Abstract
Small retrospective case series suggest that decompressive hemicraniectomy can be life saving in patients with cerebral venous thrombosis (CVT) and impending brain herniation. Prospective studies of consecutive cases are lacking. Thus, a single centre, prospective study was performed. In 2006 we adapted our protocol for CVT treatment to perform acute decompressive hemicraniectomy in patients with impending herniation, in whom the prognosis with conservative treatment was considered infaust. We included all consecutive patients with CVT between 2006 and 2010 who underwent hemicraniectomy. Outcome was assessed at 12 months with the modified Rankin Scale (mRS). Ten patients (8 women) with a median age of 41 years (range 26–52 years) were included. Before surgery 5 patients had GCS < 9, 9 patients had normal pupils, 1 patient had a unilaterally fixed and dilated pupil. All patients except one had space-occupying intracranial hemorrhagic infarcts. The median preoperative midline shift was 9 mm (range 3–14 mm). Unilateral hemicraniectomy was performed in 9 patients and bilateral hemicraniectomy in one. Two patients died from progressive cerebral edema and expansion of the hemorrhagic infarcts. Five patients recovered without disability at 12 months (mRS 0–1). Two patients had some residual handicap (one minor, mRS 2; one moderate, mRS 3). One patient was severely handicapped (mRS 5). Our prospective data show that decompressive hemicraniectomy in the most severe cases of cerebral venous thrombosis was probably life saving in 8/10 patients, with a good clinical outcome in six. In 2 patients death was caused by enlarging hemorrhagic infarcts.
Keywords: Sinus thrombosis, Intracranial, Hemicraniectomy, Stroke
Introduction
The standard treatment for cerebral venous thrombosis (CVT) is heparin and most patients recover well with this therapy. Approximately 20% of patients, however, remain handicapped or die [4]. The main cause of death is transtentorial herniation due large hemorrhagic infarcts [1].
Retrospective case series suggest that decompressive hemicraniectomy can be life saving and result in good clinical outcome in such patients [2, 9], sometimes even in seemingly hopeless cases [7]. A retrospective multinational registry of acute CVT cases undergoing decompressive surgery included 38 patients [3]. Twelve patients (32%) recovered completely [modified Rankin Scale (mRS) 0–1] and only 3 patients (8%) were severely dependent (mRS 4–5). Six patients (18%) died. Retrospective studies, however, may overestimate the efficacy because of recall and selection bias. Confirmation in prospective studies is therefore required, but at present, these studies are not available. We prospectively examined all CVT patients who underwent decompressive hemicraniectomy in our hospital since 2006. Here we report the results of the first 5 years.
Methods
Study population
We included all consecutive patients with CVT who presented to the Academic Medical Centre in Amsterdam, a tertiary care university hospital, between July 2006 and March 2011. Patients had decompressive craniectomy if they fulfilled the following criteria: (1) CVT confirmed by brain magnetic resonance imaging (MRI) with MRI venography or computed tomographic venography (CT-V). (2) Clinical signs of impending herniation, as defined by the presence of unilateral third nerve dysfunction and/or deterioration on the Glasgow Coma Scale (GCS). (3) The deterioration had to be the result of cerebral mass lesions (venous infarction or brain edema) and not attributable to seizures. The majority of the patients were transferred from other hospitals to our centre because of clinical deterioration. In 1 patient (case 10), CVT was diagnosed 1 day after evacuation of a 6 × 3 × 3 cm intracerebral hemorrhage from the left-sided temporal lobe. Because we considered decompressive craniectomy after July 2006 a standard treatment option for CVT patients with impending herniation, approval from the ethical committee was not required. As with any therapy, patients and family were counselled on the possible risks and benefits before treatment. The first cases have been published as a case report previously [2].
Medical management
Patients were admitted to the department of neurology, neurosurgery or intensive care. Patients received heparin (unfractionated or low-molecular weight heparin) in therapeutic doses immediately after they were diagnosed with CVT. During decompressive surgery, therapeutic heparin treatment was discontinued and continued only in prophylactic dose for at least 24 h post-operatively. Thereafter therapeutic dose heparin was restarted. In some patients prophylactic doses were given for a longer time because full dose heparin was considered too hazardous.
Decompressive hemicraniectomy
Decompressive hemicraniectomy consisted of the excision of a large bone flap and duraplasty. In summary, a large skin incision in the shape of a question mark based at the ear was made. A bone flap with a diameter of at least 12 cm including frontal, temporal and parietal bones was created. Since hemorrhagic infarcts in CVT are often large and frequently extend into the temporal lobe, we made special effort to extend the decompression toward the temporal skull base (Fig. 1). The dura was opened widely to ensure maximal decompression. We did not resect any hemorrhagic infarcts, hematoma or brain parenchyma, except in case 10 where the diagnosis of CVT was made after evacuation of a hematoma. The cortical surface was covered with the unapproximated dural flaps and absorbable hemostatic cellulose (Surgicel®), after which only the skin was closed. In cases of severe intra-operative brain swelling, an ipsilateral intraparenchymatous intracranial pressure (ICP) transducer was implanted. Patients underwent post-operative brain CT within 24 h. Re-implantation of the bone flap was performed within 12 months.
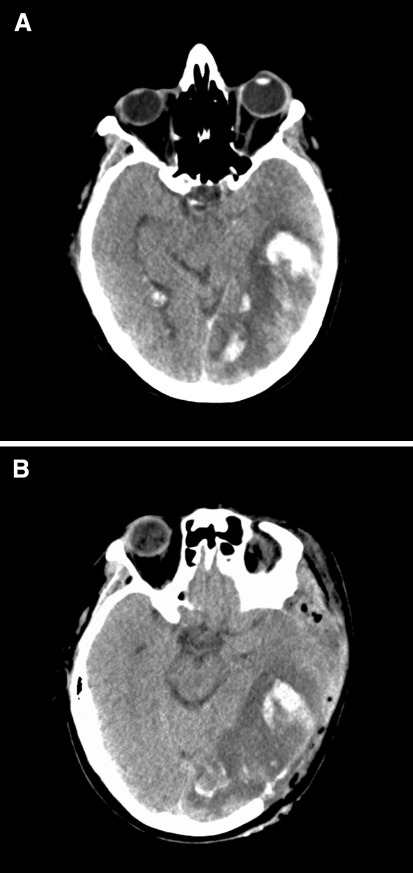
Fig. 1.
a Admission CT scan (of patient 4) shows left temporo-parieto-occipital hemorrhagic infarct, b Direct post-operative CT scan, large decompression extending toward the temporal skull base
Outcome analysis and long-term follow-up
Follow-up visits were performed at 6 and 12 months after the diagnosis. We assessed the outcome with the modified Rankin Scale (mRS; 0 = complete recovery, 6 = dead). We considered a mRS of 0, 1 or 2 (no disability or minor handicap) at 12 months the primary outcome. In case of missing values we imputed the last known score.
Statistical analysis
Continuous variables are reported as medians. We used univariate logistic regression analysis to examine the association between clinical and radiological variables and outcome. The following variables were examined: age; gender; comatose before surgery; fixed and dilated pupil(s) before surgery; comatose after surgery, fixed and dilated pupil(s) after surgery; new or increased hemorrhagic lesion after surgery.
Results
Baseline characteristics
Out of 56 CVT patients admitted in specified period, 10 patients (8 women and 2 men) were included. Compared to patients who did not receive decompressive surgery, those who underwent decompressive surgery had significantly more often intracranial hemorrhagic lesions, midline shift and were more frequently comatose (Table 1). Table 2 shows the individual baseline characteristics of the 10 included patients. The median age was 41 years (range 26–52 years). Median time from onset to diagnosis was 5 days (range 2–14 days) and from diagnosis to surgery was 2 days (range 0–4 days). Five patients were comatose before surgery and one had a unilateral fixed and dilated pupil. Six patients had one or more seizures before surgery.
Table 1.
Comparison of baseline characteristics between CVT patients who did and did not undergo decompressive hemicraniectomy during 2006–2011
| No DC | DC | |
|---|---|---|
| Number of patients | 46 | 10 |
| Median age | 42 | 41 |
| Gender (% female) | 67 | 80 |
| Comatose (GCS < 9) at admission (%) | 7 | 30* |
| Epileptic seizure(s) (%) | 22 | 40 |
| Hemorrhagic lesion(s) (%) | 35 | 100* |
| Midline shift (%) | 13 | 100* |
| Endovascular treatment (%) | 9 | 10 |
GCS Glasgow Coma Scale, DC decompressive craniectomy
* p < 0.05
Table 2.
Baseline characteristics
| Patient number | Sex (M/F) | Age (years) | Onset to diagnosis (days) | Diagnosis to surgery (days) | Before surgery GCS |
Before surgery Pupils |
Before surgery Seizures |
|---|---|---|---|---|---|---|---|
| 1 | M | 39 | 4 | 2 | E1M4Vt | +/+ | – |
| 2 | F | 36 | 3 | 2 | E3M6V4 | +/+ | + |
| 3 | F | 42 | 7 | 2 | E1M5V1 | +/+ | + |
| 4 | F | 52 | 8 | 0 | E3M5V2 | +/+ | – |
| 5 | F | 36 | 5 | 1 | E3M5V2 | +/+ | + |
| 6 | M | 52 | 2 | 0 | E1M4Vt | +/+ | + |
| 7 | F | 37 | 6 | 0 | E1M1Vt | +/+ | + |
| 8 | F | 29 | 2 | 0 | E3M6V4 | −/+ | – |
| 9 | F | 26 | 14 | 4 | E2M5V2 | +/+ | + |
| 10 | F | 52 | 4 | 4 | E1M4Va | +/+ | – |
GCS Glasgow Coma Scale, t tube, a aphasia
Radiological examinations, surgery and post-operative condition
Results of the radiological examinations are shown in Table 3. The pre- and post-operative CT scans of all patients are shown in Fig. 2. Six patients had left-sided hemorrhagic infarcts, 2 patients had right-sided hemorrhagic infarcts and 2 patients had bilateral lesions. All patients had space-occupying intracranial hemorrhagic infarcts except 1 patient. This patient (case 9) had extensive subarachnoid bleeding and obliterated basal cisterns, and a bilateral craniectomy was performed. The median preoperative midline shift was 9 mm (range 3–14, Table 4). The median diameter of the bone flap was 15 cm (range 12–17 cm). Because of considerable brain swelling, an ICP transducer was implanted at the end of the procedure in 3 patients (cases 4, 6 and 9). The median post-operative midline shift was 4 mm (range 0–9). Three patients were comatose after surgery. Post-operatively, 8 patients had normal pupils; 2 had bilaterally fixed and dilated pupils. New hemorrhagic lesions occurred in 4 patients. In 1 patient (case 4), this new lesion occurred in the region of the ICP transducer, after its removal.
Table 3.
Radiological findings
| Patient number | Thrombosed sinuses | Hemorrhagic infarcts | Volume lesion (cm3) | Midline shift pre operative (mm) | Bilateral lesions | Location hemorrhagic infarcts |
|---|---|---|---|---|---|---|
| 1 | STL, SSL, CV | + | 110 | 12 | – | Left temporal |
| 2 | SSS, CV | + | 133 | 9 | – | Right parieto-occipital |
| 3 | SSS, SR, CV | + | 39 | 4 | – | Left frontal |
| 4 | STL, SSL, SR, DCS, CL, CV | + | 78 | 14 | – | Left temporo-parieto occipital, cerebellar |
| 5 | STL, SSL, SR, DCS, CL, CV | + | 165 | 12 | – | Left temporo-occipital, cerebellar |
| 6 | SSS, SR, JL, CV | + | 102 | 7 | + | Left fronto-parietal, right parietal |
| 7 | SSS, STR, SSR, CV | + | 58 | 5 | – | Left frontal |
| 8 | STR, SSR, CV | + | 161 | 9 | – | Right temporo-occipital |
| 9 | SSS, STL, STR, SSL, SSR, SR, JL, JR, CV | + | 1a | 3 | + | Left parietal, right parietal extensive subarachnoid bleeding |
| 10 | STL, SSL, JL, CV | + | 152 | 10 | – | Left temporo-parieto-occipital |
SSS superior sagittal sinus, ST transverse sinus (R right, L left), SR sinus rectus (straight sinus), SS sigmoid sinus (R right, L left); CV cortical vein, DCS deep cerebral venous system, C cerebellar vein (R right, L left), J jugular vein (R right, L left)
aExtensive subarachnoid bleeding and obliterated basal cisterns
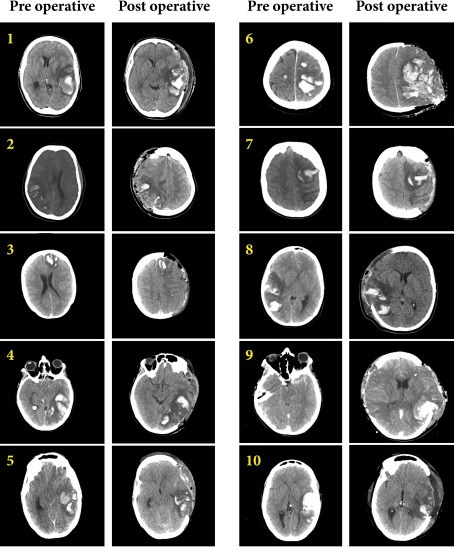
Fig. 2.
Pre- and post-operative CT scans of all 10 cases. All patients had space-occupying intracranial hemorrhagic infarcts except case 9. Case 9 had extensive subarachnoid bleeding, small bilateral hemorrhages, generalized cerebral edema and obliterated basal cisterns. Case 3 had besides the hemorrhagic infarct obliterated basal cisterns due to increased bilateral cerebral edema
Table 4.
Surgical characteristics and outcome
| Patient number | Intervention | Midline shift (mm) | GCS | Pupils | New hemorrhage | Additional interventions | mRS 6 months |
mRS 12 months |
|---|---|---|---|---|---|---|---|---|
| 1 | U | 7 | E4M6Va | +/+ | – | – | 2 | 1 |
| 2 | U | 4 | E4M6V5 | +/+ | – | – | 1 | 1 |
| 3 | U | 0 | E1M5Vt | +/+ | – | – | 1 | 1 |
| 4 | U | 3 | E3M5Vt | +/+ | + | – | 3 | 3 |
| 5 | U | 2 | E3M6V2 | +/+ | – | – | 1 | 1 |
| 6 | U | 9 | E1M1Vt | −/− | + | Enlarging hemicraniectomy | 6 | 6 |
| 7 | U | 4 | E3M5Vt | +/+ | + | Thrombosuction | 2 | 2 |
| 8 | U | 0 | E4M6V5 | +/+ | – | – | 2 | 0 |
| 9 | B | 4 | E1M1Vt | −/− | + | Enlarging hemicraniectomy | 6 | 6 |
| 10 | U | 0 | E4M6Va | +/+ | – | Evacuation subdural empyema | 5 | a |
U unilateral hemicraniectomy, B bilateral hemicraniectomy, GCS Glasgow Coma Scale, t tube, a aphasia, mRS modified Rankin Scale, a12 month outcome not yet available
Additional interventions
Patient 7 underwent endovascular trombosuction 2 days after the hemicraniectomy because of lack of clinical improvement after decompressive hemicraniectomy, despite an uncomplicated procedure. She recovered with a minor handicap (mRS 2). Four patients needed additional operations. Two patients (cases 6 and 9) required re-operation because of progressive edema, expansion of the hemorrhagic infarcts and pathologically elevated ICP. Both underwent enlargement of the decompressive craniectomy, but both died. Patient 10 developed a cerebral abscess and subdural empyema at the site of the hemicraniectomy which was surgically removed. She also developed bacterial meningitis and hydrocephalus, and needed an external ventricular drain, which was later converted to a ventricular peritoneal shunt. Patient 4 developed an epidural hematoma after re-implantation of the bone flap and subsequently had two re-operations.
Clinical outcome
Two patients died during the primary hospital admission (Table 3). Six patients had a good clinical outcome (mRS 0–2) at 12 months. Two patients had a moderate or severe residual handicap: patient 4 had a severe expressive aphasia and a right-sided homonymous hemianopia (mRS 3). Patient 10 is still bedridden and incontinent after 6 months (mRS 5). All surviving patients received oral anticoagulation for a period of 6–12 months. Six patients had seizures during follow-up, 5 of them still at 12 months. Despite the small sample size we attempted to identify prognostic variables using univariate logistic regression analysis. There was a trend that new hemorrhagic lesions after surgery was associated with a poor outcome at 12 months (odds ratio 0.07, 95% CI 0.003–1.51, p = 0.09). No other prognostic factors were identified.
Discussion
This is the first prospective cohort study of ten CVT patients with impending transtentorial herniation undergoing decompressive craniectomy. Six patients had good functional recovery after 1 year. Two patients died despite intervention. The largest study so far on this subject is a combination of a retrospective multinational registry and systematic review of published cases [3], with outcome data of 69 patients. Forty-five patients had decompressive craniectomy, 7 hematoma evacuation, and 17 patients underwent both types of surgery. Compared to our study, both mortality (16 vs. 20%) and the percentage of patients who were functionally independent at follow-up (57 vs. 60%) were similar (Table 5). However, patients from this retrospective study were generally in a worse clinical condition prior to surgery: 72% were comatose, compared to 50% in our study, and 57% had single or bilateral fixed and dilated pupils, compared to 10% in our cohort.
Table 5.
Comparison of outcomes with retrospective data
| Ferro et al. [3] | Current study | |
|---|---|---|
| Design | Retrospective | Prospective |
| Number of patients | 69 | 10 |
| Median age | 42 | 41 |
| Comatose before surgery (%) | 72 | 50 |
| Fixed and dilated pupil(s) before surgery (%) | 57 | 10 |
| Hemorrhagic lesions (%) | 90 | 100 |
| Independence at follow-up (mRS 0–2) (%) | 57 | 60 |
| Mortality (%) | 16 | 20 |
mRS modified Rankin Scale
Transtentorial herniation is the most common cause of death during the acute stage of CVT [1]. Prior to 2006, before we adopted our policy to perform decompressive craniectomy, most of our CVT patients with impending transtentorial herniation died despite maximal conservative treatment and endovascular thrombolysis [6]. A recent French study also showed a large difference in mortality between patients who did or did not undergo decompressive craniectomy, in favor of surgical intervention [9]. The most plausible explanation is that craniectomy removes the immediate threat of fatal herniation, analogous to the effect of craniectomy in ischemic stroke [10].
We tried to make the craniectomy wide and to extend decompression toward the temporal skull base. If the craniectomy is too small, the decompressive effect may not be sufficient to prevent or reverse transtentorial herniation. Re-operation to enlarge the decompression was performed in 2 patients who did not clinically improve after craniectomy and in whom post-operative ICP remained pathologically elevated. Nevertheless, both patients died from intractable expanding cerebral hemorrhagic infarcts.
The large number of patients who underwent decompressive craniectomy in our study (10 out of 56; 18%) cannot be extrapolated to CVT patients in general. Our hospital serves as a tertiary care centre for CVT patients in the Netherlands. While milder cases are usually treated at local hospitals, neurologists tend to refer severe cases to our centre. Therefore, the percentage of all CVT patients undergoing decompressive surgery is certainly much lower than the 18% in our study.
A reason why clinicians may be reluctant to perform decompressive craniectomy could be the fear that it may reduce mortality at the expense of an increase of severely disabled survivors. A randomized trial on decompressive craniectomy for ischemic stroke indeed showed a fivefold increase in severely disabled patients (mRS 4 or 5) [10]. This concern does not seem warranted in CVT patients. While the decision to perform decompressive craniectomy must still be carefully weighed in each case, the large percentage of patients with a good functional outcome justifies a positive stance regarding this intervention. Moreover, withholding craniectomy in these patients will result in death by herniation in nearly all cases. The relatively low number of severely disabled survivors is probably due to the fact that venous infarcts in general have more potential for recovery than arterial infarcts [5]. For the same reason, we prefer not to evacuate the venous infarct or hematoma during the decompressive surgery, except when this is considered the last resort to reverse the herniation process.
New hemorrhagic lesions were identified on post-operative imaging in 4 out of 10 patients and tended to be associated with poor outcome. Both patients who died had expanding hemorrhagic infarcts with new hemorrhagic areas after surgery. The etiology of new lesions is unclear. One possibility is that the elevated pre-operative ICP prevents the development, or enlargement, of hemorrhagic lesions, and that new hemorrhagic lesions develop when the ICP decreases following craniectomy. We did not systematically measure ICP during surgery, so we cannot examine whether there was an association between ICP and new hemorrhagic lesions. Another possibility is that developing collateral venous systems draining to the skull or scalp may be damaged during surgery [8]. Thirdly, we cannot exclude that new hemorrhagic lesions resulted from direct manipulation of the cortex during surgery or during post-operative removal of ICP transducers. Finally, the need to interrupt or reduce the heparin might cause extension of the thrombus and occlusion of (new) cortical veins.
The currently available evidence suggests that decompressive craniectomy often results in good functional outcome in CVT patients with impending transtentorial herniation. Obviously, additional prospective data on its efficacy are needed. However, we believe that a randomized controlled trial faces insurmountable ethical objections since most of these patients will die if left untreated [1, 6, 9]. Instead, an international prospective registry of consecutive cases would be preferable. Currently, efforts are being undertaken to initiate such a project. Until that time, however, we believe that decompressive craniectomy should be strongly considered in CVT patients who have both clinical and radiological sign of impending herniation.
Acknowledgments
We thank C.L. Vleggeert-Lankamp, MD PhD, for her contribution. S.M. Zuurbier is supported by “Netherlands Heart Foundation” grand number 2009B016. J.M. Coutinho is supported by “The Netherlands Organisation for Scientific Research” (NWO) grand number 021.001.045.
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Susanna M. Zuurbier and Jonathan M. Coutinho contributed equally.
References
- 1.Canhao P, Ferro JM, Lindgren AG, Bousser MG, Stam J, Barinagarrementeria F. Causes and predictors of death in cerebral venous thrombosis. Stroke. 2005;36:1720–1725. doi: 10.1161/01.STR.0000173152.84438.1c. [DOI] [PubMed] [Google Scholar]
- 2.Coutinho JM, Majoie CB, Coert BA, Stam J. Decompressive hemicraniectomy in cerebral sinus thrombosis: consecutive case series and review of the literature. Stroke. 2009;40:2233–2235. doi: 10.1161/STROKEAHA.108.543421. [DOI] [PubMed] [Google Scholar]
- 3.Ferro JM, Crassard I, Coutinho JM, Canhao P, Barinagarrementeria F, Cucchiara B, Derex L, Lichy C, Masjuan J, Massaro A, Matamala G, Poli S, Saadatnia M, Stolz E, Viana-Baptista M, Stam J, Bousser MG (2011) Decompressive surgery in cerebrovenous thrombosis: a multicenter registry and a systematic review of individual patient data. Stroke 42:2825–2831 [DOI] [PubMed]
- 4.Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F. Prognosis of cerebral vein and dural sinus thrombosis: results of the international study on cerebral vein and dural sinus thrombosis (ISCVT) Stroke. 2004;35:664–670. doi: 10.1161/01.STR.0000117571.76197.26. [DOI] [PubMed] [Google Scholar]
- 5.Lovblad KO, Bassetti C, Schneider J, Guzman R, El-Koussy M, Remonda L, Schroth G. Diffusion-weighted mr in cerebral venous thrombosis. Cerebrovasc Dis. 2001;11:169–176. doi: 10.1159/000047634. [DOI] [PubMed] [Google Scholar]
- 6.Stam J, Majoie CB, van Delden OM, van Lienden KP, Reekers JA. Endovascular thrombectomy and thrombolysis for severe cerebral sinus thrombosis: a prospective study. Stroke. 2008;39:1487–1490. doi: 10.1161/STROKEAHA.107.502658. [DOI] [PubMed] [Google Scholar]
- 7.Stefini R, Latronico N, Cornali C, Rasulo F, Bollati A. Emergent decompressive craniectomy in patients with fixed dilated pupils due to cerebral venous and dural sinus thrombosis: report of three cases. Neurosurgery. 1999;45:626–629. doi: 10.1097/00006123-199909000-00038. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi S, Nawashiro H (2011) Decompressive craniectomy for cerebral venous thrombosis. Platelets 22:157–159 [DOI] [PubMed]
- 9.Theaudin M, Crassard I, Bresson D, Saliou G, Favrole P, Vahedi K, Denier C, Bousser MG. Should decompressive surgery be performed in malignant cerebral venous thrombosis?: a series of 12 patients. Stroke. 2010;41:727–731. doi: 10.1161/STROKEAHA.109.572909. [DOI] [PubMed] [Google Scholar]
- 10.Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, Amelink GJ, Schmiedeck P, Schwab S, Rothwell PM, Bousser MG, van der Worp HB, Hacke W. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–222. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]