the NAc and a decrease in D2-receptor binding in the dorsal striatum (Colantuoni et al., 2001). Similar findings with D2-receptor binding, as well as an upregulation in DA transporter, have been reported by other researchers using a similar paradigm of restricted access to sucrose (Bello et al., 2002; Bello et al., 2003). Further, bingeing on sucrose results in changes in dopaminergic gene expression, with a decrease in D2-receptor mRNA in the NAc and an increase in D3 receptor mRNA in the NAc and caudate-putamen compared to chow-fed controls (Spangler et al., 2004). These brain changes are also summarized in Table 1. It is of interest to note that many of these changes in DA seen in response to binge eating sucrose are similar to the changes observed with drug dependency and obesity (Johnson and Kenny; Koob and Volkow; Wang et al., 2004; Wise, 2006), and it has been suggested that binge eating of palatable foods may result in addictive-like behavior and concomitant neurochemical changes (see Avena et al., 2008b for review).
Figure 2.
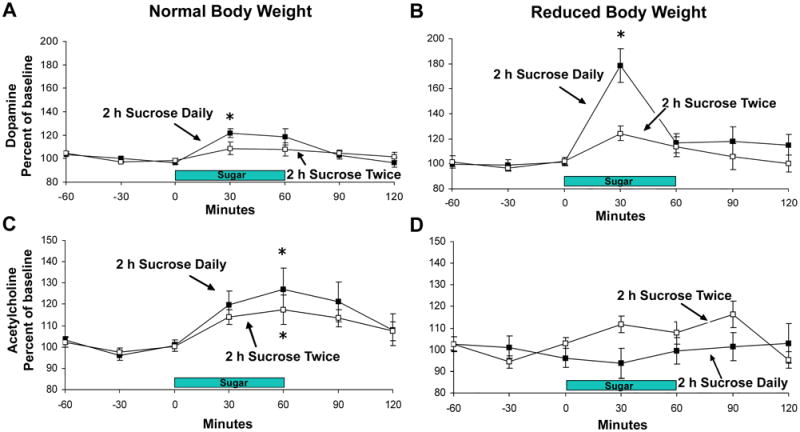
Accumbens DA and ACh release when rats binge on sucrose at a normal body weight and then again at a reduced body weight (85% body weight). The control group had access to sucrose twice (day 1 and 21), and was similarly reduced in body weight. (A) DA is released in response to drinking 10% sucrose on day 21 of access at a normal body weight, and (B) this release is enhanced (to 179% of baseline) when animals binge on sucrose at a reduced body weight. Rats with access to sucrose only two times do not show this effect. (C) ACh rises as the sucrose meal progresses for both groups when at normal body weight. (D) This effect on ACh release is blunted for the sucrose bingeing group when at a reduced body weight. * P<0.05 from baseline. Figure reprinted with permission from (Avena et al., 2008c).
Table 1.
| Selected Findings in Reward-Related Brain Areas Using Animal Models | ||||
|---|---|---|---|---|
| Disorder | Dopamine | Opioids | Serotonin | Acetylcholine |
| Binge eating |
|
|
Delayed rise in release | |
| Bulimia nervosa | ↑ release while purging | ↓ release while purging | ||
| Anorexia nervosa | Antagonists ↓ release |
|
↓release | |
| Selected Findings in Reward-Related Brain Areas in Clinical Populations | ||||
| Disorder | Dopamine | Opioids | Serotonin | Acetylcholine |
| Binge eating |
|
Mu-antagonist ↓ food palatability | ↓ transporter binding | |
| Bulimia nervosa |
|
|
||
| Anorexia nervosa |
|
gene polymorphisms of the delta-1 receptor |
|
|
Pharmacological studies using limited-access precipitation of binge eating sucrose or fat have shown that the D2 antagonist raclopride can reduce intake of binge fat, while having no effect on ad libitum intake of the same, but attenuates sucrose intake regardless of availability schedule (Corwin and Wojnicki, 2009). Further, raclopride differentially effects binge intake of sugar-fat mixtures, with the largest effect seen when a moderate amount of sucrose (10%) is added to vegetable shortening (Wong et al., 2009). These studies are important in that they suggest potential pharmaceutical DA targeting treatments, while also indicating that there may be differences in the effects of bingeing with respect to different macronutrients (Avena et al., 2009b). Further, this provides evidence that the efficacy of pharmaceutical interventions targeting the DA system may depend on the individualized sugar-to-fat ratio in any given binge episode.
4.4 Animal Models of Binge Eating: Opioids
A role for opioids in binge eating has been suggested. Rats bingeing on sucrose or a sweet-fat, liquid diet show a decrease in enkephalin expression in the NAc (Kelley et al., 2003; Spangler et al., 2004). Moreover, rats with binge access to sucrose show an increase in mu-opioid receptor binding in multiple brain regions, including the NAc (Colantuoni et al., 2001). Pharmacological studies suggest that the mu-antagonist naltrexone can suppress binge eating of sucrose and fat (Corwin and Wojnicki, 2009). In a subset of obese animals that binge eat, chronic opioid antagonism via intracerebroventricular administration of the kappa antagonist nor-binaltorphimine dynorphin has been shown to decrease body weight and food intake (Jarosz and Metzger, 2002). The paraventricular nucleus of the hypothalamus has been identified as one brain region in which a mu-agonist (DAMGO) can stimulate fat intake in rats that are binge eating, but this effect seems to be dependent on the animal's individual dietary preference (Naleid et al., 2007).
Targeting the opioid system might also be effective in treating binge eating, in that local injections of the opioid antagonist, naltrexone, in the reward related brain regions has been shown to decrease intake of preferred fat and sucrose diets (Naleid et al., 2007). Further, the opioid antagonist, naloxone, has been shown to block stress induced eating in rats with a feeding-restriction-refeeding history (Boggiano et al., 2005). Additional studies show that naloxone suppresses palatable food intake in animals eating oil emulsions, sweet solutions, solid fats, and sweet fat mixtures regardless of intake pattern, but with a larger effect in animals engaging in binge consumption (Corwin and Wojnicki, 2009; Wong et al., 2009). Nalmefene, a mu- and kappa-opioid antagonist has been shown to attenuate binge eating of a sweet-chocolate diet in rats (Cottone et al., 2008).
4.5 Animal models of binge eating: ACh
Animal models have also unveiled a role for NAc ACh (Rada et al., 2005) in binge-eating behavior. Normally, ACh release in the NAc increases as a meal progresses and peaks at the end when the animal stops eating (Mark et al., 1992). With regard to binge eating, when rats binge on sucrose there is a delay in the peak release of ACh in the NAc, as revealed by in vivo microdialysis (Rada et al., 2005). This may be related to a delay in satiety that could occur as a result of binge eating. Support for this theory comes from data showing that when rats binge eat sugar while at a reduced body weight, or when they sham feed the sucrose, accumbens ACh release is blunted (Avena et al., 2008c; Avena et al., 2006b).
4.6 Clinical Correlates of Animal Models of Binge Eating
Clinical findings have supported and enhanced the information derived from animal models of binge eating (Table 1). Brain imaging studies suggest a ventral limbic circuit comprised of the amygdalae, insula, ventral striatum and ventral regions of the anterior cingulated cortex (ACC) and orbitofrontal cortex are important in both high-level regulation of feeding behavior (Beaver et al., 2006; Holsen et al., 2005; Killgore et al., 2003; LaBar et al., 2001; Morris and Dolan, 2001) and in identifying the emotional significance of stimuli and affective response (Phillips et al., 2003). Atrophy or dysregulation in this region has been noted in some patients who binge-eat (Marsh et al., 2009; Woolley et al., 2007). Moreover, patients diagnosed with binge eating disorder show stronger orbitofrontal cortex activation while viewing pictures of food compared with normal weight controls (Schienle et al., 2009). In a recent PET study of fasted obese binge and nonbinge eaters, food stimuli specifically increased DA in the caudate and putamen in the binge eaters, indicating a role for DA in the striatum independent of obesity (Wang et al., 2011). A role for DA in binge-eating behavior has been suggested by findings of short allele 3′-UTR VNTR polymorphisms of the DA transporter gene, which also suggests that dysregulation of DA reuptake may act as a common pathophysiological mechanism in eating disorders with binge-eating behavior and substance abuse (Shinohara et al., 2004). With regard to the opioids system, there is some evidence that the mu-antagonists can reduce the subjective pleasantness of palatable food in patients who binge eat (Drewnowski et al., 1995). Single-photon emission tomography (SPECT) coupled with the radioligand 123I-labelled nor-beta-CIT, which specifically labels 5-HT transporters, showed decreased 5HT transporter binding in obese binge eaters compared to obese controls, providing another potential pharmaceutical target (Kuikka et al., 2001).
5.1 Bulimia Nervosa
Bulimia nervosa is characterized by binge eating in conjunction with inappropriate means of compensating for the excess calories consumed, and it is not always associated with weight loss (American Psychiatric Association, 2000). These patients have an unremitting drive to restrain their food intake, a fear of gaining body weight, and often a distorted perception of their body image. Bulimia nervosa is divided into two subtypes: the purging-type, which is characterized by purging behavior, usually via emesis, and the lesser-common non-purging type, which is characterized by fasting or excessive exercise as a means by which to compensate for the calories obtained from the binge. There is a noted comorbidity between bulimia nervosa and other disorders, including substance abuse, affective disorders, and attention disorders (Altman and Shankman, 2009; Hatsukami et al., 1984; Sandager et al., 2008).
5.2 Animal Models of Bulimia Nervosa
Modeling bulimia nervosa in the laboratory animal is a challenge. In part, this is due to the extreme nature of the behaviors associated with this disorder. While no “gold standard” exists, several paradigms have been used to study aspects of bulimia nervosa. The paradigms described above for binge eating can all be applied to the study of the binge-eating component of bulimia nervosa. For instance, cyclic bingeing coupled with food deprivation may serve as a useful tool in studying the type of bulimia nervosa in which fasting is involved. Models that have imposed periods of food restriction followed by periods of refeeding (Hagan and Moss, 1991, 1997) demonstrate long term effects on feeding behavior when rats are subjected to restriction–refeeding cycles for several months. When they return to a normal body weight and feeding regimen, rats with a history of restriction and refeeding continue to exhibit binge-eating behavior, even when sated. Food restriction has also been linked with decreased levels of 5-HT driven by a low rate of synthesis (Haleem and Haider, 1996) as well as down-regulates the density of 5-HT transporters (Huether et al., 1997), leading to the clinical use of fluoxetine (Prozac), a selective serotonin reuptake inhibitor (SSRI), for the treatment of bulimia (Wong et al., 2005). The restriction–refeeding model also highlights the role that stress can play in perpetuating a binge in humans (Lattimore, 2001); when laboratory animals are stressed with a footshock (Hagan et al., 2002) or confinement (Inoue et al., 2006), they become hyperphagic. When given voluntary access to palatable food paired with increasing intensity of foot shock, rats designated as binge-eating prone, consumed significantly more and tolerated higher levels of footshock for palatable food than those classified as binge-eating resistant (Oswald et al., 2011). This model may be of particular relevance to the nonpurging subtype of bulimia nervosa and may underscore ways in which negative emotions can trigger abnormal eating responses.
While many models have been proposed to assess the bingeing behavior associated with bulimia nervosa, it has been more difficult to assess the purging component. Rats do not have the emetic reflex, so regurgitation studies are not possible in this laboratory animal. Gorillas have been reported to engage in regurgitative behavior, and thus have been proposed as an animal in which to study bulimia nervosa (Gould and Bres, 1986), but others suggest it may be a better model for childhood rumination disorder as opposed to bulimia nervosa (Casper et al., 2008).
Sham feeding has been proposed as an animal model of bulimia nervosa through its simulation of a defect in satiety mechanisms (Casper et al., 2008; Davis and Campbell, 1973; Mook and Wagner, 1987; Smith, 1989). The sham feeding preparation allows for the rat to taste food but retain minimal calories or nutrients, as the food is expelled through a cannula inserted in the stomach or the esophagus (Smith, 1998). To incorporate the aspects of both binge eating and purging into one animal model, binge eating has been combined with gastric sham feeding in the laboratory rat (Avena et al., 2006b). In this paradigm, when rats are allowed to binge on a sucrose solution with an open fistula, they ingest copious amounts of sucrose.
5.3 Animal Models of Bulimia Nervosa: DA and ACh
The section on binge eating describes neurochemical findings that also relate to the binge eating that may be associated with bulimia nervosa, therefore this discussion will focus on the findings obtained with the model of binge eating combined with sham feeding to simulate the bingeing and purging that together characterize bulimia nervosa. When in vivo microdialysis was used to measure DA release in rats that were sham feeding while binge eating, it was shown that bingeing released DA in the NAc, even when the stomach contents were immediately purged (Avena et al., 2006b) (Figure 2). Since the release of DA occurs in response to the taste of the sucrose when animals are binge eating with an open gastric fistula, the release of accumbens DA is not contingent on the full digestion of the nutrients obtained from the sucrose. It may be that the orosensory reward obtained from binge eating is repeatedly stimulating the release of DA in the NAc. It was also noted that the release of ACh, which as described above is thought to be associated with satiety, was attenuated when animals were sham feeding (Figure 3). This suggests that the ACh satiety effect might be attenuated when animals purge. Elevated ACh also has been linked to aversive behavior (Hoebel et al., 2007); so perhaps purging the contents of the stomach, which reduces ACh release, invariably reduces aversion.
Figure 3.
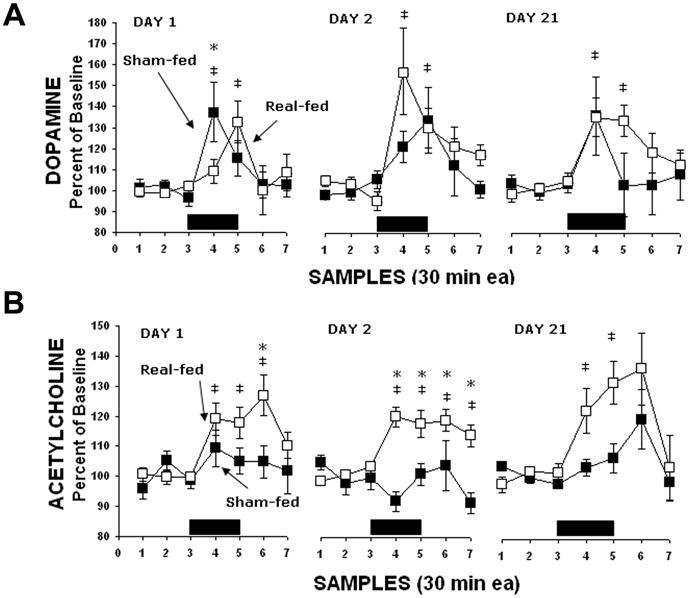
Changes in extracellular DA and ACh release in the NAc when rats are bingeing and purging via sham feeding. (A) DA release is increased in bingeing rats when both real-feeding and sham-feeding. Significant differences between groups are indicated by asterisks (P<0.05). Significant differences from baseline are indicated by ‡ (P<0.05). (B) ACh increased for real-feeding rats during sugar intake, but there was no response for sham-feeding rats during this time marked by black rectangles along the ordinate. Asterisks indicate that the ACh levels were significantly higher for the real- compared with sham-feeding rats. Differences from baseline for the real-feeding rats are indicated by ‡ (P<0.05). Figure reprinted with permission from (Avena et al., 2006b).
5.4 Clinical Correlates of Animal Models of Bulimia Nervosa
Data obtained from clinical studies support and validate the findings obtained with animal models of bulimia nervosa. Imaging studies have suggested perturbations in reward-related brain areas, including the insula (Schienle et al., 2009), and executive control and self-regulatory areas, such as the ACC (Marsh et al., 2009; Penas-Lledo et al., 2007), in bulimic patients. A role for DA is suggested by the finding that bulimic patients have low central DA activity as reflected in analysis of DA metabolites in the spinal fluid (Jimerson et al., 1992). In addition, bulimic patients have low β-endorphin levels (Brewerton et al., 1992; Waller et al., 1986), and they also have decreased mu-opioid receptor binding in the insula compared with controls, which correlates with recent fasting behavior (Bencherif et al., 2005). Bulimic patients will binge excessively on non-caloric sweeteners (Klein et al., 2006), suggesting that they derive benefits from sweet orosensory stimulation.
6.1 Anorexia Nervosa
As with binge eating, anorexia is both a disorder and a symptom of other disorders, both psychiatric and organic in nature. According to the DSM-IV, the diagnosis of anorexia nervosa is characterized by a distinct refusal to maintain a normal body weight (less than 85% of what is expected for age and height), an intense fear of gaining weight, and amenorrhea for postmenarcheal females (American Psychiatric Association, 2000). It is estimated that less than 1% females suffer from anorexia nervosa (Hoek et al., 1995; Hudson et al., 2007).
Just as some of the findings with binge eating apply to bulimia nervosa, some of the findings obtained with models of bulimia nervosa may apply to the study of anorexia nervosa. While these disorders seem to be opposites in terms of their behavioral characterization, they share the common feature originating from restrained eating behavior and dysfunctional cognitions related to body weight and body image (Kaye et al., 2005). This is particularly evident with the binge-eating/purging subtype of anorexia nervosa, in which patients regularly engage in binge-eating/purging behaviors while at a low body weight. The other subtype of anorexia nervosa, restricting type, is characterized by a lack of bingeing or purging behavior. Further, patients are also known to cross over between anorexia and bulimia nervosa (Kendler et al., 1991), and patients with these disorders tend to have increased levels of anxiety, obsessive behaviors, and perfectionism (Kaye et al., 2005; Strober, 1980).
6.2 Animal Models of Anorexia Nervosa
The most well-known animal model of anorexia is activity-based anorexia (ABA) (Routtenberg and Kuznesof, 1967). In this model, rats are maintained on a severely restricted feeding schedule, usually 1 h per day of food access, with free access to a running wheel. Over just a few days, this combination leads to excessive wheel running and a decline in body weight, amenorrhea and ultimately death in many cases without experimenter intervention. These behaviors are similar to the core features of anorexia nervosa as described above.
There are a multitude of other potential animal models that could apply to the study of anorexia. There are numerous models of food restriction and models of under nutrition that possess features that apply to aspects of anorexia nervosa (see Carr, 2007; Casper, 1998 for review). Other models that have assessed the effect of stress on appetite loss are also applicable (Wang, 2002), as anorexic patients are known to have inappropriate responses to stress (Miller et al., 2003) and stressful encounters may instigate anorectic behaviors.
6.3 Animals Models of Anorexia Nervosa: DA
It is known that food restriction and weight loss can enhance the release of DA in the limbic system in response to rewarding substances, including drugs of abuse and palatable food (Avena et al., 2008b; Cadoni et al., 2003; Carr, 2002, 2007; Pothos et al., 1995; Stuber et al., 2002). Moreover, DA has been shown to have a role in ABA, particularly in relation to reward and hyperactivity (Scheurink et al., 2010). Pharmacological experiments show that treatment with a non-selective DA antagonist, cis-flupenthixol, reduces activity levels, and increases body weight and food intake in ABA animals, but has no effect on food-anticipatory behavior (Verhagen et al., 2009a). It is interesting that the hormone leptin, which has been shown to suppress ABA behaviors (Exner et al., 2000; Hillebrand et al., 2005), is also known to attenuate the effects of DA and some motivated behaviors in reward-related brain areas (Fulton et al., 2006; Fulton et al., 2000; Shalev et al., 2001). Thus, leptin's ability to suppress ABA behavior may be explained by a direct effect of leptin on the midbrain DA system (Hillebrand et al., 2008).
6.4 Animal Models of Anorexia Nervosa: 5-HT
The discussions above for binge eating and bulimia nervosa contrasted the findings with DA with ACh in the NAc, as it has previously been reported that these neurotransmitters appear to work in balance to modulate food intake and dysphoric state (Hoebel et al., 2007). Limited research has been done with regard to cholinergic mechanisms underlying anorexia nervosa, but some studies have assessed the effects of ACh on growth hormone (Muller et al., 1995; Rolla et al., 1991; Tamai et al., 1990). In contrast, a large body of research on anorexia has focused on 5-HT systems, which have also been proposed to serve as aversive motivational systems that might oppose a DA-related appetitive system (Cools et al., 2008; Daw et al., 2002). In animals, very low-fat diets, typically consumed by anorectic patients, have been found to diminish neuronal 5-HT activity (Muldoon et al., 1992). Further, laboratory animal studies using ABA show that 5-HT release is reduced in the NAc (Verhagen et al., 2009b). Moreover, pharmacological suppression of the serotonergic systems using 8-OH-DPAT reduces hyperactivity and weight loss in ABA animals, suggesting that reduction of serotonergic activity can inhibit the development of ABA (Atchley and Eckel, 2006).
6.5 Animal Models of Anorexia Nervosa: Opioids
It has been suggested that endogenous opioid systems are disturbed in patients with anorexia nervosa whereby patients become “dependent” on the opioids released as a result of sustained starvation (Luby et al., 1987). This hypothesis has been supported by findings that female BALB/c mice that have a tendency to be hyperactive and anorexic in response to morphine (Marrazzi et al., 1990). Opioid dysregulation in brain reward systems is also suggested by findings of signs related to opiate-like drug withdrawal in ABA rats that are administered the opioid antagonist naloxone (Kanarek et al., 2009). Plasma levels of β-endorphin have been reported to be elevated in ABA rats, as have increases in hypothalamic β-endorphin and dynorphin-A (Aravich et al., 1993).
6.6 Clinical Correlates of Animal Models of Anorexia Nervosa
An interesting bridge between preclinical and clinical work on anorexia nervosa has emerged from the use of imaging in the rat. MicroPET allows cerebral glucose metabolism to be determined, and this technique has been applied to rats in the ABA model. Low metabolism has been noted in several brain areas, including the ventral striatum, along with a positive correlation noted between body weight loss and brain metabolism in the cingulate cortex (van Kuyck et al., 2007).
Clinical studies of anorexia nervosa lend support to the findings described above. It is interesting to note that patients who have recovered from anorexia nervosa have exaggerated activation in the caudate/dorsal striatum and in some cortical regions that project to this area (Wagner et al., 2007). This may underlay their strategic, rather than hedonic, approach to tasks and explain the hyperactivity in the neurocircuitry that mitigates planning and consequences (Kaye et al., 2009). The DA system has been suggested to have a role in anorexia nervosa through the observation of reduced cerebrospinal fluid levels of DA metabolites in ill and recovered patients (Kaye et al., 1999), and PET studies have shown that patients recovered from anorexia nervosa have increased D2/D3 receptor binding in the ventral striatum (Frank et al., 2005). Further, D2 gene polymorphisms have been seen in patients with anorexia nervosa (Bergen et al., 2005).
In terms of 5-HT, patients with anorexia nervosa have a reduction in basal concentrations of the 5-HT metabolite 5-HIAA in cerebrospinal fluid, but these levels are elevated in recovered patients (Kaye et al., 1984). PET studies using a radioligand for 5-HT receptors suggest that 5-HT1A and 5-HT2A receptors and the 5-HT transporter are dysregulated in cortical and limbic regions in patients recovered from anorexia nervosa (Bailer et al., 2005; Kaye et al., 2005). It has been suggested that the feeding-provoked dysphoric mood characteristic of anorexia nervosa (Kaye and Weltzin, 1991) may be due, in part, to food-induced increases in 5-HT, which drive anxiety through stimulation of the 5-HT1A receptors (Kaye et al., 2009). Polymorphisms in the 5-HT-1D receptor gene have been associated with restricting-type anorexia (Brown et al., 2007). However, SSRIs have been proven inadequate in effecting the core eating disorder symptoms. Animal models as well as clinical research indicates an interesting relationship between DA and 5-HT in anorexia, suggesting an imbalance in the two is driving the symptoms (Walter et al., 2009). For this reason, atypical antipsychotics, such as olanzapine, which have effects on both DA and 5-HT receptors, might be effective in treating anorexia (Bissada et al., 2008).
With regard to the opioids, endogenous opioids are elevated in patients with anorexia nervosa (Marrazzi et al., 1997) and gene polymorphisms of the delta-1 receptor have been linked to restricting-type of anorexia nervosa (Brown et al., 2007). Although changes are seen in opioid levels in both the ABA animal model and in some patients studies, treatment with opioid antagonists have only been successful in some individuals, particularly those with the binge–purge subtype (Marrazzi et al., 1995).
7 Summary and Conclusion
The findings reviewed here suggest that animal models of eating disorders have contributed to the growing literature on the perturbations in brain reward systems as they relate to binge eating disorder, bulimia nervosa and anorexia nervosa. Many models of binge eating have been proposed, all of which are unique in that they highlight various aspects that contribute to binge eating (e.g., stress, food deprivation). Data from animal models of binge eating have demonstrated alterations in DA, ACh, 5-HT and opioid systems, and these data are consistent with the findings of clinical studies. Further, animal models of binge eating allow for variables, such as macronutrient consumption, to be controlled, which may ultimately lead to a well-defined characterization of the effects that overeating of certain nutrients can have on brain chemistry. Further, by combining binge eating with sham feeding, the binge/purge aspect of bulimia nervosa can be modeled. When purging, animals continue to release DA in the NAc, and satiety may be delayed as reflected by the late rise accumbens ACh. Finally, models of anorexia nervosa are discussed, with the most well-characterized being activity-based anorexia. Findings reveal that both DA and 5-HT are perturbed in animals maintained on this feeding/exercise regimen, in ways that are supported by data derived from clinical cases.
Preclinical data highlight both similarities and differences that exist among eating disorders in terms of their influence on neurotransmitters and gene expression. Further, they offer the ability for translational research to build upon and complement animal models of eating disorders. Together with clinical data, the findings obtained with future studies using animal models will undoubtedly shed light on the functions of neural circuits in relationship to aberrant eating behaviors characteristic of these disorders, as well as aide in the development of pharmacotherapies targeted at specific eating disorders.
Highlights.
DA, ACh, 5-HT and opioids and all implicated in eating disorders.
Alterations are noted in reward-related brain areas in response to binge eating.
Anorexia models show alterations in DA and 5-HT due to starvation and exercise.
Acknowledgments
This work has been supported by DA-031230 (NMA), AA-019623 (fellowship to MEB), the National Eating Disorders Foundation (NMA), University of Florida, and a generous gift from Kidelhoj-Santini. The authors would like to thank Miaoyuan Wang for her assistance in developing the figures.
Abbreviations
- ABA
Activity-Based Anorexia
- ACC
Anterior Cingulated Cortex
- ACh
Acetylcholine
- DA
Dopamine
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision)
- NAc
Nucleus Accumbens
- PET
Positron Emission Tomography
- SSRI
Selective Serotonin Reuptake Inhibitor
- 5-HT
Serotonin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman SE, Shankman SA. What is the association between obsessive-compulsive disorder and eating disorders? Clin Psychol Rev. 2009;29:638–646. doi: 10.1016/j.cpr.2009.08.001. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Fouth Edition Text Revision (DSM-IV-TR) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- American Psychiatric Association. Proposed Revision: Binge Eating Disorder 2010 [Google Scholar]
- Aravich PF, Rieg TS, Lauterio TJ, Doerries LE. Beta-endorphin and dynorphin abnormalities in rats subjected to exercise and restricted feeding: relationship to anorexia nervosa? Brain Res. 1993;622:1–8. doi: 10.1016/0006-8993(93)90794-n. [DOI] [PubMed] [Google Scholar]
- Atchley DP, Eckel LA. Treatment with 8-OH-DPAT attenuates the weight loss associated with activity-based anorexia in female rats. Pharmacol Biochem Behav. 2006;83:547–553. doi: 10.1016/j.pbb.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Avena N, Rada P, Hoebel B. Unit 9.23C Sugar bingeing in rats. In: Crawley J, Gerfen C, Rogawski M, Sibley D, Skolnick P, Wray S, editors. Current Protocols In Neuroscience. John Wiley & Sons, Inc; Indianapolis: 2006a. pp. 9.23C.21–29.23C.26. [DOI] [PubMed] [Google Scholar]
- Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav. 2008a;94:309–315. doi: 10.1016/j.physbeh.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Bocarsly ME, Hoebel BG. Binge eating as a form of addiction: Evidence from an animal model of sugar addiction. In: Chambers N, editor. Binge Eating: Psychological Factors, Symptoms and Treatment. Nova Science Publishers; New York: 2009a. pp. 95–123. [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence of sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008b;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience. 2008c;156:865–871. doi: 10.1016/j.neuroscience.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009b;139:623–628. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience. 2006b;139:813–820. doi: 10.1016/j.neuroscience.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, Weissfeld L, Mathis CA, Drevets WC, Wagner A, Hoge J, Ziolko SK, McConaha CW, Kaye WH. Altered brain serotonin 5-HT1A receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [carbonyl11C]WAY-100635. Arch Gen Psychiatry. 2005;62:1032–1041. doi: 10.1001/archpsyc.62.9.1032. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993;265:1253–1260. [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Barbarich NC, Kaye WH, Jimerson D. Neurotransmitter and imaging studies in anorexia nervosa: new targets for treatment. Curr Drug Targets CNS Neurol Disord. 2003;2:61–72. doi: 10.2174/1568007033338779. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J Neurosci. 1997;17:851–861. doi: 10.1523/JNEUROSCI.17-02-00851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello NT, Lucas LR, Hajnal A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport. 2002;13:1575–1578. doi: 10.1097/00001756-200208270-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1260–1268. doi: 10.1152/ajpregu.00716.2002. [DOI] [PubMed] [Google Scholar]
- Bencherif B, Guarda AS, Colantuoni C, Ravert HT, Dannals RF, Frost JJ. Regional mu-opioid receptor binding in insular cortex is decreased in bulimia nervosa and correlates inversely with fasting behavior. J Nucl Med. 2005;46:1349–1351. [PubMed] [Google Scholar]
- Bergen AW, Yeager M, Welch RA, Haque K, Ganjei JK, van den Bree MB, Mazzanti C, Nardi I, Fichter MM, Halmi KA, Kaplan AS, Strober M, Treasure J, Woodside DB, Bulik CM, Bacanu SA, Devlin B, Berrettini WH, Goldman D, Kaye WH. Association of multiple DRD2 polymorphisms with anorexia nervosa. Neuropsychopharmacology. 2005;30:1703–1710. doi: 10.1038/sj.npp.1300719. [DOI] [PubMed] [Google Scholar]
- Berner LA, Avena NM, Hoebel BG. Bingeing, self-restriction, and increased body weight in rats with limited access to a sweet-fat diet. Obesity (Silver Spring) 2008;16:1998–2002. doi: 10.1038/oby.2008.328. [DOI] [PubMed] [Google Scholar]
- Bissada H, Tasca GA, Barber AM, Bradwejn J. Olanzapine in the treatment of low body weight and obsessive thinking in women with anorexia nervosa: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2008;165:1281–1288. doi: 10.1176/appi.ajp.2008.07121900. [DOI] [PubMed] [Google Scholar]
- Blundell JE. Is there a role for serotonin (5-hydroxytryptamine) in feeding? Int J Obes. 1977;1:15–42. [PubMed] [Google Scholar]
- Blundell JE, Lawton CL, Halford JC. Serotonin, eating behavior, and fat intake. Obes Res. 1995;3(Suppl 4):471S–476S. doi: 10.1002/j.1550-8528.1995.tb00214.x. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Lamonte N, Israel Y, Kandov Y, Ackerman TF, Khaimova E. Reciprocal opioid-opioid interactions between the ventral tegmental area and nucleus accumbens regions in mediating mu agonist-induced feeding in rats. Peptides. 2005;26:621–629. doi: 10.1016/j.peptides.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Chandler PC. Binge eating in rats produced by combining dieting with stress. Curr Protoc Neurosci. 2006 doi: 10.1002/0471142301.ns0923as36. Chapter 9, Unit9 23A. [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CR, Wauford PK. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav Neurosci. 2005;119:1207–1214. doi: 10.1037/0735-7044.119.5.1207. [DOI] [PubMed] [Google Scholar]
- Brewerton TD, Lydiard RB, Laraia MT, Shook JE, Ballenger JC. CSF beta-endorphin and dynorphin in bulimia nervosa. Am J Psychiatry. 1992;149:1086–1090. doi: 10.1176/ajp.149.8.1086. [DOI] [PubMed] [Google Scholar]
- Brown KM, Bujac SR, Mann ET, Campbell DA, Stubbins MJ, Blundell JE. Further evidence of association of OPRD1 & HTR1D polymorphisms with susceptibility to anorexia nervosa. Biol Psychiatry. 2007;61:367–373. doi: 10.1016/j.biopsych.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Bulik CM. Genetic and biological risk factors. In: Thompson JK, editor. Handbook of Eating Disorders and Obesity. John Wiley & Sons; Hoboken: 2004. [Google Scholar]
- Cadoni C, Solinas M, Valentini V, Di Chiara G. Selective psychostimulant sensitization by food restriction: differential changes in accumbens shell and core dopamine. Eur J Neurosci. 2003;18:2326–2334. doi: 10.1046/j.1460-9568.2003.02941.x. [DOI] [PubMed] [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- Carr KD. Chronic food restriction: enhancing effects on drug reward and striatal cell signaling. Physiol Behav. 2007;91:459–472. doi: 10.1016/j.physbeh.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Casper RC. Biology of Eating Disorders. Second. American Psychiatric Press; Washington, DC: 1998. [Google Scholar]
- Casper RC, Sullivan EL, Tecott L. Relevance of animal models to human eating disorders and obesity. Psychopharmacology (Berl) 2008;199:313–329. doi: 10.1007/s00213-008-1102-2. [DOI] [PubMed] [Google Scholar]
- Cattanach L, Malley R, Rodin J. Psychologic and physiologic reactivity to stressors in eating disordered individuals. Psychosom Med. 1988;50:591–599. doi: 10.1097/00006842-198811000-00005. [DOI] [PubMed] [Google Scholar]
- Chandler-Laney PC, Castaneda E, Pritchett CE, Smith ML, Giddings M, Artiga AI, Boggiano MM. A history of caloric restriction induces neurochemical and behavioral changes in rats consistent with models of depression. Pharmacol Biochem Behav. 2007;87:104–114. doi: 10.1016/j.pbb.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FH. Binge eating in rats with limited access to vegetable shortening. Curr Protoc Neurosci. 2006 doi: 10.1002/0471142301.ns0923bs36. Chapter 9, Unit9 23B. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FH. Baclofen, raclopride, and naltrexone differentially affect intake of fat and sucrose under limited access conditions. Behav Pharmacol. 2009;20:537–548. doi: 10.1097/FBP.0b013e3283313168. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1066–1076. doi: 10.1152/ajpregu.90309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JD, Campbell CS. Peripheral control of meal size in the rat. Effect of sham feeding on meal size and drinking rate. J Comp Physiol Psychol. 1973;83:379–387. doi: 10.1037/h0034667. [DOI] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord. 2000;28:436–445. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA. Naloxone, an opiate blocker, reduces the consumption of sweet high-fat foods in obese and lean female binge eaters. Am J Clin Nutr. 1995;61:1206–1212. doi: 10.1093/ajcn/61.6.1206. [DOI] [PubMed] [Google Scholar]
- Evans KR, Vaccarino FJ. Amphetamine- and morphine-induced feeding: evidence for involvement of reward mechanisms. Neurosci Biobehav Rev. 1990;14:9–22. doi: 10.1016/s0149-7634(05)80156-3. [DOI] [PubMed] [Google Scholar]
- Exner C, Hebebrand J, Remschmidt H, Wewetzer C, Ziegler A, Herpertz S, Schweiger U, Blum WF, Preibisch G, Heldmaier G, Klingenspor M. Leptin suppresses semi-starvation induced hyperactivity in rats: implications for anorexia nervosa. Mol Psychiatry. 2000;5:476–481. doi: 10.1038/sj.mp.4000771. [DOI] [PubMed] [Google Scholar]
- Fava M, Copeland PM, Schweiger U, Herzog DB. Neurochemical abnormalities of anorexia nervosa and bulimia nervosa. Am J Psychiatry. 1989;146:963–971. doi: 10.1176/ajp.146.8.963. [DOI] [PubMed] [Google Scholar]
- Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC, Mathis CA, Wagner A, Hoge J, Ziolko S, Barbarich-Marsteller N, Weissfeld L, Kaye WH. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biol Psychiatry. 2005;58:908–912. doi: 10.1016/j.biopsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Gaetani S, Kaye WH, Cuomo V, Piomelli D. Role of endocannabinoids and their analogues in obesity and eating disorders. Eat Weight Disord. 2008;13:e42–48. [PubMed] [Google Scholar]
- Galic MA, Persinger MA. Voluminous sucrose consumption in female rats: increased “nippiness” during periods of sucrose removal and possible oestrus periodicity. Psychol Rep. 2002;90:58–60. doi: 10.2466/pr0.2002.90.1.58. [DOI] [PubMed] [Google Scholar]
- Gould E, Bres M. Regurgitation in gorillas: possible model for human eating disorders (rumination/bulimia) J Dev Behav Pediatr. 1986;7:314–319. doi: 10.1097/00004703-198610000-00009. [DOI] [PubMed] [Google Scholar]
- Guertin TL, Conger AJ. Mood and forbidden foods' influence on perceptions of binge eating. Addict Behav. 1999;24:175–193. doi: 10.1016/s0306-4603(98)00049-5. [DOI] [PubMed] [Google Scholar]
- Hadigan CM, Kissileff HR, Walsh BT. Patterns of food selection during meals in women with bulimia. Am J Clin Nutr. 1989;50:759–766. doi: 10.1093/ajcn/50.4.759. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Moss DE. An animal model of bulimia nervosa: opioid sensitivity to fasting episodes. Pharmacol Biochem Behav. 1991;39:421–422. doi: 10.1016/0091-3057(91)90201-c. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Moss DE. Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int J Eat Disord. 1997;22:411–420. doi: 10.1002/(sici)1098-108x(199712)22:4<411::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- Haleem DJ, Haider S. Food restriction decreases serotonin and its synthesis rate in the hypothalamus. Neuroreport. 1996;7:1153–1156. doi: 10.1097/00001756-199604260-00011. [DOI] [PubMed] [Google Scholar]
- Halford JC, Blundell JE. Pharmacology of appetite suppression. Prog Drug Res. 2000a;54:25–58. doi: 10.1007/978-3-0348-8391-7_2. [DOI] [PubMed] [Google Scholar]
- Halford JC, Blundell JE. Separate systems for serotonin and leptin in appetite control. Ann Med. 2000b;32:222–232. doi: 10.3109/07853890008998829. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Eckert E, Mitchell JE, Pyle R. Affective disorder and substance abuse in women with bulimia. Psychol Med. 1984;14:701–704. doi: 10.1017/s0033291700015324. [DOI] [PubMed] [Google Scholar]
- Hillebrand JJ, Kas MJ, van Elburg AA, Hoek HW, Adan RA. Leptin's effect on hyperactivity: potential downstream effector mechanisms. Physiol Behav. 2008;94:689–695. doi: 10.1016/j.physbeh.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Hillebrand JJ, Koeners MP, de Rijke CE, Kas MJ, Adan RA. Leptin treatment in activity-based anorexia. Biol Psychiatry. 2005;58:165–171. doi: 10.1016/j.biopsych.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Avena NM, Rada P. Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr Opin Pharmacol. 2007;7:617–627. doi: 10.1016/j.coph.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek HW, Bartelds AI, Bosveld JJ, van der Graaf Y, Limpens VE, Maiwald M, Spaaij CJ. Impact of urbanization on detection rates of eating disorders. Am J Psychiatry. 1995;152:1272–1278. doi: 10.1176/ajp.152.9.1272. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS, Nollen NL, Savage CR. Neural mechanisms underlying food motivation in children and adolescents. Neuroimage. 2005;27:669–676. doi: 10.1016/j.neuroimage.2005.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huether G, Zhou D, Schmidt S, Wiltfang J, Ruther E. Long-term food restriction down-regulates the density of serotonin transporters in the rat frontal cortex. Biol Psychiatry. 1997;41:1174–1180. doi: 10.1016/s0006-3223(96)00265-x. [DOI] [PubMed] [Google Scholar]
- Inoue K, Iwasaki S, Muramatsu T, Yamauchi T, Kiriike N. Animal model of eating disorders. Nihon Shinkei Seishin Yakurigaku Zasshi. 2006;26:89–92. [PubMed] [Google Scholar]
- Jarosz PA, Metzger BL. The effect of opioid antagonism on food intake behavior and body weight in a biobehavioral model of obese binge eating. Biol Res Nurs. 2002;3:198–209. doi: 10.1177/10900402003004005. [DOI] [PubMed] [Google Scholar]
- Jimerson DC, Lesem MD, Kaye WH, Brewerton TD. Low serotonin and dopamine metabolite concentrations in cerebrospinal fluid from bulimic patients with frequent binge episodes. Arch Gen Psychiatry. 1992;49:132–138. doi: 10.1001/archpsyc.1992.01820020052007. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales EF. Macronutrient analysis of binge eating in bulimia. Physiol Behav. 1990;48:837–840. doi: 10.1016/0031-9384(90)90236-w. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57:1047–1060. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, D'Anci KE, Jurdak N, Mathes WF. Running and addiction: precipitated withdrawal in a rat model of activity-based anorexia. Behav Neurosci. 2009;123:905–912. doi: 10.1037/a0015896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2008;94:121–135. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Ebert MH, Raleigh M, Lake R. Abnormalities in CNS monoamine metabolism in anorexia nervosa. Arch Gen Psychiatry. 1984;41:350–355. doi: 10.1001/archpsyc.1984.01790150040007. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Frank GK, Bailer UF, Henry SE, Meltzer CC, Price JC, Mathis CA, Wagner A. Serotonin alterations in anorexia and bulimia nervosa: new insights from imaging studies. Physiol Behav. 2005;85:73–81. doi: 10.1016/j.physbeh.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Frank GK, McConaha C. Altered dopamine activity after recovery from restricting-type anorexia nervosa. Neuropsychopharmacology. 1999;21:503–506. doi: 10.1016/S0893-133X(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10:573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Weltzin TE. Serotonin activity in anorexia and bulimia nervosa: relationship to the modulation of feeding and mood. J Clin Psychiatry. 1991;52(Suppl):41–48. [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate Ensure(R)) alters striatal enkephalin gene expression. Eur J Neurosci. 2003;18:2592–2598. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, MacLean C, Neale M, Kessler R, Heath A, Eaves L. The genetic epidemiology of bulimia nervosa. Am J Psychiatry. 1991;148:1627–1637. doi: 10.1176/ajp.148.12.1627. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- Klein DA, Boudreau GS, Devlin MJ, Walsh BT. Artificial sweetener use among individuals with eating disorders. Int J Eat Disord. 2006;39:341–345. doi: 10.1002/eat.20260. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuikka JT, Tammela L, Karhunen L, Rissanen A, Bergstrom KA, Naukkarinen H, Vanninen E, Karhu J, Lappalainen R, Repo-Tiihonen E, Tiihonen J, Uusitupa M. Reduced serotonin transporter binding in binge eating women. Psychopharmacology (Berl) 2001;155:310–314. doi: 10.1007/s002130100716. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Lattimore PJ. Stress-induced eating: an alternative method for inducing ego-threatening stress. Appetite. 2001;36:187–188. doi: 10.1006/appe.2000.0387. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Hoebel BG. Behavioral neuroscience and obesity. In: Bray G, Bouchard C, James P, editors. The Handbook of Obesity. Marcel Dekker; New York: 2004. pp. 301–371. [Google Scholar]
- Leibowitz SF, Weiss GF, Shor-Posner G. Hypothalamic serotonin: pharmacological, biochemical, and behavioral analyses of its feeding-suppressive action. Clin Neuropharmacol. 1988;11(Suppl 1):S51–71. [PubMed] [Google Scholar]
- Leibowitz SF, Wortley KE. Hypothalamic control of energy balance: different peptides, different functions. Peptides. 2004;25:473–504. doi: 10.1016/j.peptides.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1236–1239. doi: 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- Luby ED, Marrazzi MA, Sperti S. Anorexia nervosa: a syndrome of starvation dependence. Compr Ther. 1987;13:16–21. [PubMed] [Google Scholar]
- Marcus MD, Kalarchian MA. Binge eating in children and adolescents. Int J Eat Disord. 2003;34(Suppl):S47–57. doi: 10.1002/eat.10205. [DOI] [PubMed] [Google Scholar]
- Mark GP, Blander DS, Hoebel BG. A conditioned stimulus decreases extracellular dopamine in the nucleus accumbens after the development of a learned taste aversion. Brain Res. 1991;551:308–310. doi: 10.1016/0006-8993(91)90946-s. [DOI] [PubMed] [Google Scholar]
- Mark GP, Rada P, Pothos E, Hoebel BG. Effects of feeding and drinking on acetylcholine release in the nucleus accumbens, striatum, and hippocampus of freely behaving rats. Journal of Neurochemistry. 1992;58:2269–2274. doi: 10.1111/j.1471-4159.1992.tb10973.x. [DOI] [PubMed] [Google Scholar]
- Mark GP, Weinberg JB, Rada PV, Hoebel BG. Extracellular acetylcholine is increased in the nucleus accumbens following the presentation of an aversively conditioned taste stimulus. Brain Res. 1995;688:184–188. doi: 10.1016/0006-8993(95)00401-b. [DOI] [PubMed] [Google Scholar]
- Marrazzi MA, Bacon JP, Kinzie J, Luby ED. Naltrexone use in the treatment of anorexia nervosa and bulimia nervosa. Int Clin Psychopharmacol. 1995;10:163–172. doi: 10.1097/00004850-199510030-00005. [DOI] [PubMed] [Google Scholar]
- Marrazzi MA, Luby ED, Kinzie J, Munjal ID, Spector S. Endogenous codeine and morphine in anorexia and bulimia nervosa. Life Sci. 1997;60:1741–1747. doi: 10.1016/s0024-3205(97)00133-1. [DOI] [PubMed] [Google Scholar]
- Marrazzi MA, Mullings-Britton J, Stack L, Powers RJ, Lawhorn J, Graham V, Eccles T, Gunter S. Atypical endogenous opioid systems in mice in relation to an auto-addiction opioid model of anorexia nervosa. Life Sci. 1990;47:1427–1435. doi: 10.1016/0024-3205(90)90521-r. [DOI] [PubMed] [Google Scholar]
- Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am J Psychiatry. 2009;166:664–674. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SP, Redlich AD, Steiner H. The stress response in anorexia nervosa. Child Psychiatry Hum Dev. 2003;33:295–306. doi: 10.1023/a:1023036329399. [DOI] [PubMed] [Google Scholar]
- Mook DG, Wagner S. Preparation and maintenance of rats with chronic esophagostomy and gastric cannula. Physiol Behav. 1987;39:417–420. doi: 10.1016/0031-9384(87)90245-9. [DOI] [PubMed] [Google Scholar]
- Morris JS, Dolan RJ. Involvement of human amygdala and orbitofrontal cortex in hunger-enhanced memory for food stimuli. J Neurosci. 2001;21:5304–5310. doi: 10.1523/JNEUROSCI.21-14-05304.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon MF, Kaplan JR, Manuck SB, Mann JJ. Effects of a low-fat diet on brain serotonergic responsivity in cynomolgus monkeys. Biol Psychiatry. 1992;31:739–742. doi: 10.1016/0006-3223(92)90285-8. [DOI] [PubMed] [Google Scholar]
- Muller EE, Rolla M, Ghigo E, Belliti D, Arvat E, Andreoni A, Torsello A, Locatelli V, Camanni F. Involvement of brain catecholamines and acetylcholine in growth hormone hypersecretory states. Pathophysiological, diagnostic and therapeutic implications. Drugs. 1995;50:805–837. doi: 10.2165/00003495-199550050-00004. [DOI] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Chimukangara M, Billington CJ, Levine AS. Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. Am J Physiol Regul Integr Comp Physiol. 2007;293:R99–105. doi: 10.1152/ajpregu.00675.2006. [DOI] [PubMed] [Google Scholar]
- Nathan PJ, Bullmore ET. From taste hedonics to motivational drive: central mu-opioid receptors and binge-eating behaviour. Int J Neuropsychopharmacol. 2009:1–14. doi: 10.1017/S146114570900039X. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Oswald KD, Murdaugh DL, King VL, Boggiano MM. Motivation for palatable food despite consequences in an animal model of binge eating. Int J Eat Disord. 2011;44:203–211. doi: 10.1002/eat.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penas-Lledo EM, Loeb KL, Martin L, Fan J. Anterior cingulate activity in bulimia nervosa: a fMRI case study. Eat Weight Disord. 2007;12:e78–82. doi: 10.1007/BF03327599. [DOI] [PubMed] [Google Scholar]
- Perry ML, Baldo BA, Andrzejewski ME, Kelley AE. Muscarinic receptor antagonism causes a functional alteration in nucleus accumbens mu-opiate-mediated feeding behavior. Behav Brain Res. 2009;197:225–229. doi: 10.1016/j.bbr.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Polivy J. Psychological consequences of food restriction. J Am Diet Assoc. 1996;96:589–592. doi: 10.1016/S0002-8223(96)00161-7. quiz 593-584. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995;15:6640–6650. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WE, Blackstone K. Nucleus accumbens acetylcholine and food intake: decreased muscarinic tone reduces feeding but not food-seeking. Behav Brain Res. 2009;198:252–257. doi: 10.1016/j.bbr.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Kelley AE. Striatal muscarinic receptor antagonism reduces 24-h food intake in association with decreased preproenkephalin gene expression. Eur J Neurosci. 2005;22:3229–3240. doi: 10.1111/j.1460-9568.2005.04489.x. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Rada PV, Hoebel BG. Supraadditive effect of d-fenfluramine plus phentermine on extracellular acetylcholine in the nucleus accumbens: possible mechanism for inhibition of excessive feeding and drug abuse. Pharmacol Biochem Behav. 2000;65:369–373. doi: 10.1016/s0091-3057(99)00219-1. [DOI] [PubMed] [Google Scholar]
- Rolla M, Andreoni A, Belliti D, Cristofani R, Ferdeghini M, Muller EE. Blockade of cholinergic muscarinic receptors by pirenzepine and GHRH-induced GH secretion in the acute and recovery phase of anorexia nervosa and atypical eating disorders. Biol Psychiatry. 1991;29:1079–1091. doi: 10.1016/0006-3223(91)90250-p. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Brain mechanisms underlying flavour and appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:1123–1136. doi: 10.1098/rstb.2006.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Sensory processing in the brain related to the control of food intake. Proc Nutr Soc. 2007;66:96–112. doi: 10.1017/S0029665107005332. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J Comp Physiol Psychol. 1967;64:414–421. doi: 10.1037/h0025205. [DOI] [PubMed] [Google Scholar]
- Sandager N, Peterson CB, Allen S, Henderson KE, Crow S, Thuras P. Tobacco use and comorbidity in bulimia nervosa. Int J Eat Disord. 2008;41:734–738. doi: 10.1002/eat.20572. [DOI] [PubMed] [Google Scholar]
- Scheurink AJ, Boersma GJ, Nergardh R, Sodersten P. Neurobiology of hyperactivity and reward in Anorexia Nervosa: Agreeable restlessness in Anorexia Nervosa. Physiol Behav. 2010 doi: 10.1016/j.physbeh.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Hermann A, Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry. 2009;65:654–661. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Shalev U, Yap J, Shaham Y. Leptin attenuates acute food deprivation-induced relapse to heroin seeking. J Neurosci. 2001;21:RC129. doi: 10.1523/JNEUROSCI.21-04-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Mizushima H, Hirano M, Shioe K, Nakazawa M, Hiejima Y, Ono Y, Kanba S. Eating disorders with binge-eating behaviour are associated with the s allele of the 3′-UTR VNTR polymorphism of the dopamine transporter gene. J Psychiatry Neurosci. 2004;29:134–137. [PMC free article] [PubMed] [Google Scholar]
- Smith GP. Animal models of human eating disorders. Ann N Y Acad Sci. 1989;575:63–72. doi: 10.1111/j.1749-6632.1989.tb53233.x. discussion 72-64. [DOI] [PubMed] [Google Scholar]
- Smith GP. Sham feeding in rats with chronic, reversible gastric fistulas. In: Crawley JN, Gerfen CR, Mc Kay R, Rogawski MA, Sibley DR, Skolnick P, editors. Current Protocols in Neruoscience. John Wiley and Sons, Inc; New York: 1998. pp. D.1–D.6. [DOI] [PubMed] [Google Scholar]
- Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124:134–142. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175–191. doi: 10.1053/j.gastro.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Strober M. Personality and symptomatological features in young, nonchronic anorexia nervosa patients. J Psychosom Res. 1980;24:353–359. doi: 10.1016/0022-3999(80)90027-6. [DOI] [PubMed] [Google Scholar]
- Strober M. Family-genetic perspectives on anorexia nervosa and bulimia nervosa. In: Bronwnell K, Fairburn C, editors. Eating Disorders and Obesity- A Comprehensive Handbook. The Guliford Press; New York: 1995. pp. 212–218. [Google Scholar]
- Stuber GD, Evans SB, Higgins MS, Pu Y, Figlewicz DP. Food restriction modulates amphetamine-conditioned place preference and nucleus accumbens dopamine release in the rat. Synapse. 2002;46:83–90. doi: 10.1002/syn.10120. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ. Eating patterns and obesity. Psychiatr Q. 1959;33:284–295. doi: 10.1007/BF01575455. [DOI] [PubMed] [Google Scholar]
- Tamai H, Komaki G, Matsubayashi S, Kobayashi N, Mori K, Nakagawa T, Truong MP, Walter RM, Jr, Kumagai LF. Effect of cholinergic muscarinic receptor blockade on human growth hormone (GH)-releasing hormone-(1-44)-induced GH secretion in anorexia nervosa. J Clin Endocrinol Metab. 1990;70:738–741. doi: 10.1210/jcem-70-3-738. [DOI] [PubMed] [Google Scholar]
- van Kuyck K, Casteels C, Vermaelen P, Bormans G, Nuttin B, Van Laere K. Motor- and food-related metabolic cerebral changes in the activity-based rat model for anorexia nervosa: a voxel-based microPET study. Neuroimage. 2007;35:214–221. doi: 10.1016/j.neuroimage.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Verhagen LA, Luijendijk MC, Hillebrand JJ, Adan RA. Dopamine antagonism inhibits anorectic behavior in an animal model for anorexia nervosa. Eur Neuropsychopharmacol. 2009a;19:153–160. doi: 10.1016/j.euroneuro.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Verhagen LA, Luijendijk MC, Korte-Bouws GA, Korte SM, Adan RA. Dopamine and serotonin release in the nucleus accumbens during starvation-induced hyperactivity. Eur Neuropsychopharmacol. 2009b;19:309–316. doi: 10.1016/j.euroneuro.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Wagner A, Aizenstein H, Venkatraman VK, Fudge J, May JC, Mazurkewicz L, Frank GK, Bailer UF, Fischer L, Nguyen V, Carter C, Putnam K, Kaye WH. Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry. 2007;164:1842–1849. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- Waller DA, Kiser RS, Hardy BW, Fuchs I, Feigenbaum LP, Uauy R. Eating behavior and plasma beta-endorphin in bulimia. Am J Clin Nutr. 1986;44:20–23. doi: 10.1093/ajcn/44.1.20. [DOI] [PubMed] [Google Scholar]
- Walter H, Kammerer H, Frasch K, Spitzer M, Abler B. Altered reward functions in patients on atypical antipsychotic medication in line with the revised dopamine hypothesis of schizophrenia. Psychopharmacology (Berl) 2009;206:121–132. doi: 10.1007/s00213-009-1586-4. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Geliebter A, Volkow ND, Telang FW, Logan J, Jayne MC, Galanti K, Selig PA, Han H, Zhu W, Wong CT, Fowler JS. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity (Silver Spring) 2011;19:1601–1608. doi: 10.1038/oby.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- Wang SW. Effects of restraint stress and serotonin on macronutrient selection: a rat model of stress-induced anorexia. Eat Weight Disord. 2002;7:23–31. doi: 10.1007/BF03354426. [DOI] [PubMed] [Google Scholar]
- Wideman CH, Nadzam GR, Murphy HM. Implications of an animal model of sugar addiction, withdrawal and relapse for human health. Nutr Neurosci. 2005;8:269–276. doi: 10.1080/10284150500485221. [DOI] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac) Nat Rev Drug Discov. 2005;4:764–774. doi: 10.1038/nrd1821. [DOI] [PubMed] [Google Scholar]
- Wong KJ, Wojnicki FH, Corwin RL. Baclofen, raclopride, and naltrexone differentially affect intake of fat/sucrose mixtures under limited access conditions. Pharmacol Biochem Behav. 2009;92:528–536. doi: 10.1016/j.pbb.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Gorno-Tempini ML, Seeley WW, Rankin K, Lee SS, Matthews BR, Miller BL. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69:1424–1433. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]


