Abstract
Evidence for the extent and nature of attentional impairment in premanifest and manifest Huntington’s disease (HD) is inconsistent. Understanding such impairments may help to better understand early functional changes in HD and could have consequences concerning care for HD patients. We investigated attentional control in both early and premanifest HD. We studied 17 early HD subjects (mean age: 51 years), 12 premanifest HD subjects (mean age: 43 years), and 15 healthy controls (mean age: 51 years), using the sustained attention to response task (SART), a simple Go/No-go test reflecting attentional and inhibitory processes through reaction time (RT) and error rates. Simultaneously recorded EEG yielded P300 amplitudes and latencies. The early HD group made more Go errors (p < 0.001) and reacted slower (p < 0.005) than the other groups. The RT pattern during the SART was remarkably different for early HD subjects compared to the other two groups (p < 0.005), apparent as significant post-error slowing. P300 data showed that for early HD the No-go amplitude was lower than for the other two groups (p < 0.05). Subjects with early HD showed a reduced capacity to effectively control attention. They proved unable to resume the task directly after having made an error, and need more time to return to pre-error performance levels. No attentional control deficits were found for the premanifest HD group.
Keywords: Huntington’s disease, Attention, Inhibition, SART, P300
Introduction
Huntington’s disease (HD) is an autosomal dominant neurological disorder characterized by progressive motor, cognitive and behavioural abnormalities. While the clinical diagnosis is based on the presence of motor signs, deficits in the other functional domains are widespread [9, 39]. The discovery of the HD gene [17] allows gene-carriers to be identified in the premanifest phase of the disease, i.e., before symptoms and signs appear. Deficits in cognition such as psychomotor slowing, memory decline and executive dysfunctioning have been reported in both manifest and premanifest individuals [23], but inconsistently [16].
Attentional processing may well be abnormal in HD, but results are conflicting: some authors reported attentional and inhibitory deficits in both patients and premanifest gene carriers [24, 38], while others did not [42]. The conflict may be due to the complex nature of the widely applied neuropsychological tests such as the symbol digit modalities test, the stroop colour-word task and the trial making test. These assess attention and inhibition, they also tap into psychomotor speed, implicit learning, and visuomotor integration.
Studies of attentional processing showed deficits in focused attention [27, 32], shifting attention [2, 8], and inhibition, with sometimes reduced inhibition [2] and sometimes increased inhibitory control [1]. Because of such methodological differences it is difficult to come to a conclusion about overall attentional processing in HD. Moreover, attention and inhibition are functionally very closely related and rely heavily on each other, i.e. attention often is a prerequisite of correct inhibition. This interdependence led us to investigate attention and inhibition together in the context of attentional control, a construct overarching the two terms [20, 26].
The sustained attention to response test
The sustained attention to response test (SART) is a test of attention control assessing both attentional and inhibitory processes [18, 31, 35]. Participants are requested to press a button when a number (1–9) appears on a screen except when that number is a three. The need to withhold responses only to rare stimuli means that the task relies heavily on attentional control. Pressing a button is simple in terms of motor control, important in HD, as motor disturbances can interfere with the determination of cognitive deficits. The SART demonstrated deficiencies in attention, in disorders such as traumatic brain injury [6], schizophrenia [7], attention deficit hyperactivity disorder [3] and narcolepsy [15].
The P300
There is a growing need in HD research for sensitive, objective and quantifiable assessments. Electroencephalography (EEG) has the advantages of low cost, non-invasiveness and high temporal resolution. Event-related potentials (ERP) are EEG-based potentials reflecting the neurophysiologic substrate of mental processes such as stimulus identification, processing and response initiation [25]. We focused on the P300 peak, which is most commonly evoked by rare stimuli interspersed in a series of frequent ones. Although the exact neural origin is not entirely clear, the P300 is often linked with processes of attention [5, 14, 22]. Some authors have suggested P300 amplitude to reflect the amount of attentional resources allocated to the stimulus, while the latency is linked to the stimulus evaluation time, or more general, speed of cognitive processing of the stimulus [30]. In previous work P300 latency was increased and its amplitude decreased in manifest HD compared to healthy controls in a visual search task [28] and a visual Go/No-go task [4]. However, no P300 abnormalities were found in premanifest HD or those at risk in auditory odd-ball paradigms [12, 19].
The P300 can be elicited by Go/No-go tasks such as the SART. Studies in healthy individuals found that simultaneous SART and EEG assessment resulted in good indexes for attentional and inhibitory processes [11]. Only one previous study has used the combination of SART and P300 in premanifest HD [21], but found no differences in P300 characteristics between the premanifest HD group and controls. They concluded that possibly their premanifest group was not yet close enough to disease onset and that with progressive basal ganglia degeneration closer to onset differences would have emerged. Indeed, magnetic resonance imaging measurements of grey and white matter structures showed changes before the appearance of overt clinical signs of HD [13, 41].
We hypothesized that both premanifest and manifest (early) HD groups show impaired attentional control in comparison with control subjects as measured by a heightened error rate on the SART. Furthermore, we aim to further strengthen this hypothesis by showing altered P300 characteristics (i.e. lowered amplitude and increased latency) in both HD groups in accordance with deviant SART results. Because of the motor disturbances in HD we expected the SART reaction times for the early HD group to be longer.
Methods
Subjects
Thirteen subjects with premanifest HD (PMHD), 18 with early manifest HD (MHD) and 17 age-matched healthy controls, relatives that were tested as gene-negative, were included, all were above the age of 18 years. Participants were recruited from the outpatient neurologic clinic of the Leiden University Medical Centre and had been genetically tested for HD. Gene carriers were considered premanifest when they had five points or less on the total motor subscale of the Unified Huntington’s Disease Rating Scale (UHDRS) [40]. The manifest group consisted of early HD subjects (Shoulson–Fahn stages 1 and 2) [34]. Disease burden was calculated using the formula ‘age (CAG-35.5)’ [29]. Exclusion criteria were major psychiatric disorders, neurological co-morbidity, a score of ≤25 on the mini-mental state examination and medication with known effects on the EEG (e.g. neuroleptics). The study was approved by the local medical ethical committee and all participants gave written informed consent.
All participants underwent neurological, SART and EEG assessments. Depression was measured using the short version of the problem behaviour assessment for HD [10]. The motor part of the UHDRS was administered by a clinician (SvdB) blinded for genetic status.
One PMHD, one MHD, and one control subject were excluded because of excessive muscle artefacts on the EEG. One additional control was excluded because of epileptiform abnormalities on the EEG. Data of 12 PMHD, 17 MHD and 15 controls were analyzed.
Sustained attention to response task
For the SART, subjects were seated in a comfortable chair 1 m from a computer screen, with a computer keyboard placed in easy access of the dominant hand. Numbers from 1 to 9 were shown 25 times on a computer screen. Subjects were asked to respond to the appearance of every number by pressing the spacebar (‘Go’ trials), except when the number 3 was shown (‘No-go’ trials). When the number 3 was displayed, participants were instructed to withhold their response. Reaction time (RT) was recorded whenever the spacebar was pressed. To ensure accurate measurement of RT a cathode ray screen was used together with a purpose built hardware device that allowed precise measurement of the build-up time of the screen information and hence of RT in relation to the visual stimulus. Subjects were instructed that accuracy and speed were equally important. Before the start of the test subjects performed a practice run consisting of 25 numbers from 1 to 9 in random order. Stimuli were shown for 250 ms followed by a blank screen for 900 ms (detailed description of the task see [31]). Outcome measures for the SART were RT and error rates. ‘Overall RT’ refers to mean RT over all trials performed. The mean RT for correct Go trials and incorrect No-go trials were also computed. Error rate data consisted of overall error rate (the total number of errors as a percentage of the total number of trials performed), error rate Go (total of Go errors as percentage of total number of trials) and error rate No-go (total of No-go errors as a percentage of total number of trials).
ERP recording and analysis
All EEGs (Nihon Kohden 2110 EEG apparatus) were recorded between 12.00 and 14.30 h, except for one control subject tested late in the morning. Twenty-one Ag/AgCl electrodes were placed according to the 10/20 convention. ECG, respiration and horizontal eye movement leads were also recorded. The EEG was band-pass filtered from 0.16–70 Hz before display and analysis. Sample frequency was 200 Hz and A–D precision 12 bits. For the P300 analysis we used the midline sites Fz (frontal), Cz (central) and Pz (parietal) with linked mastoids as reference. The computer controlling the SART paradigm wrote synchronization signals to the EEG machine, allowing averaging to take place offline after controlling for signal quality. Data were averaged over epochs of 1200 ms, starting 200 ms before stimulus onset. Individual trials with eye blink artefacts or suspected muscle artefacts (peak amplitudes more than 75 μV) were excluded from P300 analysis. The P300 component was defined as the maximum positivity between 350 and 650 ms. This time-frame was based on visual inspection of the averaged ERPs. ERP analysis, including peak detection, was performed automatically using an in-house developed program written in MATLAB (MathWorks, Natick, USA). Peak amplitudes were measured relative to a 200 ms baseline before stimulus onset. Outcome measures consisted of amplitude and latency data. The mean amplitudes and latencies for all trials, all Go trials and all No-go trials were calculated averaging the data from the midline electrodes.
Statistical analysis
SPSS for Windows version 17.0 was used for data analysis. Analyses of demographic variables were performed using parametric and non-parametric tests where appropriate. Group differences for mean SART RT and error rate and P300 amplitude and latency data were calculated using univariate analysis of covariance (ANCOVA) with age as a covariate. Upon visual inspection of the RT patterns for the four trials preceding and the four trials following a No-go trial, different patterns were observed. To investigate possible group differences in these patterns a secondary analysis was performed. For this purpose the difference (delta) between the mean RT just before and just after both correct and incorrect No-go trials was calculated for each subject. The delta scores for the three groups were analyzed again using ANCOVA with age as covariate. The Bonferroni method was used to correct for multiple testing. The level of significance was set at p ≤ 0.05. P values >0.05 < 0.1 were reported as trend significant.
Results
Clinical characteristics
There were no differences between groups for sex, age, IQ and level of education (Table 1). Disease burden differed significantly between PMHD and MHD (p < 0.0001). Two subjects (one premanifest, one early HD subject) were rated as mildly depressed; depression, however, did not differ between groups (data not shown in table).
Table 1.
Descriptive statistics of controls, premanifest and manifest participants
| Characteristic | Controls N = 15 | PMHD N = 12 | MHD N = 17 |
|---|---|---|---|
| Age (years) | 51 (10) | 43 (10) | 50 (11) |
| CAG | 20 (3) | 42 (2) | 44 (3) |
| Male/femalea, b | 7/8 | 6/6 | 8/9 |
| Level of educationa, b (lower/middle/higher) | 1/9/5 | 0/9/3 | 2/11/12 |
| Intelligence Quotient (IQ) | 107 (8) | 105 (8) | 101 (12) |
| Disease burden | 251 (75) | 404 (81) |
Descriptive statistics are presented as mean (standard deviation), except for a which is total number. IQ was measured by the National Adult Reading Test (Dutch version). Disease burden is age (CAG-35.5)
bPearson Chi-square test
SART
Table 2 shows SART error rate and RT data. The overall error rate (i.e. all errors made, not differentiated for type) differed significantly between groups (p < 0.05) (Table 2). The MHD group made significantly more errors of all types than both PMHD and controls (p < 0.05). Further analysis revealed that only for Go-errors (i.e., subjects did not press the spacebar when they ought to) there was a group difference (p < 0.001). Surprisingly, the number of No-go errors (pressing the spacebar when the number 3 appears) did not differ between groups. Concerning mean RT (i.e. mean RT for all pressed trials, not differentiated for correct or erroneous trials), there was a group difference (p < 0.005), where the overall RT was significantly longer for MHD than for PMHD (p < 0.005) and controls (p < 0.001).
Table 2.
Main and post-hoc effects for SART error rate and mean reaction time data and P3 mean amplitude and latency data
| Controls N = 15 | PMHD N = 12 | MHD N = 17 | p value | ||||
|---|---|---|---|---|---|---|---|
| Main effect | MHD––Controls | PMHD––Controls | MHD–PMHD | ||||
| SART | |||||||
| Error rate | 4.3 (1.8) | 3.9 (2.5) | 6.3 (2.8) | 0.021 | 0.027 | ns | 0.014 |
| Error rate No-go | 27.7 (11.9) | 25.8 (13.8) | 32.9 (15.9) | ns | ns | ns | ns |
| Error rate Go | 1.4 (0.98) | 1.2 (1.3) | 3.0 (1.4) | <0.001 | 0.001 | ns | 0.001 |
| Mean RT | 381 (34) | 388 (48) | 462 (77) | 0.001 | < 0.001 | ns | 0.004 |
| P300 | |||||||
| Amplitude | 8.7 (2.8) | 8.3 (4.4) | 5.9 (3.2) | 0.058 | ns | ns | ns |
| Amplitude Go | 7.9 (2.8) | 7.8 (4.6) | 5.4 (3.1) | ns | ns | ns | ns |
| Amplitude No-go | 16.3 (4.9) | 1.61 (4.5) | 1.20 (5.6) | 0.046 | 0.023 | ns | 0.064 |
| Latency | 414 (37) | 401 (37) | 439 (57) | ns | ns | ns | ns |
| Latency Go | 408 (30) | 405 (43) | 445 (62) | 0.059 | ns | ns | ns |
| Latency No-go | 430 (38) | 424 (47) | 447 (50) | ns | ns | ns | ns |
Data are mean (standard deviation), Errors are percentage of errors out of total number of stimuli, Amplitude is μV, Latency is milliseconds. Bold values are statistically significant (p < 0.05)
RT Reaction time in milliseconds, ns not significant
P300
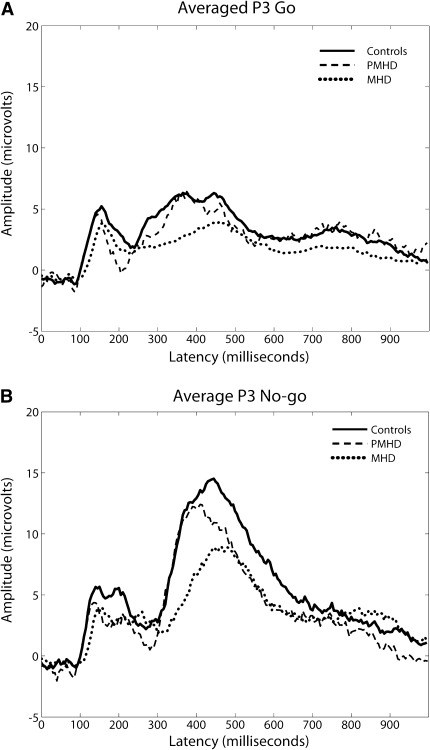
The number of epochs used for P300 analyses were not different between groups. P300 amplitudes were larger for No-go trials than Go trials (Table 2; Fig. 1a, b). Overall P300 amplitude proved only trend significant between groups (p < 0.06). Amplitude in No-go trials differed significantly between groups, with lower amplitude in MHD than in the other groups (p < 0.05). For mean latency only a trend towards significant group differences for Go trials was observed (p < 0.06).
Fig. 1.
a Go P300 waves per group (averaged). Note P300 waves for the three groups during SART Go trials, averaged over the midline electrodes. Time point 0 denotes the point of stimulus presentation. b No-go P300 waves per group (averaged). Note P300 waves for the three groups during SART No-go trials, averaged over the midline electrodes. Time point 0 denotes the point of stimulus presentation
Reaction time patterns before and after correct and incorrect No-go
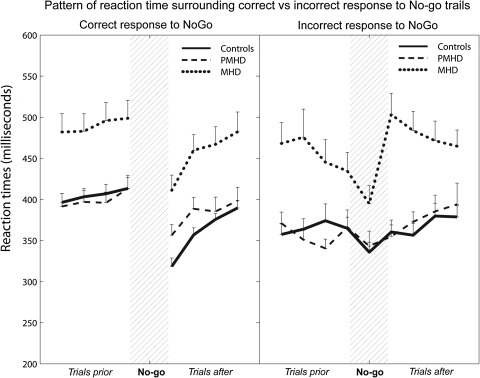
Almost all Go errors (not pressing on 1–9) occurred directly following a No-go error (incorrectly pressing on 3), with MHD making significantly more of these errors than the PMHD group (p < 0.05). The RT patterns for the four trials preceding and following both correct and incorrect No-go trials are shown in Fig. 2. Analysis of covariance on the difference between the RT of the last trial before and the first trial after both correct and incorrect No-go responses revealed a significant result for incorrect No-go trials only (p < 0.01). MHD had a significantly slower response to the first Go trial following an incorrect No-go trial compared to controls (p < 0.005) and PMHD (p < 0.05). P300 amplitude did not differ significantly for trials surrounding correct and incorrect No-go trials.
Fig. 2.
RT patterns for trials before and after correct and incorrect No-go trial. Note The reaction time for the four trials preceding and the four trials following No-go trails. Data are separated for RT patterns surrounding correct responses (i.e. not pressing at No-go stimulus) and incorrect responses (i.e. pressing at No-go stimulus), averaged per group
Discussion
The main finding of this study was that attentional control is deficient in MHD, evident primarily through a heightened error rate on the SART. This behavioural deficit was corroborated by abnormalities of P300 characteristics.
SART error rate
As expected, MHD made more errors of any type than the other groups, indicative of defective attentional mechanisms. Unexpectedly, this was not caused by a high rate of No-go errors, but by significantly more Go errors. The only study using the SART in HD did not report about the type of errors made [21]. Even though they used different Go/No-go paradigms other studies also report on attentional deficits in manifest HD as measured by more Go errors for manifest HD compared to controls, but all have concomitantly also found more No-go errors, contrary to our findings [27, 36]. Our findings are partly in line with studies using the SART in other brain disorders with known attentional deficits. Schizophrenic patients have also been found to largely make Go errors and not No-go errors [7]. However, patients with traumatic brain injury made significantly more errors of both types, with stronger evidence for No-go than Go errors [3, 31]. So, our findings cannot be easily attributed to attentional deficits alone as earlier findings in other studies were not replicated. Therefore, they were further investigated in reaction time pattern analyses.
Reaction time patterns surrounding No-go errors
As the SART is likely to provoke No-go errors due to the repetitive nature of the task and the rarity of No-go stimuli, we further investigated the significant amount of Go errors made by the early HD group. Examination of these Go errors in MHD revealed that most were made directly following a No-go error. Analysis of the reaction time patterns for trials directly preceding and following a correct No-go trial (correctly withholding response to a three) revealed identical patterns for the three groups, although the MHD group reacted significantly slower than the PMHD or control groups. Prior to correct responses to a No-go trial RT was relatively stable for all three groups. Directly after such a correctly withheld response, RT was noticeably shorter. This speeding most likely represents action anticipation that is evoked by the repetitive nature of the SART. This primes the motor response; after having correctly withheld the response at the No-go trial, the response to the next Go trial is more quickly accessed, resulting in a quicker response [37]. Although the MHD group reacted slower, the general pattern was the same as for the other groups. This suggests that the cause of slowing is due to motor disturbances and not to different cognitive processing.
Interestingly, a different pattern emerged concerning incorrect No-go responses, i.e. when participants incorrectly pressed the space bar in response to a 3. For all three groups the trials directly preceding such a No-go error showed a shortened RT. We hypothesize that this pre-error speeding could mean that the task was performed fairly automatically, with less attentional control, eventually resulting in an error [31]. Remarkably, this pre-error speeding was more prominent in MHD than in the other two groups. This could indicate that subjects with MHD can sustain attention less well than the other groups. After such a No-go error RT returned to the pre-error level almost immediately for the PMHD and control groups, but not for the MHD group, showing a dramatic post-error slowing.
One proposition for this is that a No-go error induces MHD subjects to slow down in response time in the hope of making fewer errors. This is an unconscious cognitive strategy known as ‘speed accuracy trade-off’ (SAT): low speed allows high accuracy [33]. That healthy controls performing the SART use this SAT strategy has also been put forward by Helton and colleagues [18]. At first glance one would then expect that subjects who choose ‘accuracy over speed’ would make fewer errors, but this was not the case. A more likely explanation is that there is an intrinsic deficit of attentional control in MHD. This is seen in the obvious drop in RT trials preceding an error. This could possibly reflect a drop in attentional control, in turn causing the No-go error. The post-error RT pattern shows that the PMHD and control groups are able to return to the task immediately and perform on pre-error level. The pattern of the MHD subjects, however, reflects a difficulty in recovery; it takes this group several trials to return to pre-error performance. This difficulty could be due to the realization of having made an error, i.e., the response evaluation, causes confusion; the subsequent quick return to a Go trial adds to this confusion, leading to a slower return to pre-error performance. Alternatively, this post-error slowing does not reflect cognitive confusion, but could be indicative of an inability to switch from a No-go to a Go response, and thus from inhibiting the response to activating it. Together with the fact that directly following a No-go error significantly more Go errors are made in the MHD group than in the other two groups we speculate that attentional and inhibitory deficits are the probable causes of inadequate attentional control in MHD. Adding to this theory of impaired attentional control we found, on further analysis, that in the trial directly following a No-go error trial, the early group made significantly more go-errors (8%) than both the premanifest (0.5%) and control groups (3%). Similar results in a task-switch and stop-signal task in MHD have been reported [2]. Post-error slowing was interpreted in that study as task-switch cost and a deficit in the ‘inhibition of the just-performed response’ respectively. The authors attributed these phenomena to deficient inhibition. These explanations are not mutually exclusive in that early HD subjects can use the speed accuracy trade-off strategy to avoid making further errors, but that their cognitive abilities are deficient and they cannot use this strategy successfully.
Even though constructs such as attention and inhibition are not directly measurable and can only be derived from secondary measurements, we hypothesize that the RT pattern around No-go errors in the MHD group seems to reflect a cognitive rather than a motor process as subjects with early HD are able to respond in the same manner as PMHD and controls in correctly withheld No-go trials, albeit slower. This similar pattern for all groups demonstrates that it is not a No-go trial per se that elicits a deviant reaction from early subjects. The problem seems to lie purely in the fact that an error was made.
P300 amplitude and latency
As stated before, P300 amplitude is hypothesized to reflect the amount of attentional capacity that is being allocated to a stimulus [30]. If so, then P300 amplitude would be lower for incorrectly performed No-go trials. This was indeed the case for the MHD group, confirming a lowered attentional control during presentation of No-go stimuli. Our findings correspond well to those of Beste et al. [4] and Jurgens et al. [21]. Münte et al. [28] also reported lowered P300 amplitude; however, not in the context of a Go/no-go task.
P300 latency is thought to be linked to the speed of attentional processing [30]. In accordance with Münte et al. [28], P300 latency was significantly longer in MHD compared to the other groups for Go trials. This implies a low speed of attentional processing during Go trials is lessened for MHD. Together with a lowered attention during No-go trials this strengthens our hypothesis that the disturbed pattern observed surrounding No-go errors is of a cognitive rather than a motor nature.
Premanifest HD results
PMHD did not exhibit any attentional or inhibitory deficits. Explanations for this are that no attentional control deficits are yet present or that subtle changes in attentional control capacity are already present in PMHD, but that they are still too subtle to be measured with this method. Possibly these deficits gradually worsen and are better picked up in subjects closer to expected onset. This interpretation seems plausible as both SART and P300 data did show a nonsignificant trend towards worse performance in the premanifest group. The only reverse pattern concerned SART error rate, where PMHD subjects made fewer errors than controls. We hypothesize that this reflects a high motivation. Clinical experience suggests that PMHD subjects are highly motivated to perform to their best on the tests, as they may wish to prove that there they are still in the premanifest phase.
Practical implications and limitations
Patients with HD may experience more distress from the decline of their cognitive functions rather than the presence of motor disturbances. The results from this study indicate that patients with HD experience difficulties with recovering after an error and maintaining attentional control for a longer period, which adds to the knowledge about cognition in HD and could have implication for daily care.
A limitation to the present study is the relative small number of subjects in the PMHD group and therefore having less statistical power. This could have obscured possible subtle differences from controls.
We conclude that there is an attentional control deficit in MHD. MHD subjects are cognitively not able to directly resume task requirements after having made an error and that they need more time to return to pre-error performance level. No attentional control deficits were found for the PMHD group.
Acknowledgments
We thank P.J. van Someren, F.I. Kerkhof, M.J. Stijl-Pek, G.H. van Beurkering-Louwes, J.G. van Vliet-de Regt, J.J.M. Witteman and W. Huyser for their help in EEG registration. We also thank our participants for their time and effort.
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Aron AR, Schlaghecken F, Fletcher PC, Bullmore ET, Eimer M, Barker R, Sahakian BJ, Robbins TW. Inhibition of subliminally primed responses is mediated by the caudate and thalamus: evidence from functional MRI and Huntington’s disease. Brain. 2003;126:713–723. doi: 10.1093/brain/awg067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aron AR, Watkins L, Sahakian BJ, Monsell S, Barker RA, Robbins TW. Task-set switching deficits in early-stage Huntington’s disease: implications for basal ganglia function. J Cogn Neurosci. 2003;15:629–642. doi: 10.1162/jocn.2003.15.5.629. [DOI] [PubMed] [Google Scholar]
- 3.Bellgrove MA, Hawi Z, Gill M, Robertson IH. The cognitive genetics of attention deficit hyperactivity disorder (ADHD): sustained attention as a candidate phenotype. Cortex. 2006;42:838–845. doi: 10.1016/S0010-9452(08)70426-X. [DOI] [PubMed] [Google Scholar]
- 4.Beste C, Saft C, Andrich J, Gold R, Falkenstein M. Response inhibition in Huntington’s disease––a study using ERPs and sLORETA. Neuropsychologia. 2008;46:1290–1297. doi: 10.1016/j.neuropsychologia.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Beste C, Willemssen R, Saft C, Falkenstein M. Response inhibition subprocesses and dopaminergic pathways: basal ganglia disease effects. Neuropsychologia. 2010;48:366–373. doi: 10.1016/j.neuropsychologia.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Chan RC. A further study on the sustained attention response to task (SART): the effect of age, gender and education. Brain Inj. 2001;15:819–829. doi: 10.1080/02699050110034325. [DOI] [PubMed] [Google Scholar]
- 7.Chan RC, Chen EY, Cheung EF, Chen RY, Cheung HK. A study of sensitivity of the sustained attention to response task in patients with schizophrenia. Clin Neuropsychol. 2004;18:114–121. doi: 10.1080/13854040490507208. [DOI] [PubMed] [Google Scholar]
- 8.Couette M, Bachoud-Levi AC, Brugieres P, Sieroff E, Bartolomeo P. Orienting of spatial attention in Huntington’s Disease. Neuropsychologia. 2008;46:1391–1400. doi: 10.1016/j.neuropsychologia.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Craufurd D, Thompson JC, Snowden JS. Behavioural changes in Huntington Disease. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:219–226. [PubMed] [Google Scholar]
- 10.Craufurd D, Thompson JC, Snowden JS. Behavioral changes in Huntington Disease. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:219–226. [PubMed] [Google Scholar]
- 11.Datta A, Cusack R, Hawkins K, Heutink J, Rorden C, Robertson IH, Manly T (2007) The p300 as a marker of waning attention and error propensity. Comput Intell Neurosci 93968 [DOI] [PMC free article] [PubMed]
- 12.de Tommaso M, Sciruicchio V, Specchio N, Difruscolo O, Vitale C, Specchio LM, Puca F. Early modifications of auditory event-related potentials in carriers of the Huntington’s disease gene. Acta Neurol Belg. 2003;103:192–198. [PubMed] [Google Scholar]
- 13.Dumas EM, van den Bogaard SJ, Ruber ME, Reilman RR, Stout JC, Craufurd D, Hicks SL, Kennard C, Tabrizi SJ, van Buchem MA, van der Grond J, Roos RA (2011) Early changes in white matter pathways of the sensorimotor cortex in premanifest Huntington’s disease. Hum Brain Mapp. doi:10.1002/hbm.2120510.1002/hbm.21205 [DOI] [PMC free article] [PubMed]
- 14.Duncan CC, Barry RJ, Connolly JF, Fischer C, Michie PT, Naatanen R, Polich J, Reinvang I, Van PC. Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300 and N400. Clin Neurophysiol. 2009;120:1883–1908. doi: 10.1016/j.clinph.2009.07.045. [DOI] [PubMed] [Google Scholar]
- 15.Fronczek R, Middelkoop HA, van Dijk JG, Lammers GJ. Focusing on vigilance instead of sleepiness in the assessment of narcolepsy: high sensitivity of the Sustained Attention to Response Task (SART) Sleep. 2006;29:187–191. [PubMed] [Google Scholar]
- 16.Giordani B, Berent S, Boivin MJ, Penney JB, Lehtinen S, Markel DS, Hollingsworth Z, Butterbaugh G, Hichwa RD, Gusella JF. Longitudinal neuropsychological and genetic linkage analysis of persons at risk for Huntington’s disease. Arch Neurol. 1995;52:59–64. doi: 10.1001/archneur.1995.00540250063014. [DOI] [PubMed] [Google Scholar]
- 17.RG HDC. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-E. [DOI] [PubMed] [Google Scholar]
- 18.Helton WS, Kern RP, Walker DR. Conscious thought and the sustained attention to response task. Conscious Cogn. 2009;18:600–607. doi: 10.1016/j.concog.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Homberg V, Hefter H, Granseyer G, Strauss W, Lange H, Hennerici M. Event-related potentials in patients with Huntington’s disease and relatives at risk in relation to detailed psychometry. Electroencephalogr Clin Neurophysiol. 1986;63:552–569. doi: 10.1016/0013-4694(86)90143-4. [DOI] [PubMed] [Google Scholar]
- 20.Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- 21.Jurgens CK, van der Hiele K, Reijntjes RH, van de Wiel L, Witjes-Ane MN, van der Grond J, Roos RA, Middelkoop HA, van Dijk JG. Basal ganglia volume is strongly related to P3 event-related potential in premanifest Huntington’s disease. Eur J Neurol. 2011;18:1105–1108. doi: 10.1111/j.1468-1331.2010.03309.x. [DOI] [PubMed] [Google Scholar]
- 22.Kok A. Event-related-potential (ERP) reflections of mental resources: a review and synthesis. Biol Psychol. 1997;45:19–56. doi: 10.1016/S0301-0511(96)05221-0. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence AD, Hodges JR, Rosser AE, Kershaw A, ffrench-Constant C, Rubinsztein DC, Robbins TW, Sahakian BJ. Evidence for specific cognitive deficits in preclinical Huntington’s disease. Brain. 1998;121(Pt 7):1329–1341. doi: 10.1093/brain/121.7.1329. [DOI] [PubMed] [Google Scholar]
- 24.Lemiere J, Decruyenaere M, Evers-Kiebooms G, Vandenbussche E, Dom R. Cognitive changes in patients with Huntington’s disease (HD) and asymptomatic carriers of the HD mutation––a longitudinal follow-up study. J Neurol. 2004;251:935–942. doi: 10.1007/s00415-004-0461-9. [DOI] [PubMed] [Google Scholar]
- 25.Luck SJ. An introduction to the event-related potential technique . London, England: The MIT Press; 2005. [Google Scholar]
- 26.Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- 27.Muller SV, Jung A, Preinfalk J, Kolbe H, Ridao-Alonso M, Dengler R, Munte TF. Disturbance of “extrinsic alertness” in Huntington’s disease. J Clin Exp Neuropsychol. 2002;24:517–526. doi: 10.1076/jcen.24.4.517.1043. [DOI] [PubMed] [Google Scholar]
- 28.Munte TF, Ridao-Alonso ME, Preinfalk J, Jung A, Wieringa BM, Matzke M, Dengler R, Johannes S. An electrophysiological analysis of altered cognitive functions in Huntington disease. Arch Neurol. 1997;54:1089–1098. doi: 10.1001/archneur.1997.00550210027009. [DOI] [PubMed] [Google Scholar]
- 29.Penney JB, Jr, Vonsattel JP, MacDonald ME, Gusella JF, Myers RH. CAG repeat number governs the development rate of pathology in Huntington’s disease. Ann Neurol. 1997;41:689–692. doi: 10.1002/ana.410410521. [DOI] [PubMed] [Google Scholar]
- 30.Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. ‘Oops!’: performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;35:747–758. doi: 10.1016/S0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- 32.Roman MJ, Delis DC, Filoteo JV, Demadura TL, Paulsen J, Swerdlow NR, Swenson MR, Salmon D, Butters N, Shults C. Is there a “subcortical” profile of attentional dysfunction? A comparison of patients with Huntington’s and Parkinson’s diseases on a global-local focused attention task. J Clin Exp Neuropsychol. 1998;20:873–884. doi: 10.1076/jcen.20.6.873.1111. [DOI] [PubMed] [Google Scholar]
- 33.Samavatyan H, Leth-Steensen C. The time course of task switching: a speed-accuracy trade-off analysis. Mem Cognit. 2009;37:1051–1058. doi: 10.3758/MC.37.7.1051. [DOI] [PubMed] [Google Scholar]
- 34.Shoulson I, Fahn S. Huntington disease: clinical care and evaluation. Neurology. 1979;29:1–3. doi: 10.1212/WNL.29.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Smilek D, Carriere JS, Cheyne JA. Failures of sustained attention in life, lab, and brain: ecological validity of the SART. Neuropsychologia. 2010;48:2564–2570. doi: 10.1016/j.neuropsychologia.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Sprengelmeyer R, Lange H, Homberg V. The pattern of attentional deficits in Huntington’s disease. Brain. 1995;118(Pt 1):145–152. doi: 10.1093/brain/118.1.145. [DOI] [PubMed] [Google Scholar]
- 37.Sterr A. Preparing not to move: does no-response priming affect advance movement preparation processes in a response priming task? Biol Psychol. 2006;72:154–159. doi: 10.1016/j.biopsycho.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Stout JC, Weaver M, Solomon AC, Queller S, Hui S, Johnson SA, Gray J, Beristain X, Wojcieszek J, Foroud T. Are cognitive changes progressive in prediagnostic HD? Cogn Behav Neurol. 2007;20:212–218. doi: 10.1097/WNN.0b013e31815cfef8. [DOI] [PubMed] [Google Scholar]
- 39.Tabrizi SJ, Scahill RI, Durr A, Roos RA, Leavitt BR, Jones R, Landwehrmeyer GB, Fox NC, Johnson H, Hicks SL, Kennard C, Craufurd D, Frost C, Langbehn DR, Reilmann R, Stout JC. Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet Neurol. 2011;10:31–42. doi: 10.1016/S1474-4422(10)70276-3. [DOI] [PubMed] [Google Scholar]
- 40.RS UHD. Unified Huntington’s disease rating scale: reliability and consistency. Huntington study group. Mov Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 41.van den Bogaard SJ, Dumas EM, Acharya TP, Johnson H, Langbehn DR, Scahill RI, Tabrizi SJ, van Buchem MA, van der Grond J, Roos RA (2010) Early atrophy of pallidum and accumbens nucleus in Huntington’s disease. J Neurol 258(3):412–420 [DOI] [PMC free article] [PubMed]
- 42.Witjes-Ane MN, Vegter-van der Vlis M, van Vugt JP, Lanser JB, Hermans J, Zwinderman AH, van Ommen GJ, Roos RA. Cognitive and motor functioning in gene carriers for Huntington’s disease: a baseline study. J Neuropsychiatry Clin Neurosci. 2003;15:7–16. doi: 10.1176/appi.neuropsych.15.1.7. [DOI] [PubMed] [Google Scholar]