Abstract
An IL-10 family cytokine IL-22 is characterized by several unique biological properties, including 1) the target restricted to innate cells, 2) the distinct expression pattern between large and small intestines, 3) alteration of the cellular source depending on several factors, 4) the dual abilities to serve as protective versus proinflammatory mediators in inflammatory responses, and 5) the close association with some major IBD susceptibility genes. The major functions of IL-22 in the intestine are the stimulation of epithelial cells to produce a wide variety of antibacterial proteins, the reinforcement of mucus barrier through stimulation of mucin 1 production under intestinal inflammatory conditions, and the enhancement of epithelial regeneration with goblet cell restitution. Through these beneficial functions, IL-22 contributes to the improvement of some types of experimental chronic colitis, which are mediated by Th1 or Th2 responses. Most importantly, studies using both loss-of-function and gain-of-function approaches have clearly demonstrated the ability of IL-22 to promote intestinal wound healing from acute intestinal injury. These findings highlight IL-22 as an attractive and promising target for future IBD therapy. Alternatively, the enormous progress in the field of IL-22 biology has also suggested more complicated mechanism with IL-22 pathway than previously predicted. This review article briefly summarizes previous and current knowledge on IL-22 particularly associated with intestinal inflammation.
Keywords: epithelial defense, mucin 1, antibacterial proteins, epithelial regeneration, susceptibility genes, inflammatory bowel disease
Introduction
Interleukin (IL)-22 is an IL-10 cytokine family member that was originally discovered in 2000 from a mouse T cell line stimulated with IL-9 (1). Enormous progress in this area has since been seen particularly during last five years (2–6). The growing body of evidence highlights several characteristic properties with IL-22. First characteristic is the cellular targets. IL-22 can specifically target innate cells such as intestinal and respiratory epithelial cells, keratinocytes, and hepatocytes – this is due to the restricted expression of IL-22 receptor (IL-22R) on these innate, but not adaptive immune, cells (7). Second characteristic is the unique expression pattern in the intestine. IL-22 expression in the large intestine of mice and humans is elicited under inflammatory conditions (8–10), whereas IL-22 is constitutively expressed in the normal small intestine (11,12). Third characteristic is the dual abilities to serve as protective versus proinflammatory factors -presumably depending upon the tissue targeted, the disease mechanism involved, or the local cytokine environment exposed. For example, IL-22 contributes to the improvement of experiment colitis and hepatitis (9,10,13), but it also causes psoriasis (14,15). Therefore, IL-22 has been metaphorically called as “a sheep in wolf's clothing” (16) or “a two-headed cytokine” (2). Fourth characteristic is the close association of IL-22 with IBD susceptibility genes. Several IBD susceptibility genes such as IL-23R, IL-10R2, and STAT3 are functionally associated with IL-22 directly or indirectly (17). Therefore, these findings suggest that further works on IL-22 would have the potential to not only dissect the fundamental mechanism of IBD but also provide important rationale to develop novel therapeutic measures for this disorder.
Distinct expression pattern of IL-22 in large versus small intestines
IL-22 is constitutively expressed in the small intestine of humans and mice to preserve the intestinal homeostasis against enteric microorganisms (11,12,18). In contrast, IL-22 expression is hardly detectable in the normal colon of humans (8,9,19,20) and mice (9,10,21). Diverse intestinal inflammatory conditions, which range from IBD to infectious colitis, elicit the expression of IL-22 in the colon (8–10,19–21). Interestingly, the expression level of IL-22 in the inflamed colon tends to be higher in CD patients as compared to UC patients (8,9,19). In CD, serum IL-22 levels are significantly higher in patients with risk-increasing il23r variants than in those with risk-decreasing il23r variants (22).
In mice, IL-22 is highly expressed in the inflamed colon of Th1-mediated colitis model (CD45RB model) as compared to Th2-mediated colitis model (TCRα KO mice) (9). CD45RB model has widely been used for the study of human CD (23), and TCRα KO model shares some characteristic features with human UC (24). These findings suggest that colonic IL-22 expression, which can be elicited by inflammatory insults, is further conditioned by the local cytokine environments in the inflamed colon (e.g. Th1 versus Th2). Indeed, in skin, atopic dermatitis with significant contribution from Th2 responses exhibits lower levels of IL-22 expressions as compared to psoriasis characterized by enhanced Th1/Th17 responses (25).
Cellular sources of IL-22 in the small intestine
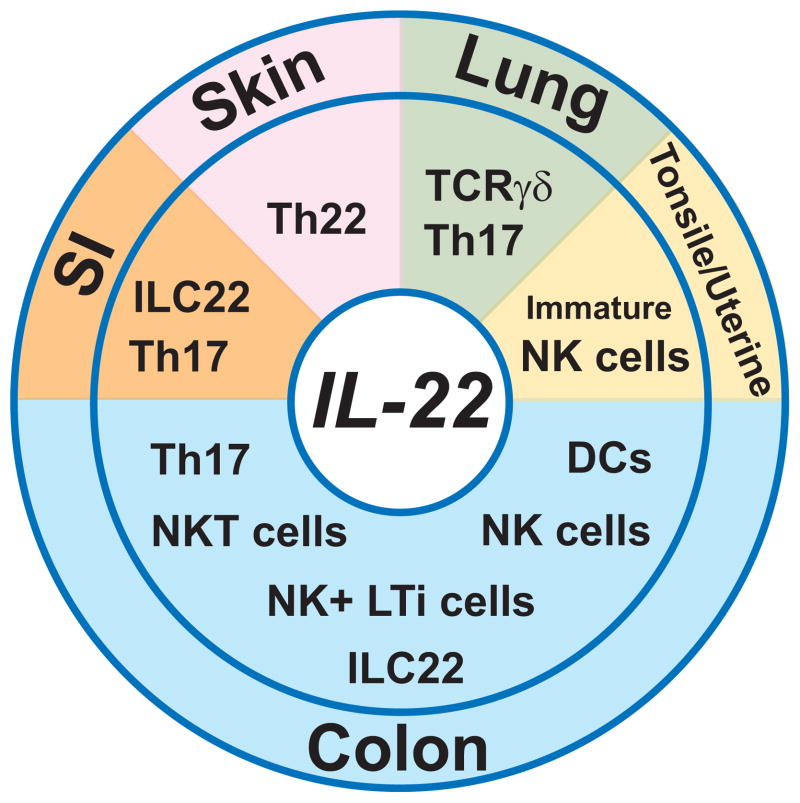
IL-22 can be produced by a wide variety of innate and adaptive immune cells, including Th17 CD4+ T cells, Th22 CD4+ T cells, CD8+ T cells, TCRγδ T cells, dendritic cells (DCs), NK cells, lymphoid tissue inducer (LTi) cells, and innate lymphoid cells (ILCs) (2–6). Interestingly, the major cellular source of IL-22 seems to differ depending upon the tissue involved (4) (Fig. 1). For example, Th22 cells, which express skin homing receptors (CCR4 and CCR10) and produce IL-22 but not IL-17, represent the major source of IL-22 in the skin (26,27). In pulmonary immune response, TCRγδ T cells and Th17 cells primarily contribute to the production of IL-22 (28,29). In human tonsile, stage-3 immature conventional NK cells serve as the source of IL-22 (30). IL-1β is required to retain the expression of aryl hydrocarbon receptor (AHR) and thus IL-22 in the immature NK cells (30). Similarly, such immature NK cells are also the source of IL-22 in human uterine mucosa (31).
Figure 1. Cellular sources of IL-22.
Potential major cellular sources in the small intestine (SI, orange), skin (pink), lung (green), tonsile and uterine (yellow), and colon (blue) are shown. Some innate cells in the colon may represent a same linage with different differentiation stages.
In the small intestine under healthy steady state and also under an inflammatory condition caused by Citrobacter rodentium infection, RAR-related orphan receptor (ROR)γt-dependent innate lymphoid cells (ILCs), which share some characteristics with NK cells and lymphoid tissue inducer (LTi) cells (Lin− Thy1+ cKit+ CD4+ CD3−), are primarily responsible for the production of IL-22 (11,12,18,32,33). These IL-22-producing ILCs are referred as “ILC22 cells” (32). The ILC22 cells include NK receptor-expressing LTi cells, ILCs (Lin−Thy1+CD127+cKit+CD3−) expressing a natural cytotoxicity receptor NKp46 with low to no NK1.1 expression, and human NK22 cells expressing NKp44, CD56 and CCR6 with low expression of NKp46 (32). Recent studies suggest that NKp46+ ILCs are progeny of LTi cells (33). Gut commensals promote the activation of RORγt in ILC22 cells to enhance their development (11), but microbiota are not strictly required for the development of ILC22 cells as indicated by the presence of these cells in germ-free mice (18,34). In response to Toxoplasma gondii-infection, major source of IL-22 in the small intestine may shift from ILC22 cells to CD4+ T cells (35). Commensals may be required for the shift, as IL-22 becomes undetectable in the small intestine of germ-free mice after a mono-associated infection with T. gondii (35).
Cellular sources of IL-22 in the large intestine
As compared to other tissues mentioned above, cellular sources of IL-22 in the inflamed colon seem to differ depending upon the type of inflammation. NK cells and Th17 CD4+ T cells have been reported to produce IL-22 in the inflamed colon of UC patients (20). In contrast, CD4+ T cells expressing natural killer group 2 member D (NKG2D) have been proposed to produce IL-22 in the inflamed intestine of CD patients (36). In addition, intestinal NKp46+ NKp44−, but not NKp46− NKp44+, NK cells have also been demonstrated to produce IL-22 (37). Interestingly, IL-22-producing NKp46+ NKp44− NK cells are more increased in the inflamed colon of CD patients as compared to that of UC patients (37). In addition to such NK cells, ILCs, which are characterized by linage marker (Lin) −CD45+, CD3−, IL-7Rα+ phenotype, have recently been identified to exist in human colon (38). These ILCs are further classified into two subsets depending on the expression pattern of neural cell adhesion molecule (NCAM, CD56). CD56+ ILCs produce IL-22 and IL-26 in response to IL-23, while CD56− ILCs produce IL-17A and IL-17F. Interestingly, increased number of both IL-22-producing CD56+ ILCs and IL-17-producing CD56− ILCs are observed in the inflamed colon of CD patients as compared to UC patients (38). These findings indicate that innate cell populations (NK cells and CD56+ ILCs) producing IL-22 more expand in CD as compared to UC. The enhanced expansion of these NK cells and ILCs may contribute for maintaining higher levels of IL-22 expressions in CD mucosa as compared to UC mucosa.
Like observed in IBD patients, major cellular sources of IL-22 in the inflamed colon of mouse IBD models also tend to alter depending upon the fundamental disease mechanisms. In Th1-mediated experimental colitis model (CD45RB model), both NK cells and Th17 cells are primarily responsible for the production of IL-22 in the inflamed colon (10). The NK cells are further classified into CD27high versus CD27low NK cell subsets. Interestingly, IL-23 can induce the IL-22 expression in both NK subsets, whereas IL-12 and IL-18 induce IL-22 expression only in CD27high NK cell subset (10). CD11c+ dendritic cells (DCs) may represent a major source of IL-22 in an acute intestinal injury model induced by oral administration of dextran sulfate sodium (DSS) (21). In C. rodentium-induced infectious colitis model, DCs may also serve as a major source of IL-22 (39). Alternatively, a recent study demonstrates in the C. rodentium infectious model that RORγt+ LTi cells, but not DCs, produce IL-22 in the inflamed colon, and DCs rather contribute to the activation of the naïve LTi cells through production of IL-23 (40). Similarly, IL-23-dependent CD4+ LTi cells, which are characterized by the expression of NKp46 and CD90 and by the localization within the intraepithelial compartment of colon, have been proposed to represent the dominant source of colonic IL-22 in the context of C. rodentium infection (41).
Signaling machinery to induce IL-22 expression
IL-22 production by Th17 T cells is induced by IL-23 or IL-6 (14). Notably, TGF-β, although critical for Th17 differentiation, inhibits the IL-22 production (14). The inhibition of IL-22 production by TGF-β is mediated by a transcription factor c-Maf (42). Like observed in Th17 cells, IL-23 also acts on ILCs to induce the expression of IL-22 (43). Aryl hydrocarbon receptor (AHR), which serves as a cytosolic sensor of aromatic hydrocarbons and plays critical roles in the metabolism of benzopyrene as well as in the acute toxicity of halogenated dioxins, is also required for the ability of Th17 T cells to produce IL-22 (44). Significantly decreased expression of AHR is observed in CD patients as compared to UC patients (45). In addition, AHR sustains the expression of NKp46 in small intestinal ILCs through activation of Notch pathway and consequently drives the development of IL-22-producing ILCs (18). AHR is also required for the development of colonic ILCs (46). In DSS-induced colonic injury model, IL-22 expression in the colon may be induced through TLR4 (LPS), TLR9 (CpG), or to a lesser extent TLR2 (lipteichoic acid) (21).
Responder cells to IL-22
IL-22 is recognized by a heterodimeric receptor consisting of IL-22R1 and IL-10Rβ (2–6). IL-10Rβ is ubiquitously expressed by majority of cell types, while the expression of IL-22R1 is restricted to innate cells such as epithelial cells, hepatocytes, and keratinocytes (2–6). Therefore, this expression pattern of IL-22R1 allows IL-22 to specifically target innate, but not adaptive immune, cell populations (7). In the intestine of mice and humans, IL-22R1 is expressed by epithelial cells and myofibroblasts (8,9,20). However, the responsiveness of myofibroblasts to IL-22 may be much weaker as compared to that of epithelial cells, as indicated by the requirement of high (200 ng/ml) versus low (5ng/ml) doses of IL-22 for the activation of myofibroblasts versus epithelial cells, respectively (8,9).
Activation of STAT3 by IL-22
IL-22 stimulation can activate STAT3, to a lesser extent STAT1, and in certain cells STAT5 (47,48). In addition, ability of IL-22 to activate Erk1/2, JNK, and p38 MAP kinase pathways has been proposed in a rat hepatoma cell line (47,48). High dose of IL-22 also induces a transient activation of Erk1/2 in colonic cancer cell line HT29 (19). However, the concept on the Erk1/2 activation by IL-22 has been challenged by some reports showing the inability of IL-22 to activate Erk/1/2 in a HepG2 cell line (49) and in primary epithelial cells from human and mouse colons (9). In contrast, the strong activity of IL-22 to stimulate STAT3 has been well confirmed using human colonic biopsies, human colonic cancer cell lines, and primary mouse colonic epithelial cells (9,10,20,21). Most importantly, epithelial STAT3 activation under DSS-induced colitis has been demonstrated to depend more on IL-22 rather than IL-6, a well known STAT3 activator (21). This observation may be unexpected but consistent with a recent discovery that IL-22R1 uses a novel mechanism to “constitutively” activate STAT3 through constitutive interaction of its C-terminal tail with the coiled-coil domain of STAT3 (50). Indeed, IL-22 has recently been shown to have a strong ability to activate STAT3 as compared to IL-6 (51).
IL-22 and antibacterial proteins
IL-22 has been implicated in several important functions in innate cells, including the host defense against microorganisms and the wound healing (2–6). One of major functions played by IL-22 is the induction of antibacterial protein expressions. IL-22 stimulates kerationcytes to produce S100A7 and S100A8 (52). In collaboration with IL-17, IL-22 also synergistically promotes the expression of β-defensin 2 and S100A9 in kerationocytes (53).
Interestingly, even though the expression levels of S100A8 (also called MRP8), S100A9 (also called MRP14), regenerating gene (Reg) IIIγ and RegIIIβ are differerent, they are significantly enhanced and commonly observed in the colonic epithelial cells of different types of experimental “chronic” colitis models, including Th1-mediated and Th2-mediated colitis models (54). Alternatively, in DSS-induced “acute” colonic injury model, the expression pattern of these molecules differs in the acute versus recovery phases (54). RegIIIγ was initially thought to play a role in cell regeneration, but subsequent studies have identified the ability to serve as an antibacterial protein against gram (+) bacteria (55). Interestingly, IL-22 stimulates epithelial cells to produce RegIIIγ and RegIIIβ in the context of infection with a gram (-) bacteria C. rodentium, (39), whereas Listeria monocytogenes stimulates the production of RegIIIγ in a MyD88-dependent manner (56). In DSS-induced colonic injury model, IL-22-dependent induction of RegIII γ and β expressions through STAT3 has been proposed (21), but a recent study suggests that IL-23 (the inducer of IL-22 expression) rather than IL-22 directly induces the expression of RegIII γ and β (57). An IL-17 family member IL-17C, which is produced by intestinal epithelial cells, has recently been shown to act in synergy with IL-22 to induce the expression of anti-bacterial proteins, including S100A8, S100A9, RegIIIβ, and RegIIIγ (58). Ability of IL-22 to promote the expression of β-defensin 2 has also been shown using a colonic cancer cell line HT29 (19). In CD patients, IL-22 induces the production of lipopolysaccharide (LPS)-binding protein by hepatocytes, which may contribute to the prevention of LPS-induced systemic responses (49).
IL-22 and intestinal mucus barrier
Intestinal mucus functions as a lubricant and a physiological barrier between luminal contents and mucosal surface (59). Mucin (Muc) 1, which is a heavily O-glycosylated membrane-bound mucin, represents one of the major components in the intestinal mucus (59). Bacteria-derived adenosine 5’-triphosphate (ATP) stimulates the development of intestinal Th17 T cells (60), and a segmented filamentous bacterium also leads to the appearance of intestinal Th17 cells (61). Interestingly, a recent study proposes the ability of Muc1 to suppress the expansion of both Th17 cells and IL-17-producing ILCs presumably by blocking the translocation of bacterial products from intestinal lumen into intestinal lamina propria (62).
Protective roles of Muc1 in both Th2-mediated colitis of T cell receptor (TCR) α knockout (KO) mice and Th1-mediated colitis of CD45RB model have recently been shown using loss-of-function systems (Muc1 KO mice) (62). Alternatively, forced overexpressions (Muc1 transgenic mouse system) of “hypoglycosylated” Muc1 make breaches in the static mucus barrier (63) and exacerbate Th1/Th17-mediated colitis in IL-10 KO mice (64), suggesting that complete glycosylation of Muc1 is required for eliciting the protective function in colitis. Indeed, impaired glycosylation in the mucus exacerbates or causes colitis (65,66). Interestingly, the ability of IL-22 to promote the production of functional Muc1 through activation of STAT3, but not STAT1, has been demonstrated using human colonic cancer cell lines (T84 and HT29) and primary colonic epithelial cells from mice (9,67). Consistent with these findings, binding of STAT3 within the promoter region of muc1 gene has been demonstrated (68). Most notably, Muc1 expression is abolished in a patient with early onset form of IBD, who has a functional polymorphism within il10rb, a receptor for IL-22 (51).
IL-22 and epithelial regeneration
IL-22 has been demonstrated to promote the epithelial cell regeneration with goblet cell restitution under intestinal inflammatory condition, but it may play no obvious role in normal colonic epithelial homeostasis in the healthy state (9,21). Consistent with these observations, STAT3 activation through IL-22 enhances the transcription of anti-apoptotic and pro-proliferative genes such as birc5, pla2g5, smo, myc, and mcl1 under inflammatory conditions (69). In addition, IL-22 stimulates a colonic cancer cell line to express a molecule termed “deleted in malignant brain tumor 1 (DMBT1)” that may play a role in epithelial cell differentiation (70). RegIα, which serves as a trophic and anti-apoptotic factor, is also reported to be induced in the inflamed colon of UC patients by IL-22 (20).
Protective versus proinflammatory roles of IL-22 in inflammatory responses
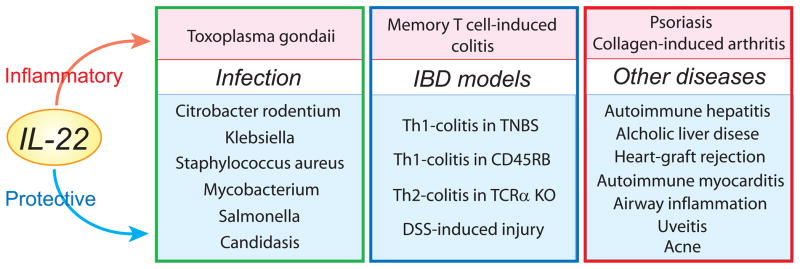
Recent accumulating evidence indicates dual abilities of IL-22 to serve as protective versus inflammatory mediators depending on several factors (Fig 2). Protective role of IL-22 has been shown in ConA-induced hepatitis (13), alcoholic fatty liver and liver damage (71), GVHD associated with heart allografts (72), autoimmune myocarditis (73), allergic airway inflammation (74), and uveitis (75). In contrast, proinflammatory role of IL-22 has also been demonstrated in collagen-induced arthritis (76). In skin, IL-22 causes psoriasis (15), but it may also suppress acne vice versa (77). IL-22 may play no role in experimental autoimmune encephalomyelitis (78). In infectious models, deficiency of IL-22 makes mice highly susceptible to broad-spectrum of pathogens, including Klebsiella pneumoniae (29), Mycobacterium tuberculosis (79), and cutaneous Staphylococcus aureus (80).
Figure 2. Role of IL-22 in inflammatory responses.
Experimental diseases, in which IL-22 has been shown to play a proinflammatory role, are indicated in pink boxes. Experimental diseases, in which IL-22 has been demonstrated to play a protective role, are listed in blue boxes.
Roles of IL-22 in intestinal inflammations
In addition, IL-22 plays dual roles in intestinal infection presumably depending upon the type of pathogens. IL-22 provides protective role against Citrobacter rodentium-induced colitis (18,39,41,46), systemic infection with Salmonella enterica (81), and gastrointestinal Candidasis in absence of IL-17R (82). In contrast, IL-22 is required for the development of Toxoplasma gondii-induced immunopathology in the small intestine (35).
Potential role of IL-22 in IBD has also been explored using mouse model system (Fig. 2). In a Th2-mediated chronic colitis model (TCRα KO mice), supplementation of IL-22 expressions in the inflamed colon through a local gene delivery system improves the colitis by reinforcing intestinal mucus barrier function (9). In a Th1-mediated colitis model (CD45RB model), there is no significant difference in colitis severity when IL-22-deficient versus IL-22-intact CD4+ CD45RBhigh T cells are transferred into recipient RAG1 KO mice that have endogenous IL-22 productions by innate cells (10,83). In contrast, IL-22-deficient CD4+ CD45RBhigh T cells cause more severe colitis as compared to IL-22-intact CD4+ CD45RBhigh T cells when IL-22-deficient and RAG1-deficient double knockout mice are used as the recipient (10). These findings suggest that both donor T cell-derived and recipient (NK cell)-derived IL-22 participate in the suppression of this colitis, but host innate cell-derived IL-22 has more prominent role in this regard (10). In contrast, proinflammatory role of memory CD4+ T cell-derived IL-22 in a new colitis model has also been proposed (84). The authors propose that adoptive transfer of CD25+ cell-depleted CD45RBlow memory CD4+ T cells can induce colitis in recipient RAG1 KO mice, but IL-22-deficient CD25+ cell-depleted CD45RBlow memory CD4+ T cells are unable to do it.
Unlike IBD models mentioned above, expression of IL-22 is not elicited in trinitrobenzene sulfonic acid (TNBS)-induced colitis model, and thus a strategy to induce the expression of IL-22 can be applied for the treatment of this colitis (45). Administration of an AHR agonist Ficz augments the colonic IL-22 expressions and suppresses the TNBS colitis (45). Neutralization of the IL-22 activity by treatment with anti-IL-22 mAbs abolishes the beneficial effect of Ficz on this colitis. Ficz also has therapeutic effect on Th1-mediated colitis of CD45RB model (45).
Role of IL-22 in intestinal wound healing
DSS-induced acute colonic injury spontaneously recovers after termination of DSS treatment, allowing us to closely examine the intestinal wound healing process (24,85). In this model, ability of IL-22 to promote intestinal wound healing has been well proven using different experimental approaches (9,10,21,45,57). IL-22 KO mice exhibit impaired recovery from DSS-induced acute colonic injury (21). Administration of neutralizing anti-IL-22 Abs also delays the recovery in WT mice (9). No recovery is observed in IL-22-deficient and RAG1-deficient double knockout mice lacking both T and B cells (10).
In addition to these loss-of-function systems, the role of IL-22 in the intestinal wound healing after exposure to DSS has been explored using gain-of-function systems that may be applicable for IBD patients. Supplementation of IL-22 expressions in the colon through a local gene delivery system promotes the recovery with enhanced goblet cell restitution (9). In addition, treatment with Ficz, which is capable of augmenting the IL-22 expression, suppresses the development of DSS-induced chronic colitis that is induced by repeated administrations of DSS with each interval (45). Interestingly, recovery from DSS-induced acute colonic injury is significantly impaired in IL-23R-deficient and RAG2-deficient double knockout mice that lack IL-22 expressions, and administration of recombinant IL-22 rescues the recovery in these mice (57).
Regulation of IL-22 function
The function of IL-22 is positively or negatively regulated by some other factors. In psoriasis, co-operative role of IL-22, IL-17, and IFN-γ may be required to fully establish the pathogenesis (86). IL-22 can induce psoriasis-like skin alteration, including acanthosis and hypogranularity, and IL-17 and IFN-γ are also necessary to induce the recruitment of inflammatory cells such as T cells and neutrophils into the region (86). In bleomycin-induced airway inflammation, IL-22 plays a proinflammatory role in the presence of IL-17A and, in turn, plays a protective role in the absence of IL-17A (87). This finding suggests that IL-17A governs the proinflammatory versus tissue-protective properties of IL-22 (87). In addition, lymphotoxin (LT) pathway, which is induced by the interaction of LTβR with LTβ but not with LIGHT, controls the protective function of IL-22 against C. rodentium (40).
Importantly, soluble class II cytokine receptor designated IL-22Ra2 (also called IL-22 binding protein, IL-22BP or CRF2-10) exists in our bodies (88-90). IL-22BP has high affinity binding capability to IL-22 and serves as an endogenous inhibitor of IL-22 activity (88–90). The cellular source of IL-22BP has not been fully established, but IL-22BP is highly expressed in placenta, spleen, skin and lung and to lesser extent in large and small intestine of humans (88). IL-22BP expressions are also detectable in normal colon of mice (9). Interestingly, distinct expression patterns of IL-22 versus IL-22BP are observed in DSS-induced acute colonic injury – IL-22 expressions increase whereas IL-22BP expressions decrease (9). Supplementation of IL-22BP expressions in the inflamed colon through a local gene delivery system significantly delays the recovery from the DSS-induced acute colonic injury and also suppresses goblet cell restitution (9). These findings indicate that IL-22 activity in the colon is controlled by its endogenous inhibitor IL-22BP.
Association of IL-22 with IBD susceptibility genes
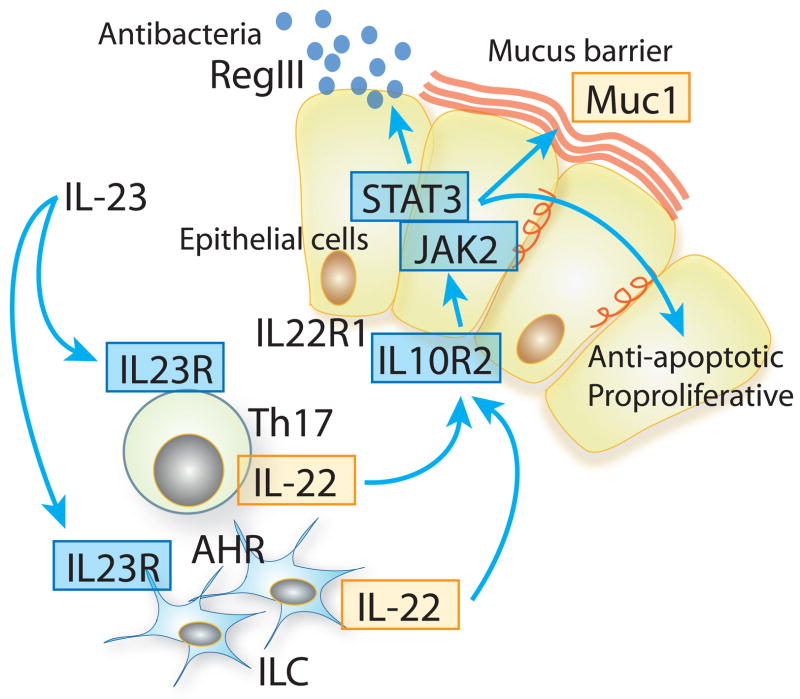
An attractive biological property with IL-22 is the functional association with some major IBD susceptibility genes (Fig. 3). Interaction of IL-23 with IL-23R has been implicated in the maintenance of IL-22-producing Th17 cells (91,92) and in the development of IL-22-producing innate cells, including ILCs, LTi cells, and NK cells (10,37,38,41,43). Functional polymorphisms within Il23r gene are negatively associated with the development of both CD and UC (91,92). IL-22 binds to a heterodimeric receptor composed of IL-10Rβ and IL-22R1. Polymorphisms of il10rb are positively associated with both CD and UC (93). In addition, il22 is located within a UC-risk locus on chromosome 12q15 (94). The receptor ligation by IL-22 induces rapid activation of STAT3 through JAK2 and TYK2. These stat3, jak2 and tyk2 all are well-defined susceptibility genes of CD and to lesser extent UC (91,92). The STAT3 activation then stimulates epithelial cells to produce Muc1 (9,67,68). A recent genome-wide association study proposes muc1 as a potential candidate gene associated with CD (95)
Figure 3. Functional linkage of IL-22 to some IBD susceptibility genes.
Ligations of IL-23R by IL-23 maintain the expansion of IL-22-producing Th17 cells and induce the development of IL-22-producing ILCs. Subsequent ligation of IL-10R2/IL-22R1 receptor complex by IL-22 activates JAK2/STAT3 pathway in epithelial cells. The activated STAT3 then stimulates epithelial cells to produce RegIII and Muc1 and also promotes epithelial cell regeneration. Well-defined IBD-association genes are indicated in blue boxes, and potential IBD-association genes indicated in yellow boxes.
Conclusion
Despite only ten years-history since the discovery, enormous efforts and rapid progression in the field of IL-22 have been seen. Indeed, as mentioned above, several unique biological properties with IL-22 have been defined. Importantly, the ability of IL-22 to promote intestinal wound healing in mice has been reproducibly confirmed by independent groups using different experimental approaches, and recent advance in genome-wide association studies has suggested the close association of IL-22 pathway with some major IBD susceptibility genes. These facts clearly highlight IL-22 as a promising target for IBD therapy. However, given the potential of IL-22 to serve as both protective and proinflammatory mediators depending on several factors, further extensive works on IL-22 would be necessary to bring novel and practical intervention for improving the lives of patients with IBD in effective and safety manner.
Acknowledgments
We thank Ms. Cindy W. Lau for her excellent editorial helps. This study was supported by Crohn’s and Colitis Foundation of America, the Harry B. and Leona Helmsley Charitable Trust, and NIH RC1DK086242.
Abbreviation used
- AHR
aryl hydrocarbon receptor
- DCs
dendritic cells
- DSS
dextran sulfate sodium
- IL-22R
interleukin-22 receptor
- ILCs
innate lymphoid cells
- JAK
Janus kinase
- KO
knockout
- LT
lymphotoxin
- LTi
lymphoid tissue inducer
- Muc1
mucin 1
- RAG
recombination activation gene
- Reg
regenerating gene
- RORγt
RAR-related orphan receptor
- STAT
signal transducer and activator of transcription
- TCR
T cell receptor
- Th
T helper
- TNBS
trinitrobenzene sulfonic acid
References
- 1.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–1819. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 2.Seiderer J, Brand S. IL-22: a two-headed cytokine in IBD? Inflamm Bowel Dis. 2009;15:473–474. doi: 10.1002/ibd.20625. [DOI] [PubMed] [Google Scholar]
- 3.Witte E, Witte K, Warszawska K, et al. Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev. 2010;21:365–379. doi: 10.1016/j.cytogfr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int Immunol. 2011;23:159–163. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- 5.Ouyang W, Rutz S, Crellin NK, et al. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 6.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 7.Wolk K, Kunz S, Witte E, et al. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Andoh A, Zhang Z, Inatomi O, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–984. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 9.Sugimoto K, Ogawa A, Mizoguchi E, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zenewicz LA, Yancopoulos GD, Valenzuela DM, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanos SL, Bui VL, Mortha A, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cella M, Fuchs A, Vermi W, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zenewicz LA, Yancopoulos GD, Valenzuela DM, et al. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 15.Ma HL, Liang S, Li J, Napierata L, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurence A, O'Shea JJ, Watford WT. Interleukin-22: a sheep in wolf's clothing. Nat Med. 2008;14:247–249. doi: 10.1038/nm0308-247. [DOI] [PubMed] [Google Scholar]
- 17.Mizoguchi A, Mizoguchi E. Animal models of IBD: linkage to human disease. Curr Opin Pharmacol. 2010;10:578–87. doi: 10.1016/j.coph.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JS, Cella M, McDonald KG, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2011 doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brand S, Beigel F, Olszak T, et al. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 20.Sekikawa A, Fukui H, Suzuki K, et al. Involvement of the IL-22/REG Ialpha axis in ulcerative colitis. Lab Invest. 2010;90:496–505. doi: 10.1038/labinvest.2009.147. [DOI] [PubMed] [Google Scholar]
- 21.Pickert G, Neufert C, Leppkes M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmechel S, Konrad A, Diegelmann J, et al. Linking genetic susceptibility to Crohn's disease with Th17 cell function: IL-22 serum levels are increased in Crohn's disease and correlate with disease activity and IL23R genotype status. Inflamm Bowel Dis. 2008;14:204–212. doi: 10.1002/ibd.20315. [DOI] [PubMed] [Google Scholar]
- 23.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizoguchi A. Animal models of inflammatory bowel disease. Prog Mol Biol Transl Sci. 2012;105:263–320. doi: 10.1016/B978-0-12-394596-9.00009-3. [DOI] [PubMed] [Google Scholar]
- 25.Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol. 2008;181:7420–7427. doi: 10.4049/jimmunol.181.10.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duhen T, Geiger R, Jarrossay D, et al. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 27.Trifari S, Kaplan CD, Tran EH, et al. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 28.Simonian PL, Wehrmann F, Roark CL, et al. γδ T cells protect against lung fibrosis via IL-22. J Exp Med. 2010;207:2239–2253. doi: 10.1084/jem.20100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aujla SJ, Chan YR, Zheng M, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes T, Becknell B, Freud AG, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32:803–814. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Male V, Hughes T, McClory S, et al. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J Immunol. 2010;185:3913–3918. doi: 10.4049/jimmunol.1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 33.Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229–34. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- 34.Sawa S, Cherrier M, Lochner M, et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 35.Muñoz M, Heimesaat MM, Danker K, et al. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009;206:3047–59. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pariente B, Mocan I, Camus M, et al. Activation of the receptor NKG2D leads to production of Th17 cytokines in CD4+ T cells of patients with Crohn's disease. Gastroenterology. 2011;141:217–226. doi: 10.1053/j.gastro.2011.03.061. [DOI] [PubMed] [Google Scholar]
- 37.Takayama T, Kamada N, Chinen H, et al. Imbalance of NKp44(+)NKp46(−) and NKp44(−)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn's disease. Gastroenterology. 2010;139:882–892. doi: 10.1053/j.gastro.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 38.Geremia A, Arancibia-Cárcamo CV, Fleming MP, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng Y, Valdez PA, Danilenko DM, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 40.Tumanov AV, Koroleva EP, Guo X, et al. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe. 2011;10:44–53. doi: 10.1016/j.chom.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonnenberg GF, Monticelli LA, Elloso MM, et al. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutz S, Noubade R, Eidenschenk C, et al. Transcription factor c-Maf mediates the TGF-β-dependent suppression of IL-22 production in T(H)17 cells. Nat Immunol. 2011;12:1238–45. doi: 10.1038/ni.2134. [DOI] [PubMed] [Google Scholar]
- 43.Takatori H, Kanno Y, Watford WT, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 45.Monteleone I, Rizzo A, Sarra M, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237–248. doi: 10.1053/j.gastro.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Qiu J, Heller JJ, Guo X, et al. The Aryl Hydrocarbon Receptor Regulates Gut Immunity through Modulation of Innate Lymphoid Cells. Immunity. 2012 doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pestka S, Krause CD, Sarkar D, et al. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 48.Nagalakshmi ML, Rascle A, Zurawski S, et al. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int Immunopharmacol. 2004;4:679–691. doi: 10.1016/j.intimp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Wolk K, Witte E, Hoffmann U, et al. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn's disease. J Immunol. 2007;178:5973–5981. doi: 10.4049/jimmunol.178.9.5973. [DOI] [PubMed] [Google Scholar]
- 50.Dumoutier L, de Meester C, Tavernier J, et al. New activation modus of STAT3: a tyrosine-less region of the interleukin-22 receptor recruits STAT3 by interacting with its coiled-coil domain. J Biol Chem. 2009;284:26377–26384. doi: 10.1074/jbc.M109.007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Begue B, Verdier J, Rieux-Laucat F, et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. Am J Gastroenterol. 2011;106:1544–1555. doi: 10.1038/ajg.2011.112. [DOI] [PubMed] [Google Scholar]
- 52.Wolk K, Witte E, Wallace E, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 53.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mizoguchi E, Xavier RJ, Reinecker HC, et al. Colonic epithelial functional phenotype varies with type and phase of experimental colitis. Gastroenterology. 2003;125:148–161. doi: 10.1016/s0016-5085(03)00665-6. [DOI] [PubMed] [Google Scholar]
- 55.Cash HL, Whitham CV, Behrendt CL, et al. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brandl K, Plitas G, Schnabl B, et al. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cox JH, Kljavin NM, Ota N, et al. Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunol. 2012;5:99–109. doi: 10.1038/mi.2011.54. [DOI] [PubMed] [Google Scholar]
- 58.Song X, Zhu S, Shi P, et al. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat Immunol. 2011;12:1151–1158. doi: 10.1038/ni.2155. [DOI] [PubMed] [Google Scholar]
- 59.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–57. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 60.Atarashi K, Nishimura J, Shima T, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 61.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nishida A, Lau CW, Zhang M, et al. The Membrane-Bound Mucin Muc1 Regulates Th17-Cell Responses and Colitis in Mice. Gastroenterology. doi: 10.1053/j.gastro.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kadayakkara DK, Beatty PL, Turner MS, et al. Inflammation driven by overexpression of the hypoglycosylated abnormal mucin 1 (MUC1) links inflammatory bowel disease and pancreatitis. Pancreas. 2010;39:510–515. doi: 10.1097/MPA.0b013e3181bd6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beatty PL, Plevy SE, Sepulveda AR, et al. Transgenic expression of human MUC1 in IL-10−/− mice accelerates inflammatory bowel disease and progression to colon cancer. J Immunol. 2007;179:735–739. doi: 10.4049/jimmunol.179.2.735. [DOI] [PubMed] [Google Scholar]
- 65.An G, Wei B, Xia B, McDaniel JM, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;204:1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stone EL, Ismail MN, Lee SH, et al. Glycosyltransferase function in core 2-type protein O glycosylation. Mol Cell Biol. 2009;29:3770–3782. doi: 10.1128/MCB.00204-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andoh A, Shioya M, Nishida A, et al. Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. J Immunol. 2009;183:687–695. doi: 10.4049/jimmunol.0804169. [DOI] [PubMed] [Google Scholar]
- 68.Yuan ZL, Guan YJ, Wang L, et al. Central role of the threonine residue within the p+1 loop of receptor tyrosine kinase in STAT3 constitutive phosphorylation in metastatic cancer cells. Mol Cell Biol. 2004;24:9390–9400. doi: 10.1128/MCB.24.21.9390-9400.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neufert C, Pickert G, Zheng Y, et al. Activation of epithelial STAT3 regulates intestinal homeostasis. Cell Cycle. 2010;9:652–655. doi: 10.4161/cc.9.4.10615. [DOI] [PubMed] [Google Scholar]
- 70.Fukui H, Sekikawa A, Tanaka H, et al. DMBT1 is a novel gene induced by IL-22 in ulcerative colitis. Inflamm Bowel Dis. 2011;17:1177–1188. doi: 10.1002/ibd.21473. [DOI] [PubMed] [Google Scholar]
- 71.Ki SH, Park O, Zheng M, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kapessidou P, Poulin L, Dumoutier L, et al. Interleukin-22 deficiency accelerates the rejection of full major histocompatibility complex-disparate heart allografts. Transplant Proc. 2008;40:1593–1597. doi: 10.1016/j.transproceed.2008.03.151. [DOI] [PubMed] [Google Scholar]
- 73.Chang H, Hanawa H, Liu H, et al. Hydrodynamic-based delivery of an interleukin- 22-Ig fusion gene ameliorates experimental autoimmune myocarditis in rats. J Immunol. 2006;177:3635–3643. doi: 10.4049/jimmunol.177.6.3635. [DOI] [PubMed] [Google Scholar]
- 74.Nakagome K, Imamura M, Kawahata K, et al. High expression of IL-22 suppresses antigen-induced immune responses and eosinophilic airway inflammation via an IL-10-associated mechanism. J Immunol. 2011;187:5077–5089. doi: 10.4049/jimmunol.1001560. [DOI] [PubMed] [Google Scholar]
- 75.Ke Y, Sun D, Jiang G, et al. IL-22-induced regulatory CD11b+ APCs suppress experimental autoimmune uveitis. J Immunol. 2011;187:2130–2139. doi: 10.4049/jimmunol.1100482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geboes L, Dumoutier L, Kelchtermans H, et al. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum. 2009;60:390–395. doi: 10.1002/art.24220. [DOI] [PubMed] [Google Scholar]
- 77.Wolk K, Warszawska K, Hoeflich C, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228–1239. doi: 10.4049/jimmunol.0903907. [DOI] [PubMed] [Google Scholar]
- 78.Kreymborg K, Etzensperger R, Dumoutier L, et al. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J Immunol. 2007;179:8098–8104. doi: 10.4049/jimmunol.179.12.8098. [DOI] [PubMed] [Google Scholar]
- 79.Dhiman R, Indramohan M, Barnes PF, et al. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J Immunol. 2009;183:6639–45. doi: 10.4049/jimmunol.0902587. [DOI] [PubMed] [Google Scholar]
- 80.Cho JS, Pietras EM, Garcia NC, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schulz SM, Köhler G, Schütze N, et al. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J Immunol. 2008;181:7891–7901. doi: 10.4049/jimmunol.181.11.7891. [DOI] [PubMed] [Google Scholar]
- 82.De Luca A, Zelante T, D'Angelo C, et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010;3:361–373. doi: 10.1038/mi.2010.22. [DOI] [PubMed] [Google Scholar]
- 83.Leppkes M, Becker C, Ivanov II, et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 84.Kamanaka M, Huber S, Zenewicz LA, et al. Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J Exp Med. 2011;208:1027–1040. doi: 10.1084/jem.20102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimomura Y, Ogawa A, Kawada M, et al. A unique B-2 B cell subset in the intestine. J Exp Med. 2008;205:1357–1368. doi: 10.1084/jem.20071572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolk K, Haugen HS, Xu W, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med. 2009;87:523–536. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 87.Sonnenberg GF, Nair MG, Kirn TJ, et al. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207:1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu W, Presnell SR, Parrish-Novak J, et al. A soluble class II cytokine receptor, IL- 22RA2, is a naturally occurring IL-22 antagonist. Proc Natl Acad Sci U S A. 2001;98:9511–9516. doi: 10.1073/pnas.171303198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dumoutier L, Lejeune D, Colau D, et al. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J Immunol. 2001;166:7090–7095. doi: 10.4049/jimmunol.166.12.7090. [DOI] [PubMed] [Google Scholar]
- 90.Kotenko SV, Izotova LS, Mirochnitchenko OV, et al. Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J Immunol. 2001;166:7096–7103. doi: 10.4049/jimmunol.166.12.7096. [DOI] [PubMed] [Google Scholar]
- 91.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066– 2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–45. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Silverberg MS, Cho JH, Rioux JD, et al. Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet. 2009;41:216–220. doi: 10.1038/ng.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Franke A, McGovern DP, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]