Abstract
Objectives
WISP3/CCN6 is mutated in progressive pseudorheumatoid dysplasia and may have effects on cartilage homeostasis. In order to uncover further roles for WISP3/CCN6 its expression was explored in osteoarthritic cartilage. Effects of WISP3/CCN6 on cartilage-relevant metalloproteinase expression were investigated in immortalised (C-28/I2) and primary chondrocytes.
Methods
Cartilage steady state levels of WISP3/CCN6 mRNA and protein production were determined by quantitative RT-PCR and immunohistochemistry respectively. WISP3/CCN6 was over-expressed in C-28/I2 cells and resultant stable clones analysed by real time RT-PCR for metalloproteinase expression and signalling pathways involved explored with pharmacological inhibition. Effects of WISP3/CCN6 on metalloproteinase expression in primary chondrocytes were investigated by an siRNA approach.
Results
WISP3/CCN6 was highly expressed in osteoarthritic cartilage compared to undamaged cartilage at RNA and protein levels. WISP3/CCN6 over-expression in C-28/I2 cells resulted in unexpected dual regulation of metalloproteinases: the expression of the potent aggrecanase, ADAMTS5, was down-regulated 9-fold, whilst MMP10 was up-regulated 14-fold, responses accentuated by suspension culture. MMP10 up-regulation was dependent on several MAP kinases but WISP3/CCN6-mediated ADAMTS5 repression was independent of these pathways and partially relieved by activation of β-catenin signalling. WISP3/CCN6 also suppressed ADAMTS5 expression in C-28/I2 cells treated with cytokines. In cytokine-treated primary chondrocytes gene silencing of WISP3/CCN6 resulted in enhanced ADAMTS5 expression whilst MMP10 expression was suppressed.
Conclusion
WISP3/CCN6 was highly expressed in end-stage osteoarthritic cartilage suggesting a role for this growth factor in cartilage homeostasis. WISP3/CCN6 repression of ADAMTS5 expression and regulation of MMP10 expression suggests complex and context-dependent roles for WISP3/CCN6 in cartilage biology.
Introduction
Wnt-1 inducible secreted protein 3 (WISP3, also known as CCN6) is a member of the Connective Tissue Growth Factor/Cysteine Rich 61/Nephroblastoma Overexpressed (CCN) family of matricellular proteins (1). Little is known about the biological activity of WISP3/CCN6, but different mutations in the WISP3 gene have been identified in several unrelated cases of Progressive Pseudorheumatoid Dysplasia (PPRD), a rare non-inflammatory skeletal disease that manifests in childhood with loss of the articular cartilage in multiple joints and bone abnormalities (2). A single nucleotide polymorphism in WISP3 has also been correlated with the incidence of polyarticular juvenile idiopathic arthritis (JIA), a disease clinically similar to PPRD (3). WISP3/CCN6 may therefore have a role in articular cartilage homeostasis. Interestingly, WISP3/CCN6 is chemotactic for mesenchymal stromal cells (4), which may also suggest it is involved in tissue repair. Over-expression of WISP3/CCN6 in immortalised chondrocytic cell lines leads to increased expression of the two major matrix components of articular cartilage, aggrecan and type II collagen (5), suggesting that WISP3/CCN6 is a potential stimulator of anabolic pathways in cartilage. Further, expression of WISP3/CCN6 is reduced in the chondrocytes of mice lacking connective tissue growth factor (CTGF/CCN2), another CCN family member known to promote chondrocyte differentiation (6). Given the severe articular cartilage degeneration observed in PPRD patients, it is also possible that WISP3/CCN6 regulates cartilage degradation through effects on proteinase expression. Indeed, chondrocytes from one PPRD patient with mutant WISP3/CCN6 show dysregulated expression and abnormal secretion of matrix metalloproteinases (MMPs) (7).
Aggrecan degradation is an important event in joint disease. Cartilage aggrecan may protect type II collagen from degradation and aggrecan degradation precedes type II collagen degradation (8). The proteinases primarily involved in cartilage matrix degradation are ADAMTS (A disintegrin and metalloprotease with a thrombospondin-like motif) proteinases and the MMPs (reviewed in (9)). Once there is excess loss of aggrecan over synthesis, type II collagen becomes accessible to collagenases such as MMP13 and the situation may be exacerbated by exposure to chondrocyte type II collagen receptors such as DDR2, which can up-regulate MMP13 expression upon ligand binding (10). Following collagen degradation, the process of cartilage destruction becomes irreversible. Thus, the enzymes responsible for pathological aggrecan degradation play a key role in initiating cartilage degeneration in joint disease.
The major aggrecan proteolytic fragment identified in osteoarthritis (OA), inflammatory joint disease, and joint injury is that resulting from cleavage of aggrecan at the ADAMTS interglobular site (11;12). Of all the ADAMTSs that have been demonstrated to cleave aggrecan, ADAMTS4 and ADAMTS5 appear to be those with the strongest activity at the interglobular domain site (13;14). In particular, ADAMTS5 has emerged as an important aggrecanase because ADAMTS5−/− mice are protected against cartilage degradation in induced inflammatory arthritis (15). ADAMTS5−/− mice also have substantially reduced cartilage degradation after surgical induction of joint instability compared to wild type or ADAMTS4−/− mice (16). Importantly, human ADAMTS5 has a thousand-fold greater aggrecanolytic activity on aggrecan under physiological conditions than ADAMTS4 (17) and immunolocalisation studies show ADAMTS5 in pericellular and intercellular locations in human OA cartilage, which are also the sites of the most intense staining for ADAMTS-generated NITEGE aggrecan fragments (18).
Due to the association of WISP3/CCN6 mutation with cartilage destruction in PPRD, a possible role for WISP3/CCN6 in OA was explored. Our results suggest that WISP3/CCN6 is highly expressed in cartilage and elevated in damaged cartilage compared to undamaged cartilage. Importantly, over-expression of WISP3/CCN6 in C-28/I2 cells dramatically reduced the expression of ADAMTS4 and ADAMTS5 whilst expression of MMP1 and MMP10 was elevated. In a loss-of-function approach WISP3/CCN6 knockdown in cytokine-stimulated primary chondrocytes resulted in elevated expression of ADAMTS5 but repression of MMP10. Taken together our results suggest that WISP3/CCN6 may have multiple effects on cartilage metabolism.
Materials and Methods
Tissue collection
Approval was obtained from the North Nottinghamshire Health Authority Local Research Ethics Committee (projects NNHA/420, NNHA/544 and NNHA/673). After informed consent, articular surfaces were collected from patients fulfilling American College of Rheumatology revised criteria for tibiofemoral OA at the time of total knee joint replacement (TKR) surgery. Postmortem (PM) cartilage was recovered from the knee joints of donors who had no history of arthritis or knee pain according to both next-of-kin and medical records, were not diagnosed with OA and had not had surgery or fracture to that joint. PM cases had no Heberden’s nodes, rheumatoid nodules or visible osteophytes. Primary chondrocytes were isolated, as previously described (19), from intact regions of femoral condyles of OA patients undergoing total knee replacement, with approval by the Institutional Review Board (IRB) and patient consent, cultured to confluence in DMEM/Ham’s F-12 containing 10% FCS and used for experimental purposes at passage one.
Real time RT-PCR
RNA was extracted from the articular cartilage (pooled from femoral condyles and tibial plateau) of OA patients or PM donors using phenol/chloroform extraction following Spexmill powdering of cartilage into Trizol (Invitrogen). WISP3/CCN6 mRNA transcript levels were analysed by one-step Taqman real-time RT-PCR using Quantitect Probe RT-PCR kit (Qiagen). For two-step Taqman real time RT-PCR analysis of C-28/I2 gene expression, RNA was harvested from cells using an RNeasy Mini kit (Qiagen) and reverse transcribed with MMLV reverse transcriptase (Promega) according to the manufacturer’s instructions. For MMP/TIMP and ADAMTS primer/probe sequences see (20). WISP3/CCN6 primer and probe sequences were: forward: 5′-ctccaaagctgaaaaatttgtcttt-3′, reverse: 5′-tttttgtggaatatgcttggataaga-3′, probe: 5′-ctggatgctcaagtactcagagttacaaaccca-3′. Expression of each gene was normalised to the expression of 18S from the same cDNA preparation using primers and probe specific for 18S (Applied Biosystems). Statistical analysis of gene expression changes between two sets of data was performed using the two tailed student’s T-test on sample groups no smaller than n = 3.
Immunohistochemistry
Human articular cartilage was obtained from the femoral condyle of knees from subjects with OA (3 male 60–73 yrs, 2 female 75 and 82 yrs) or from aged-matched PM donors (1 male 61 yrs, 3 female 59–63 yrs). Serial 7μM cryostat sections were fixed in cold acetone for 10 min, washed twice in Tris buffered saline + 0.05% Tween (TBST) for 5 min, incubated with 0.5mg/ml (175–350 U/ml) hyaluronidase (Sigma-Aldrich) in PBS for 20 min, washed in TBST, incubated with methanol + 3% hydrogen peroxide for 30 min, washed in TBST, blocked for 1h in 20% normal sheep serum (DAKO), 1% bovine serum albumin and incubated overnight at 4°C with primary polyclonal rabbit antibody directed against residues 1–100 of human WISP3/CCN6 (Abcam; 0.6μg/ml) or a non-immune rabbit isotype control (DAKO). Sections were washed and incubated with secondary biotinylated sheep anti-rabbit antibody (Serotec) for 30 min at room temperature, washed, incubated with streptavidin/HRP (StreptABC-HRP, DAKO) and then 3,3′-diaminobenzidine (DAB; Chromogen System, DAKO). The DAB chromogen was allowed to develop for an equal time on all sections and cell nuclei were counterstained with haematoxylin.
Cloning
The human WISP3/CCN6 gene was cloned from a cocktail of brain/placenta/testis/chondrocyte RNA by one-step RT-PCR using a QIAGEN® OneStep RT-PCR kit (Qiagen) with 50 picomoles of forward (5′-cgcgatgcaggggctcctctt-3′) and reverse (5′-tatattacagaatcttgagctcag-3′) primers. The 1kb PCR product was cloned into a pCR®2.1-TOPO® vector using TOPO®10 “One shot® Escherichia Coli” Cells and the TOPO® Cloning 5-minute PCR cloning kit (all Invitrogen™ Life Technologies) according to the manufacturer’s protocol. For stable transfection in C-28/I2 cells, which are resistant to geneticin (21), the WISP3/CCN6 insert was subcloned into the plasmid pcDNA3.1 Hygro (Invitrogen™ Life Technologies) that confers hygromycin resistance.
Cell culture
The generation of the immortalised chondrocytic cell line C-28/I2 is described in (21). C-28/I2 cells were maintained in DMEM (Gibco™ Invitrogen Corporation), 10% FCS (Biosera), 100U penicillin/streptomycin (Gibco-Invitrogen) and with 250μg/ml hygromycin B (Invitrogen) for transfected cells at 37°C, 5% CO2.
Stable transfection of C-28/I2 cells
5μg of pcDNA3.1 Hygro or pcDNA3.1 Hygro WISP3/CCN6 plasmid DNA was linearised with FspI and phenol/chloroform extracted. Twenty-four hours prior to transfection C-28/I2 cells were seeded at 100,000 cells per well into 6-well plates. Cells were transfected overnight in DMEM + 10% FBS with 1μg of pcDNA3.1 Hygro or pcDNA3.1 Hygro WISP3/CCN6 using FuGENE 6 transfection reagent (Roche) according to the manufacturer’s protocol. Forty-eight hours post-transfection, cells were changed to selective media and hygromycin-resistant cells were dilution plated to establish single cell clonal cell lines.
Suspension cell culture
For suspension culture 24-well tissue culture plates were coated with 0.1ml/cm2 of 12mg/ml polyHEMA (SigmaUltra, Sigma Aldrich) in ethanol (22) and allowed to dry. Cells were seeded at 190,000 cells/well in full medium and grown for 48h, centrifuged and re-suspended in 1ml serum-free medium and treated with 5ng/ml Interleukin 1α (IL-1α) and 10ng/ml oncostatin M (OSM) (both R&D Systems). In parallel cells were seeded in monolayer (38,000 cells/well; 24-well plates) in full growth media and after 48h incubated in serum-free medium for 1.5h, then stimulated with IL-1α and OSM as described above. RNA was extracted 24h post-cytokine treatment. For lithium chloride (LiCl) treatment, cells in monolayer were incubated with serum-free medium containing 40mM LiCl (SigmaUltra, Sigma Aldrich) or 40mM NaCl (as an osmolarity control) for 24h. For signal pathway analysis one control clone and one WISP3/CCN6 clone were seeded in monolayer and treated 24h later with 10μM U0126 (MEK1/2 inhibitor, Sigma-Aldrich), 10μM SB202190 (p38 MAPK inhibitor, Calbiochem) or 5μg/ml BMS 354451 (IκB kinase inhibitor, Calbiochem) as indicated.
Western blotting
Clonal C-28/I2 cell lines were seeded at the same density in T25 flasks and 24h later changed to serum-free media with 5μM BB94 (British Biotech Pharmaceuticals) and 10μg/ml Aprotinin, Leupetin, pepstatin A and soybean trypsin inhibitor (all Sigma), then incubated for a further 72h before cell lysis in RIPA buffer. Equal volumes of protein were investigated by Western blotting with primary rabbit anti-WISP3/CCN6 antibody (Abcam) and peroxidase conjugated donkey anti-rabbit secondary antibody (Jackson Laboratories) then developed with ECL™ Western blotting analysis reagents (Amersham Biosciences).
WISP3/CCN6 gene silencing
Primary chondrocytes were transfected with non-targeting control siRNA (Dharmacon) or siRNAs against WISP3/CCN6 (Dharmacon) at a final concentration of 50 nM using DharmaFECT transfection reagent. At 48h post-transfection cells were stimulated with IL-1α and OSM for 48h. Samples were lysed and reverse transcribed using Cells-to-cDNA (Ambion) protocol. WISP3/CCN6 knockdown and any subsequent effects on metalloproteinase expression was assessed by real time RT-PCR.
Results
Expression of WISP3/CCN6 is up-regulated in OA cartilage compared to post-mortem cartilage
WISP3/CCN6 steady state mRNA expression was assessed by real time RT-PCR on RNA extracted from different human tissues and cells. WISP3/CCN6 mRNA was highly expressed in cartilage and steady state levels of WISP3/CCN6 mRNA were 2-fold higher in OA cartilage than in post-mortem (PM) cartilage samples (Figure 1a). WISP3/CCN6 expression was considerably lower in the 27 other tissues studied (data not shown). To explore the elevated expression of WISP3/CCN6 in OA cartilage further, we performed immunohistochemistry for WISP3/CCN6 in OA and PM cartilage. Immunostaining for WISP3/CCN6 was observed in OA cartilage sections, often localised to pericellular areas (Figure 1b). Immunostaining for WISP3/CCN6 was barely detectable in undamaged PM cartilage (Figure 1b) or in undamaged cartilage from one OA knee (Figure 1c), while strong pericellular staining was observed in samples of damaged cartilage from the same OA knee (Figure 1c). Although PM donors used in this study were not diagnosed with or undergoing treatment for OA, some histological sections from PM donors displayed signs of cartilage damage. Interestingly, WISP3/CCN6 immunostaining was positive in these PM donors with OA-like changes and WISP3/CCN6 immunostaining was confined to damaged areas of the cartilage (Figure 1d). These results suggest WISP3/CCN6 protein is most highly expressed in damaged cartilage.
Figure 1. WISP3/CCN6 expression in OA versus PM cartilage.
(A) Real time RT-PCR for WISP3/CCN6 on mRNA from PM cartilage (20 different post-mortem samples) and OA cartilage (18 different osteoarthritic samples). * = P < 0.001. (B–D) Immunohistochemical staining for WISP3/CCN6 protein in cartilage sections. (B) Top: Pericellular staining in extensively damaged OA cartilage. Bottom: No staining in undamaged PM cartilage. (C) No staining in undamaged cartilage (top) but staining in damaged cartilage (bottom) from the knee of an OA donor with focal cartilage damage. (D) No staining in undamaged (top) but diffuse staining in damaged (bottom) areas of partially damaged PM cartilage. Scale bar = 100μm.
Stable transfection of WISP3/CCN6 in chondrocytic C-28/I2 cells
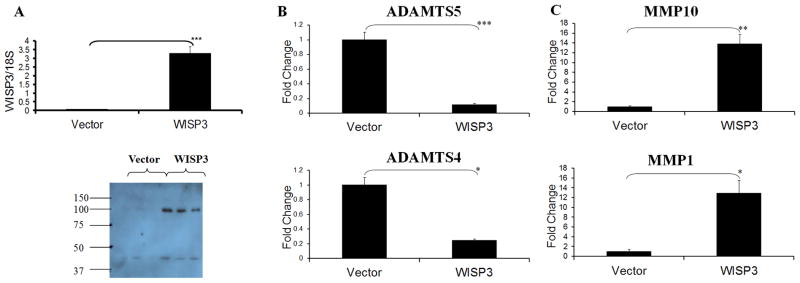
In order to explore the biological effects of elevated WISP3/CCN6 expression in chondrocytic cells, we stably transfected the WISP3/CCN6 gene into the immortalised chondrocytic cell line, C-28/I2. Three clonal cell lines stably over-expressing WISP3/CCN6 or stably transfected with the empty vector alone were generated. Real time RT-PCR confirmed that the clonal cell lines transfected with WISP3/CCN6 were over-expressing WISP3/CCN6 mRNA compared to the empty vector transfected clones (Figure 2a). Expression of the WISP3/CCN6 protein was confirmed by western blotting of lysates from clonal cell lines grown in the presence of protease inhibitors (Figure 2a). A protein band was observed at the predicted size of 39 KDa in WISP3/CCN6 clones as well as a larger band at 100 KDa, which may represent dimers or trimers of the protein. In one empty vector clone a low level of WISP3/CCN6 protein expression was observed. Note that in the absence of protease inhibitors, WISP3/CCN6 protein was undetectable (data not shown). Other CCNs are known to be cleaved by MMPs (23).
Figure 2. WISP3/CCN6 and metalloproteinase expression in clonal cell lines.
WISP3/CCN6 and metalloproteinase expression were determined in clonal cell lines stably over-expressing the empty vector pcDNA3.1 hygro (vector) or pcDNA3.1 hygro + WISP3/CCN6 (WISP3). (A) Top: WISP3/CCN6 steady state mRNA expression measured by real time RT-PCR. Bottom: anti-WISP3/CCN6 western blot of cell lysates. (B–C) Real time RT-PCR for metalloproteinase mRNA. (B) ADAMTS5 (top) and ADAMTS4 (bottom) expression. (C) MMP10 (top) and MMP1 (bottom) expression. *, **, *** = a significant difference where P is ≤ 0.05, 0.01 and 0.001 respectively. All real time RT-PCR was normalised to expression of the house keeping gene 18S. Each bar is the mean of the fold change in three clones ± s.e.m compared to the clones stably over-expressing the empty vector.
Metalloproteinase expression in clonal C-28/I2 cell lines stably transfected with WISP3/CCN6
To determine the consequences of WISP3/CCN6 over-expression in C-28/I2 cells, we evaluated the steady state levels of mRNA for metalloproteinase genes in the three clonal WISP3/CCN6 over-expressing cell lines and the three clonal empty vector transfected cell lines (Table 1). Of particular interest was the strong down-regulation of the levels of ADAMTS5 mRNA (9-fold, P ≤0.001, Figure 2b) and ADAMTS4 mRNA (4-fold, P ≤0.05, Figure 2b) in WISP3/CCN6 over-expressing clones. Conversely, the steady state mRNA levels of MMP1 and MMP10 were elevated 13-fold (P ≤0.05) and 14-fold (P ≤0.01) respectively in the WISP3/CCN6 clones compared to empty vector clones (Figure 2c). We confirmed that the induction of MMP10 was clearly observed at the protein level (Supplementary Figure 1) and the repression of ADAMTS5 may be detectable at the protein level but requires further investigation (data not shown).
Table 1. Changes in steady state mRNA levels of metalloproteinases in WISP3/CCN6 over-expressing clonal cell lines versus control clones.
The expression of MMP and ADAMTS mRNA in three empty vector C-28/I2 clones and three WISP3/CCN6 over-expressing C-28/I2 clones was measured by real time RT-PCR and normalised to 18S expression. Fold changes in gene expression in the WISP3/CCN6 clones compared to the empty vector clones are indicated where differences in expression were significant (p ≤ 0.05).
| Metalloprotienase | Regulation in WISP3/CCN6 over-expressing cells | |
|---|---|---|
| MMP1 | ↑ 13 Fold | * P<0.05 |
| MMP2 | ↓ 2 Fold | * P<0.05 |
| MMP3 | No effect | |
| MMP7 | No effect | |
| MMP8 | No expression | |
| MMP9 | No effect | |
| MMP10 | ↑ 14 Fold | ** P<0.01 |
| MMP11 | ↓ 1.5 Fold | * P<0.05 |
| MMP12 | No expression | |
| MMP13 | No effect | |
| MMP14 | ↓2 Fold | * P<0.05 |
| MMP15 | ↓ 2.5 Fold | ** P<0.01 |
| ADAMTS1 | No effect | |
| ADAMTS4 | ↓ 4 Fold | * P<0.05 |
| ADAMTS5 | ↓ 9 Fold | *** P<0.001 |
| ADAMTS8 | No expression | |
| ADAMTS9 | No effect | |
| ADAMTS15 | No effect | |
It was particularly interesting that WISP3/CCN6 over-expression modulated ADAMTS5 and MMP10 expression in C-28/I2 cells, as ADAMTS5 is a major aggrecanase and MMP10 may be involved in formation of bony osteophytes in OA (24). We determined if the modulation of ADAMTS5 and MMP10 expression by WISP3/CCN6 was still observed when cells were grown in 3D rather than in monolayer, since C-28/I2 cells grown in 3D culture have a more chondrocytic phenotype (25). Three WISP3/CCN6 over-expressing clones and three empty vector clones were seeded on tissue culture plastic coated with polyHEMA in order to prevent cell adhesion to the substratum. Cells grew in large groups forming nodule-like structures (data not shown). Suppression of ADAMTS5 expression was maintained in WISP3/CCN6-expressing clones relative to expression in the empty vector transfected clones (Figure 3a). In fact, growth in suspension significantly increased ADAMTS5 expression in the empty vector clones, but it did not increase in the WISP3/CCN6 clones (Figure 3a). Meanwhile, MMP10 expression was further enhanced in the WISP3/CCN6 clones grown in suspension (Figure 3a).
Figure 3. ADAMTS5 and MMP10 expression in monolayer versus 3D culture in the presence and absence of cytokines.
Real time RT-PCR normalised to 18S. (A) ADAMTS5 and MMP10 expression in clonal cell lines stably over-expressing the empty vector (vector) or in clonal cell lines stably over-expressing WISP3/CCN6 (WISP3) grown in monolayer or in suspension over polyhema. Each bar is the mean of the fold change in three clones ± s.e.m compared to the clones stably over-expressing the empty vector in monolayer culture. (B) ADAMTS5 and MMP10 expression in one empty vector clone (vector) and one WISP3/CCN6 clone (WISP3) grown in monolayer in serum free medium (SF) or SF supplemented with 5ng/ml IL-1α and 10ng/ml OSM (IL-1+OSM). (C) ADAMTS5 and MMP10 in one empty vector clone (vector) and one WISP3/CCN6 clone (WISP3) grown in suspension over polyhema in serum free medium (SF) or SF supplemented with 5ng/ml IL-1α and 10ng/ml OSM (IL-1+OSM). Each bar in (B) and (C) is the mean of the fold change in three wells ± s.e.m compared to cells stably over-expressing the empty vector in control conditions (SF, monolayer culture). *, **, *** = significant difference in expression where P is ≤ 0.05, 0.01 and 0.001 respectively.
Inflammation can contribute to the progression of OA (26). To determine the effects of WISP3/CCN6 over-expression in an inflammatory environment, cells were treated with interleukin 1α (IL-1α) and oncostatin M (OSM), which are known to stimulate metalloproteinase expression and matrix catabolism in cartilage explant cultures (27). In both monolayer and suspension cultures these cytokines stimulated the expression of ADAMTS5 mRNA in empty vector cells and in WISP3/CCN6-cells, partially reversing WISP3/CCN6 suppression of ADAMTS5 in both monolayer (Figure 3b) and suspension (Figure 3c) culture. However, overall expression of ADAMTS5 still remained significantly lower in the cytokine-treated WISP3/CCN6-expressing cells compared to the cytokine-treated empty vector cells (Figure 3b, 3c). Meanwhile, cytokines completely de-repressed the suppression of ADAMTS4 expression in WISP3/CCN6-expressing cells in monolayer culture (Supplementary Figure 2). Cytokine treatment had no effect on MMP10 mRNA levels in the empty vector cell line, but completely abrogated induction of MMP10 to control levels in WISP3/CCN6-expressing cells (Figure 3b, 3c). MMP1 expression in WISP3/CCN6-expressing cells grown in suspension and treated with cytokines was slightly but not significantly repressed (Supplementary Figure 2).
To determine potential pathway(s) involved in WISP3/CCN6 regulation of ADAMTS5 and MMP10 expression, cells were treated with inhibitors of MEK1/2, p38 MAPK and IκB kinase (IKK). These inhibitors did not reverse the suppression of ADAMTS5 expression in WISP3/CCN6-expressing cells, but each reduced WISP3/CCN6 induced MMP10 expression by 50% (Figure 4a), suggesting that WISP3/CCN6 regulation of MMP10 and ADAMTS5 expression may be operated via different pathways. Meanwhile, the IKK inhibitor reduced ADAMTS5 expression in both the empty vector and WISP3/CCN6-expressing cells, suggesting that NFκB signalling regulates ADAMTS5 expression in C-28/I2 cells.
Figure 4. ADAMTS5 and MMP10 expression in clonal cell lines treated with pathway inhibitors or LiCl.
(A) ADAMTS5 and MMP10 expression measured by real time RT-PCR in one empty vector clone (vector) and one WISP3/CCN6 clone (WISP3) incubated in low serum (0.5%) growth medium (control) or 10μM U0126 (U0126, a MEK1/2 inhibitor), 10μM SB202190 (SB, a p38 MAPK inhibitor) or 5μg/ml BMS 354451 (BMS, a IκB kinase inhibitor) in low serum (0.5%) growth medium for 6.5h. Each bar is the mean of the fold change in three wells ± s.e.m compared to control cells stably over-expressing the empty vector in control conditions. (B) ADAMTS5 and MMP10 expression in three empty vector clones (vector) and three WISP3/CCN6 clones (WISP3) incubated in 40mM NaCl (as an osmolarity control) or 40mM LiCl for 24h. Each bar is the mean of the fold change in three clones ± s.e.m compared to the clones stably over-expressing the empty vector in NaCl. MMP10 and ADAMTS5 expression is normalised to 18S. *, **, *** = a significant difference where P is ≤ 0.05, 0.01 and 0.001 respectively.
WISP3/CCN6 has previously been shown to inhibit Wnt/β-catenin signalling in zebrafish and in human embryonic kidney and mouse embryo cell lines (28). We treated our clonal cell lines with lithium chloride (LiCl), which can stimulate β-catenin signalling by inhibiting glycogen synthase kinase 3β (GSK3β) (29). LiCl had no effect on empty vector clones, but partially reversed WISP3/CCN6 suppression of ADAMTS5 and enhanced MMP10 expression in WISP3/CCN6 clones (Figure 4b). This suggests that WISP3/CCN6 and β-catenin signalling may interact in C-28/I2 cells and this may affect MMP10 and ADAMTS5 expression. In agreement with these findings, treatment of the clonal cell lines with a GSK3β inhibitor, SB216763 (29), produced similar results. SB216763 more potently enhanced MMP10 expression in WISP3/CCN6 over-expressing cells (Supplementary Figure 3) and also increased ADAMTS5 expression in WISP3/CCN6 over-expressing cells, although this did not reach statistical significance (P = 0.056, Supplementary Figure 3).
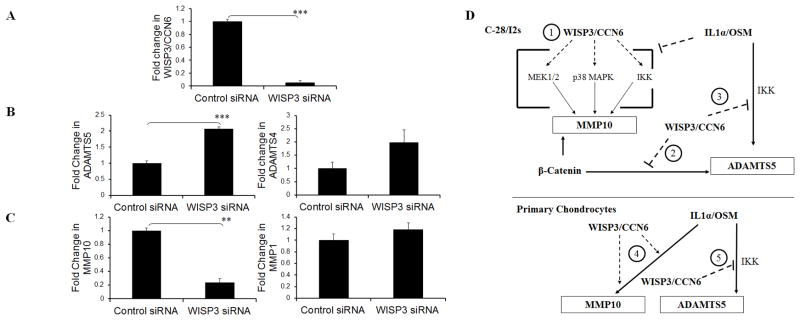
We took a gene silencing approach in order to determine if modulation of WISP3/CCN6 expression also affected metalloproteinase expression in primary chondrocytes. WISP3/CCN6 siRNA transfection resulted in a 90% reduction of WISP3/CCN6 mRNA (Figure 5a) and elevation of ADAMTS5 expression in 4 independent cell isolates treated with cytokines (representative cell isolate, Figure 5b). ADAMTS4 expression showed a trend towards increased expression (Figure 5b). Conversely, MMP10 expression was suppressed (Figure 5c) but MMP1 was slightly increased (Figure 5c).
Figure 5. Effect of WISP3/CCN6 knockdown in primary chondrocytes and potential model for WISP3/CCN6 function in C-28/I2 cells and chondrocytes.
(A–C): Silencing of WISP3/CCN6 expression (A, WISP3 siRNA) significantly increased ADAMTS5 (B) and decreased MMP10 (C) expression in primary chondrocytes treated with IL-1α/OSM. Each bar is the mean expression (Real time PCR normalized to 18S) of the fold change in three replicates ± s.e.m relative to cells transfected with control siRNA, treated with IL-1α/OSM (Control siRNA). **, *** = a significant difference where P is ≤ 0.01 and 0.001 respectively. (C): Model: C-28/I2 cells, (1): MEK1/2, p38 MAPK and IKK are involved in WISP3/CCN6-induction of MMP10 expression but in presence of IL1α /OSM, WISP3/CCN6 does not induce MMP10 expression. (2) WISP3/CCN6 suppression of ADAMTS5 expression may involve suppression of β-catenin signalling, although another pathway, perhaps opposing cytokine signalling, may be involved (3). β-catenin enhances WISP3/CCN6 induction of MMP10 by an unknown mechanism. This also demonstrates the complex and context dependent regulation of gene expression by WISP3/CCN6, which may interact with canonical Wnt/β-catenin signalling in both positive and negative ways. In primary chondrocytes, WISP3/CCN6 induces MMP10 expression (4) and suppresses ADAMTS5 expression (5) in the presence of cytokines by unknown mechanisms.
Discussion
We have shown that WISP3/CCN6 is significantly elevated in end stage OA cartilage compared to clinically normal cartilage. A key finding in this study is that stable WISP3/CCN6 over-expression in chondrocytic C-28/I2 cells led to a down-regulation in the steady state levels of ADAMTS4 and ADAMTS5 mRNA. If WISP3/CCN6 has the same activity in vivo, this may suggest that WISP3/CCN6 is up-regulated in response to cartilage damage as a feedback mechanism following early induction of ADAMTS5, in order to suppress aggrecanase mRNA levels, which are known to decrease in end stage OA (30;31).
ADAMTS5 seems to be a key player in aggrecan degradation (reviewed in (32)) and few agents are known to suppress ADAMTS5 expression. Glucosamine and chondroitin sulphate supplements can decrease IL-1 induced ADAMTS5 expression in bovine cartilage explants (33) and incorporation of the n-3 polyunsaturated fatty acid eicosapentaenoic acid into bovine articular chondrocyte membranes significantly decreases ADAMTS5 mRNA expression induced by IL-1α (34). Importantly, several recent studies show a key role for fibroblast factor (FGF)-2 in ADAMTS5 suppression. FGF-2 suppresses IL-1α-induced ADAMTS5 expression in human chondrocytes and inhibits GAG release in cartilage explants induced by cytokines or retinoic acid (35), and reduces basal ADAMTS5 mRNA expression in un-stimulated human chondrocytes (36). Furthermore, FGF2−/− mice have elevated levels of ADAMTS5 mRNA and accelerated OA following surgical induction of joint instability, and expression can be reduced to wild type levels by subcutaneous administration of recombinant FGF-2 (37).
WISP3/CCN6 suppression of ADAMTS4 and ADAMTS5 may explain the development of postnatal cartilage damage in PPRD patients lacking wild type WISP3/CCN6. Metalloproteinase expression has only been examined in chondrocytes from one PPRD patient to date and ADAMTS5 expression was not increased in these cells lacking wild type WISP3/CCN6 compared to a control (7). It would be interesting to investigate the induction of aggrecanases in PPRD chondrocytes upon mechanical injury or loading of chondrocytes, because this may be the key event that induces WISP3/CCN6 activity and later suppresses ADAMTS4 and ADAMTS5. Interestingly, mice lacking full length WISP3/CCN6 protein do not show any phenotypic similarities to PPRD patients (38). Similarly, unchallenged mice deficient in ADAMTS5 activity do not have an obvious phenotype (15;16). One possible explanation for the lack of a phenotype in WISP3/CCN6-deficient mice is that WISP3/CCN6 is induced as part of a repair response. A potential mechanism for induction of WISP3/CCN6 expression in response to injury is via FGF-2, which is stored in the pericellular matrix surrounding cartilage chondrocytes (39) and is released in response to porcine cartilage injury (40) and mechanical loading (41).
In our initial screen, MMP1 and MMP10 expression were strongly up-regulated in WISP3/CCN6 over-expressing C-28/I2 cells. MMP1 (collagenase-1) expression is elevated in OA knee cartilage compared to normal cartilage (42). MMP10 mRNA is also up-regulated by cytokines in primary human articular chondrocytes, synovial fibroblasts and chondrosarcoma cells (43;44). Moreover, recombinant MMP10 enhances IL-1α/OSM induced collagenolysis of bovine cartilage explants, probably relating to MMP10 activation of pro-MMP1 (43). The up-regulation of MMP10 in WISP3/CCN6 over-expressing cells therefore complements the up-regulation of MMP1. MMP10 protein is present in the synovial fluid from OA, RA, and JIA patients and in the cartilage and synovial tissues from OA and RA patients (43). It may therefore seem surprising that WISP3/CCN6 over-expression is associated with up-regulation of MMP1 and MMP10 when WISP3/CCN6 is hypothesised above to be a chondroprotective gene. However, chondroprotective FGF-2 suppresses ADAMTS5 but also induces MMP1 expression in porcine chondrocytes (40), human articular cartilage explants (35) and human chondrocytes (36). It thus could be argued that MMP1 and MMP10 are involved in matrix remodelling for attempted reparative/anabolic responses in OA. In support of this hypothesis, MMP10 expression is associated with osteophyte formation (24). Interestingly, WISP3/CCN6 promotes the migration of mesenchymal stromal cells (4). Although chondrocyte migration in vivo remains little studied, one could envisage that WISP3/CCN6-mediated induction of MMP1 and MMP10 may promote migration of chondrocytes in an attempted wound healing response through release of matrix-bound factors such as FGF-2.
ADAMTS5 expression has previously been shown to increase in chondrocytes cultured in alginate beads (45). In the current study, culture in a 3D environment elevated the expression of ADAMTS5 in empty vector clones, but not in the WISP3/CCN6 clones, suggesting that the suppressive effect of WISP3/CCN6 on ADAMTS5 expression can inhibit or out-compete stimulatory signalling induced by cell shape changes. Conversely, 3D culture further enhanced the inductive effect of WISP3/CCN6 on MMP10 expression in C-28/I2 cells and increased MMP10 expression in empty vector clones. Treatment of cells with IL-1α/OSM increased ADAMTS5 expression in both empty vector and WISP3/CCN6 expressing cells cultured either in 2D or 3D environments. However, ADAMTS5 expression remained significantly lower in WISP3/CCN6 clones treated with IL-1α/OSM compared to IL-1α/OSM-treated empty vector clones, suggesting that WISP3/CCN6 can still have a suppressive effect on ADAMTS5 expression in an inflammatory environment. Surprisingly, IL-1α/OSM strongly suppressed WISP3/CCN6-induced MMP10 expression, but had no effect on this proteinase in empty vector cells. It is possible that in C-28/I2 cells the WISP3/CCN6 pathway that induces MMP10 expression is suppressed in the presence of inflammatory cytokines as a protective mechanism. Taken together, these results suggest that different signalling pathways are involved in WISP3/CCN6 regulation of these two metalloproteinases in C-28/I2 cells.
We took a pharmacological approach to begin to uncover potential signalling pathways involved in WISP3/CCN6 modulation of metalloproteinase expression. The IKK inhibitor BMS354451 suppressed basal ADAMTS5 expression in vector cells and further reduced ADAMTS5 expression in WISP3/CCN6 over-expressing cells, indicating ADAMTS5 expression is normally stimulated by the NFκB pathway in C-28/I2 cells, mediated possibly by endogenous stress responses. ADAMTS5 expression is also induced by NFκB in nucleus pulposus cells (46). Partial suppression of WISP3/CCN6-induced MMP10 expression by all signalling pathway inhibitors tested suggests that this growth factor can impact on multiple regulatory networks.
We hypothesised that WISP3/CCN6 may suppress ADAMTS5 expression by inhibition of canonical Wnt/β-catenin signalling, since WISP3/CCN6 harbouring the mutations associated with cartilage degeneration lacks the ability to inhibit canonical Wnt signalling (28). Others have shown that canonical Wnt/β-catenin signalling induces ADAMTS5 expression in chick and rabbit chondrocytes (47;48). An interpretation of our data is that WISP3/CCN6 suppresses ADAMTS5 expression by repression of basal β-catenin signalling, which is relieved by LiCl. Stimulation of Wnt/β-catenin signalling did not affect MMP10 expression in empty vector clones but strongly enhanced MMP10 expression in WISP3/CCN6 over-expressing cells. Thus, in contrast to ADAMTS5 expression, β-catenin may be positively involved in WISP3/CCN6 induction of MMP10 or can synergise with the WISP3/CCN6 signalling pathway that induces MMP10 expression. Interestingly, Wnt/β-catenin signalling induces osteophyte formation in animal models (49) and MMP10 is expressed in osteophytes (24). WISP3/CCN6 could interact with Wnt/β-catenin activated processes in both positive and negative ways to modulate the outcome of Wnt/β-catenin signalling reflecting the context-dependence of this pathway. Other CCN proteins can affect Wnt/β-catenin activated pathways both positively and negatively in Xenopus Laevis (50).
In primary chondrocytes, WISP3/CCN6 gene silencing revealed effects which partially mirrored those seen by overexpression of WISP3/CCN6 in C-28/I2 cells. Figure 5d summarizes WISP-3/CCN6 regulation of ADAMTS5 and MMP10 in C-28/I2 cells and primary chondrocytes. Mechanisms underlying the changes in ADAMTS5 and MMP10, following depletion of WISP3/CCN6 in primary chondrocytes, remain to be determined. The contrasting effects of WISP3/CCN6 on MMP10 and MMP1 expression in the presence of cytokines in primary chondrocytes and C-28/I2 cells may reflect differences in signalling pathways and/or epigenetic effects in these two cellular contexts. Importantly the effect of WISP3/CCN6 on ADAMTS5 expression was confirmed in primary chondrocytes and the overall anti-catabolic effects suggest important future areas of investigation with this CCN family member in OA.
In conclusion, WISP3/CCN6 is over-expressed in end stage OA cartilage and our in vitro studies suggest this late expression may be an attempt by cartilage to reduce further damage through inhibition of aggrecan breakdown. The mechanisms by which WISP3/CCN6 regulates metalloproteinase expression are unknown, but the current study suggests that in certain circumstances WISP3/CCN6 can regulate ADAMTS5 and MMP10 by distinct pathways and could modulate existing signalling cascades, thereby resulting in complex context-dependent control of metalloproteinase expression.
Supplementary Material
MMP10 protein is induced by WISP3/CCN6 over-expression in C-28/I2 cells. Clonal cell lines stably over-expressing the empty vector pcDNA3.1 hygro (vector) or pcDNA3.1 hygro + WISP3/CCN6 (WISP3) were changed to serum-free medium during the logarithmic phase of growth and conditioned for 3 days. Conditioned media samples were concentrated by TCA precipitation and re-suspended in reducing sample buffer prior to separation on a 10% SDS-PAGE gel, transfer and incubation with sheep-anti-MMP10 antibody (Hembry et al., 1995, Annals Rheum Dis, 54: 25–32) and a peroxidase-conjugated donkey anti-sheep secondary antibody and subsequent ECL analysis according to the manufacturer’s instructions.
ADAMTS4 and MMP1 expression in monolayer and 3D culture in the presence and absence of cytokines. Real time RT-PCR normalised to 18S. (A) ADAMTS4 and MMP1 expression in one empty vector clone (vector) and one WISP3/CCN6 clone (WISP3) grown in monolayer in serum free medium (SF) or SF supplemented with 5ng/ml IL-1α and 10ng/ml OSM (IL-1+OSM). (B) ADAMTS4 and MMP1 expression in one empty vector clone (vector) and one WISP3/CCN6 clone (WISP3) grown in suspension over polyhema in serum free medium (SF) or SF supplemented with 5ng/ml IL-1α and 10ng/ml OSM (IL-1+OSM). Each bar is the mean of the fold change in three wells ± s.e.m compared to cells stably over-expressing the empty vector in control conditions (SF, monolayer culture). *, **, *** = significant difference in expression where P is ≤ 0.05, 0.01 and 0.001 respectively.
MMP10 expression is further induced by a GSK3β inhibitor in a WISP3/CCN6 over-expressing clone. Two-step Taqman real time PCR analysis was performed as described in Methods. Expression of MMP10 in the WISP3/CCN6 over–expressing clone is further enhanced by incubation with the GSK3β inhibitor, SB216763 (5μM). ADAMTS5 expression is partially de-repressed in the presence of this inhibitor but this does not quite reach statistical significance (p= 0.0506). Incubation of this clone with LiCl is shown for comparison.
Acknowledgments
We thank our colleagues in the Gavrilovic lab and in the Cellular Protease Group at UEA and in Astra Zeneca, RIRA, especially Dr WM Abbot and MJ Snow for helpful discussions throughout this project.
Work in Jelena Gavrilovic’s laboratory was supported by a BBSRC Industrial Partner CASE Studentship with Astra Zeneca and an Action Arthritis grant. Work in Mary Goldring’s laboratory was supported by National Institutes of Health Grants R01-AG022021 and RC4 AR060546, and an Arthritis Foundation Postdoctoral Fellowship. Paul Sharpe, Peter Newham, Wendy Barker, Kristen M Clements and Caroline J Langham have stock or stock options in AstraZeneca.
Contributor Information
Natasha Baker, School of Biological Sciences, University of East Anglia, Norwich, UK.
Paul Sharpe, RIRA, Alderley Park, AstraZeneca Discovery, UK.
Kirsty Culley, Hospital for Special Surgery, Weill Cornell Medical College, New York, NY, USA.
Miguel Otero, Hospital for Special Surgery, Weill Cornell Medical College, New York, NY, USA.
Damon Bevan, School of Biological Sciences, University of East Anglia, Norwich, UK.
Peter Newham, RIRA, Alderley Park, AstraZeneca Discovery, UK.
Wendy Barker, RIRA, Alderley Park, AstraZeneca Discovery, UK.
Kristen M Clements, RIRA, Alderley Park, AstraZeneca Discovery, UK.
Caroline J Langham, RIRA, Alderley Park, AstraZeneca Discovery, UK.
Mary B Goldring, Hospital for Special Surgery, Weill Cornell Medical College, New York, NY, USA.
Jelena Gavrilovic, School of Biological Sciences, University of East Anglia, Norwich, UK.
References
- 1.Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, et al. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci U S A. 1998;95(25):14717–14722. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurvitz JR, Suwairi WM, Van Hul W, El Shanti H, Superti-Furga A, Roudier J, et al. Mutations in the CCN gene family member WISP3 cause progressive pseudorheumatoid dysplasia. Nat Genet. 1999;23(1):94–98. doi: 10.1038/12699. [DOI] [PubMed] [Google Scholar]
- 3.Lamb R, Thomson W, Ogilvie E, Donn R. Wnt-1-inducible signaling pathway protein 3 and susceptibility to juvenile idiopathic arthritis. Arthritis Rheum. 2005;52(11):3548–3553. doi: 10.1002/art.21392. [DOI] [PubMed] [Google Scholar]
- 4.Schutze N, Schenk R, Fiedler J, Mattes T, Jakob F, Brenner RE. CYR61/CCN1 and WISP3/CCN6 are chemoattractive ligands for human multipotent mesenchymal stroma cells. BMC Cell Biol. 2007;8:45. doi: 10.1186/1471-2121-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sen M, Cheng YH, Goldring MB, Lotz MK, Carson DA. WISP3-dependent regulation of type II collagen and aggrecan production in chondrocytes. Arthritis Rheum. 2004;50(2):488–497. doi: 10.1002/art.20005. [DOI] [PubMed] [Google Scholar]
- 6.Kawaki H, Kubota S, Suzuki A, Lazar N, Yamada T, Matsumura T, et al. Cooperative regulation of chondrocyte differentiation by CCN2 and CCN3 shown by a comprehensive analysis of the CCN family proteins in cartilage. J Bone Miner Res. 2008;23(11):1751–1764. doi: 10.1359/jbmr.080615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou HD, Bu YH, Peng YQ, Xie H, Wang M, Yuan LQ, et al. Cellular and molecular responses in progressive pseudorheumatoid dysplasia articular cartilage associated with compound heterozygous WISP3 gene mutation. J Mol Med. 2007;85(9):985–996. doi: 10.1007/s00109-007-0193-2. [DOI] [PubMed] [Google Scholar]
- 8.Pratta MA, Yao W, Decicco C, Tortorella MD, Liu RQ, Copeland RA, et al. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278(46):45539–45545. doi: 10.1074/jbc.M303737200. [DOI] [PubMed] [Google Scholar]
- 9.Murphy G, Nagase H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheumatol. 2008;4(3):128–135. doi: 10.1038/ncprheum0727. [DOI] [PubMed] [Google Scholar]
- 10.Xu L, Peng H, Glasson S, Lee PL, Hu K, Ijiri K, et al. Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007;56(8):2663–2673. doi: 10.1002/art.22761. [DOI] [PubMed] [Google Scholar]
- 11.Sandy JD, Flannery CR, Neame PJ, Lohmander LS. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest. 1992;89(5):1512–1516. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohmander LS, Neame PJ, Sandy JD. The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 1993;36(9):1214–1222. doi: 10.1002/art.1780360906. [DOI] [PubMed] [Google Scholar]
- 13.Abbaszade I, Liu RQ, Yang F, Rosenfeld SA, Ross OH, Link JR, et al. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem. 1999;274(33):23443–23450. doi: 10.1074/jbc.274.33.23443. [DOI] [PubMed] [Google Scholar]
- 14.Tortorella MD, Burn TC, Pratta MA, Abbaszade I, Hollis JM, Liu R, et al. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science. 1999;284(5420):1664–1666. doi: 10.1126/science.284.5420.1664. [DOI] [PubMed] [Google Scholar]
- 15.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434(7033):648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 16.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 17.Gendron C, Kashiwagi M, Lim NH, Enghild JJ, Thogersen IB, Hughes C, et al. Proteolytic activities of human ADAMTS-5: comparative studies with ADAMTS-4. J Biol Chem. 2007;282(25):18294–18306. doi: 10.1074/jbc.M701523200. [DOI] [PubMed] [Google Scholar]
- 18.Plaas A, Osborn B, Yoshihara Y, Bai Y, Bloom T, Nelson F, et al. Aggrecanolysis in human osteoarthritis: confocal localization and biochemical characterization of ADAMTS5-hyaluronan complexes in articular cartilages. Osteoarthritis Cartilage. 2007;15(7):719–734. doi: 10.1016/j.joca.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Ijiri K, Zerbini LF, Peng H, Otu HH, Tsuchimochi K, Otero M, et al. Differential expression of GADD45beta in normal and osteoarthritic cartilage: potential role in homeostasis of articular chondrocytes. Arthritis Rheum. 2008;58(7):2075–2087. doi: 10.1002/art.23504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koshy PJ, Lundy CJ, Rowan AD, Porter S, Edwards DR, Hogan A, et al. The modulation of matrix metalloproteinase and ADAM gene expression in human chondrocytes by interleukin-1 and oncostatin M: a time-course study using real-time quantitative reverse transcription-polymerase chain reaction. Arthritis Rheum. 2002;46(4):961–967. doi: 10.1002/art.10212. [DOI] [PubMed] [Google Scholar]
- 21.Goldring MB, Birkhead JR, Suen LF, Yamin R, Mizuno S, Glowacki J, et al. Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J Clin Invest. 1994;94(6):2307–2316. doi: 10.1172/JCI117595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aggeler J, Frisch SM, Werb Z. Changes in cell shape correlate with collagenase gene expression in rabbit synovial fibroblasts. J Cell Biol. 1984;98(5):1662–1671. doi: 10.1083/jcb.98.5.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem. 2002;277(39):36288–36295. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- 24.Bord S, Horner A, Hembry RM, Compston JE. Stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10) expression in developing human bone: potential roles in skeletal development. Bone. 1998;23(1):7–12. doi: 10.1016/s8756-3282(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 25.Goldring MB. Culture of immortalized chondrocytes and their use as models of chondrocyte function. Methods Mol Med. 2004;100:37–52. doi: 10.1385/1-59259-810-2:037. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39(1–2):237–246. [PubMed] [Google Scholar]
- 27.Cawston TE, Ellis AJ, Humm G, Lean E, Ward D, Curry V. Interleukin-1 and oncostatin M in combination promote the release of collagen fragments from bovine nasal cartilage in culture. Biochem Biophys Res Commun. 1995;215(1):377–385. doi: 10.1006/bbrc.1995.2476. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura Y, Weidinger G, Liang JO, Aquilina-Beck A, Tamai K, Moon RT, et al. The CCN family member Wisp3, mutant in progressive pseudorheumatoid dysplasia, modulates BMP and Wnt signaling. J Clin Invest. 2007;117(10):3075–3086. doi: 10.1172/JCI32001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7(10):793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 30.Kevorkian L, Young DA, Darrah C, Donell ST, Shepstone L, Porter S, et al. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50(1):131–141. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
- 31.Davidson RK, Waters JG, Kevorkian L, Darrah C, Cooper A, Donell ST, et al. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res Ther. 2006;8(4):R124. doi: 10.1186/ar2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fosang AJ, Rogerson FM, East CJ, Stanton H. ADAMTS-5: the story so far. Eur Cell Mater. 2008;15:11–26. doi: 10.22203/ecm.v015a02. [DOI] [PubMed] [Google Scholar]
- 33.Chan PS, Caron JP, Orth MW. Effect of glucosamine and chondroitin sulfate on regulation of gene expression of proteolytic enzymes and their inhibitors in interleukin-1-challenged bovine articular cartilage explants. Am J Vet Res. 2005;66(11):1870–1876. doi: 10.2460/ajvr.2005.66.1870. [DOI] [PubMed] [Google Scholar]
- 34.Zainal Z, Longman AJ, Hurst S, Duggan K, Caterson B, Hughes CE, et al. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthritis Cartilage. 2009;17(7):896–905. doi: 10.1016/j.joca.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Sawaji Y, Hynes J, Vincent T, Saklatvala J. Fibroblast growth factor 2 inhibits induction of aggrecanase activity in human articular cartilage. Arthritis Rheum. 2008;58(11):3498–3509. doi: 10.1002/art.24025. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Song Y, Jacobi JL, Tuan RS. Inhibition of histone deacetylases antagonized FGF2 and IL-1beta effects on MMP expression in human articular chondrocytes. Growth Factors. 2009;27(1):40–49. doi: 10.1080/08977190802625179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chia SL, Sawaji Y, Burleigh A, McLean C, Inglis J, Saklatvala J, et al. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 2009;60(7):2019–2027. doi: 10.1002/art.24654. [DOI] [PubMed] [Google Scholar]
- 38.Kutz WE, Gong Y, Warman ML. WISP3, the gene responsible for the human skeletal disease progressive pseudorheumatoid dysplasia, is not essential for skeletal function in mice. Mol Cell Biol. 2005;25(1):414–421. doi: 10.1128/MCB.25.1.414-421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vincent TL, McLean CJ, Full LE, Peston D, Saklatvala J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthritis Cartilage. 2007;15(7):752–763. doi: 10.1016/j.joca.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Vincent T, Hermansson M, Bolton M, Wait R, Saklatvala J. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc Natl Acad Sci U S A. 2002;99(12):8259–8264. doi: 10.1073/pnas.122033199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vincent TL, Hermansson MA, Hansen UN, Amis AA, Saklatvala J. Basic fibroblast growth factor mediates transduction of mechanical signals when articular cartilage is loaded. Arthritis Rheum. 2004;50(2):526–533. doi: 10.1002/art.20047. [DOI] [PubMed] [Google Scholar]
- 42.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44(3):585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 43.Barksby HE, Milner JM, Patterson AM, Peake NJ, Hui W, Robson T, et al. Matrix metalloproteinase 10 promotion of collagenolysis via procollagenase activation: implications for cartilage degradation in arthritis. Arthritis Rheum. 2006;54(10):3244–3253. doi: 10.1002/art.22167. [DOI] [PubMed] [Google Scholar]
- 44.Barksby HE, Hui W, Wappler I, Peters HH, Milner JM, Richards CD, et al. Interleukin-1 in combination with oncostatin M up-regulates multiple genes in chondrocytes: implications for cartilage destruction and repair. Arthritis Rheum. 2006;54(2):540–550. doi: 10.1002/art.21574. [DOI] [PubMed] [Google Scholar]
- 45.Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46(10):2648–2657. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- 46.Seguin CA, Bojarski M, Pilliar RM, Roughley PJ, Kandel RA. Differential regulation of matrix degrading enzymes in a TNFalpha-induced model of nucleus pulposus tissue degeneration. Matrix Biol. 2006;25(7):409–418. doi: 10.1016/j.matbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Tamamura Y, Otani T, Kanatani N, Koyama E, Kitagaki J, Komori T, et al. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280(19):19185–19195. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- 48.Yuasa T, Otani T, Koike T, Iwamoto M, Enomoto-Iwamoto M. Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration. Lab Invest. 2008;88(3):264–274. doi: 10.1038/labinvest.3700747. [DOI] [PubMed] [Google Scholar]
- 49.Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13(2):156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 50.Latinkic BV, Mercurio S, Bennett B, Hirst EM, Xu Q, Lau LF, et al. Xenopus Cyr61 regulates gastrulation movements and modulates Wnt signalling. Development. 2003;130(11):2429–2441. doi: 10.1242/dev.00449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MMP10 protein is induced by WISP3/CCN6 over-expression in C-28/I2 cells. Clonal cell lines stably over-expressing the empty vector pcDNA3.1 hygro (vector) or pcDNA3.1 hygro + WISP3/CCN6 (WISP3) were changed to serum-free medium during the logarithmic phase of growth and conditioned for 3 days. Conditioned media samples were concentrated by TCA precipitation and re-suspended in reducing sample buffer prior to separation on a 10% SDS-PAGE gel, transfer and incubation with sheep-anti-MMP10 antibody (Hembry et al., 1995, Annals Rheum Dis, 54: 25–32) and a peroxidase-conjugated donkey anti-sheep secondary antibody and subsequent ECL analysis according to the manufacturer’s instructions.
ADAMTS4 and MMP1 expression in monolayer and 3D culture in the presence and absence of cytokines. Real time RT-PCR normalised to 18S. (A) ADAMTS4 and MMP1 expression in one empty vector clone (vector) and one WISP3/CCN6 clone (WISP3) grown in monolayer in serum free medium (SF) or SF supplemented with 5ng/ml IL-1α and 10ng/ml OSM (IL-1+OSM). (B) ADAMTS4 and MMP1 expression in one empty vector clone (vector) and one WISP3/CCN6 clone (WISP3) grown in suspension over polyhema in serum free medium (SF) or SF supplemented with 5ng/ml IL-1α and 10ng/ml OSM (IL-1+OSM). Each bar is the mean of the fold change in three wells ± s.e.m compared to cells stably over-expressing the empty vector in control conditions (SF, monolayer culture). *, **, *** = significant difference in expression where P is ≤ 0.05, 0.01 and 0.001 respectively.
MMP10 expression is further induced by a GSK3β inhibitor in a WISP3/CCN6 over-expressing clone. Two-step Taqman real time PCR analysis was performed as described in Methods. Expression of MMP10 in the WISP3/CCN6 over–expressing clone is further enhanced by incubation with the GSK3β inhibitor, SB216763 (5μM). ADAMTS5 expression is partially de-repressed in the presence of this inhibitor but this does not quite reach statistical significance (p= 0.0506). Incubation of this clone with LiCl is shown for comparison.