Abstract
The demand for salinity-tolerant turfgrasses is increasing due to augmented use of effluent or low-quality water (sea water) for turf irrigation and the growing turfgrass industry in coastal areas. Experimental plants, grown in plastic pots filled with a mixture of river sand and KOSASR peat (9 : 1), were irrigated with sea water at different dilutions imparting salinity levels of 0, 8, 16, 24, 32, 40, or 48 dS m−1. Salinity tolerance was evaluated on the basis of leaf firing, shoot and root growth reduction, proline content, and relative water content. Paspalum vaginatum was found to be most salt tolerant followed by Zoysia japonica and Zoysia matrella, while Digitaria didactyla, Cynodon dactylon “Tifdwarf,” and Cynodon dactylon “Satiri” were moderately tolerant. The results indicate the importance of turfgrass varietal selection for saline environments.
1. Introduction
Salinity is a major abiotic environmental stress that is reported to be responsible for reducing plant growth across the globe. Sea water intrusion, in coastal states, has imposed salinity problems in turfgrass culture [1, 2]. Sodium chloride (NaCl) is the major compound contributing salinity in soils, and more salt-tolerant turfgrasses are required to cope this problem [3]. Therefore, development of salt-tolerant turfgrasses is becoming increasingly necessary in many parts of the world including Malaysia. Salt accumulation in soils, limitations on use of groundwater, and salt water intrusion into groundwater may restrict cultivation of glycophytic crops in these areas [4]. Salinity lowers water potential and restricts of water to plants [5]. Presence of excessive salt (NaCl) outside the cell can induce an osmotic stress, which may adversely affect the plant growth [6]. Hence, osmotic balance or osmoregulation is certainly a crucial factor for the survival of a plant under salt-stressed conditions. Generally, plants have developed different adaptive mechanisms to mitigate salinity under the saline environments [7–9]. Among these, salt exclusion is considered to be the most important adaptive feature of nonhalophytic plants, whilst most tolerant halophytes are salt accumulators [5]. Salt-accumulating halophytes are very crucial for osmotic adjustment. It could be achieved in the following ways: (i) by accumulating inorganic osmolyte (K+) and/or (ii) accumulating organic osmolytes such as proline. Therefore, salt-tolerant halophytic plants have the capability to minimize the detrimental effects by morphological means and physiological or biochemical processes [10].
Some of the turfgrass species are halophytic in nature. So salt-tolerant turf varieties would allow landscape development in saline environments and would be ideal in such environments, where limited or no fresh water is available for irrigation and salt water is the only option for irrigation practices. In addition, the use of sea water is also a good strategy for weed control in seashore paspalum worldwide. The native bermudagrass (Cynodon dactylon) here is quite salt-tolerant and grows vigorously, other salt-tolerant turfgrass species may also grow in the saline environments. In our previous reports [11, 12], several turfgrass species were identified in the coastal areas of Malaysia. Interestingly, the development of turfgrass industry especially in the coastal areas of Malaysia is an emerging field. To the best of our knowledge, published literatures are very scanty on salt tolerance studies in turfgrass species, which have been or being conducted in Malaysia. Therefore, this study was framed to determine the relative salinity tolerance and growth response of six important turfgrass species to salinity.
2. Materials and Methods
Glasshouse experiments were conducted at Faculty of Agriculture, University Putra Malaysia. Plastic pots (14 × 15 cm) were filled up with sandy soil (a mixture of river sand and peat; 9 : 1, v/v). The sandy soil had electrical conductivity (EC) 0.3 dS m−1, organic carbon 0.69%, sand 97.93%, silt 1.89%, and clay 0% with pH 5.23. The glasshouse temperature, relative humidity, and light intensity in morning time were 32°C, 80%, and 110 micromol m−2 s−1, and after noon 36°C, 70%, and 175 micromol m−2 s−1, respectively. The temperature was measured using a laboratory thermometer, and light intensity was monitored using a heavy duty light meter (Extech model 407026). Based on earlier findings of [13, 14], the three most salt-tolerant and three medium salt tolerant turfgrass species (Table 1) were used in this study.
Table 1.
Turfgrass species used in this study.
| Scientific name | Common name | Salt tolerance |
|---|---|---|
| Paspalum vaginatum Sw. | Seashore paspalum | Salt tolerant |
| Zoysia japonica Steud. | Japanese lawn grass | Salt tolerant |
| Zoysia matrella (L.) Merrill | Manila grass | Salt tolerant |
| Cynodon dactylon x. Cynodon transvaalensis. | Hybridbermuda grass (Satiri) | Medium salt tolerant |
| Cynodon dactylon x. Cynodon transvaalensis. | Hybridbermuda grass (Tifdwarf) | Medium salt tolerant |
| Digitaria didactyla Willd. | Serangoon grass | Medium salt tolerant |
The native soil was washed off the sods, and the sods were then transplanted into the plastic pots and grown for 8 weeks under nonsaline irrigation to achieve full growth. Three plants were transplanted in each pot. All species were narrow leaf and were clipped weekly at a cutting height of 5 mm. After 8 weeks thereafter, salinity treatments were initiated. Salinity treatments of 0, 8, 16, 24, 32, 40, and 48 dS m−1 (sea water) were applied. The control grasses were irrigated with distilled water. Sea water was diluted by adding distilled water to achieve different treatments. To avoid salinity shock, salinity levels were increased gradually by 8 dSm−1 day−1 for each treatment until the final salinity levels were achieved. After that, irrigation water was applied daily upto four weeks. The amount of water applied was 200 mL per pot. Data on leaf firing, proline, chlorophyll, relative water content, shoot and root dry weight were recorded 4 weeks after application of salinity treatment.
2.1. Determination of Leaf Firing
Leaf firing was estimated as total percentage of chlorotic leaf area, with 0% corresponding to no leaf firing and 100% for total brown leaves [15].
2.2. Determination of Shoot and Root Dry Weight
At the end of experiment (four weeks after salt initiation), shoots above the soil surface were harvested and washed with tap water and then distilled water to remove all soil particles. After harvesting the shoots, roots were removed from the soil, washed with tap water, and rinsed with distilled water. The shoot and root samples were then oven-dried to a constant weight at 70°C for 3 days. The dry weight (g/plant) was recorded for each treatment.
2.3. Determination of Proline Content
Proline was estimated following method of [16]. Fresh leaf tissue (0.5 g) was homogenized in 10 mL of 3% sulfosalicylic acid, and the homogenate was filtered through Whatman no. 2 filter paper. Two milliliters of the filtrate were brought to reaction with 2 mL acid ninhydrin solution (1.25 g ninhydrin in 30 mL glacial acetic acid), 20 mL orthophosphoric acid (6 M), and 2 mL of glacial acetic acid for 1 h at 100°C. The reaction was terminated in an ice bath. The reaction mixture was extracted with 4 mL toluene, mixed vigorously by passing a continuous stream of air for 1-2 min. The chromophore containing toluene was aspirated from the aqueous phase, warmed at room temperature, and the absorbance was recorded spectrophotometrically (Model UV-3101PC, UV-VIS NIR) at 520 nm. The proline concentration was determined from a standard curve and calculated on fresh weight basis as follows:
| (1) |
2.4. Determination of Chlorophyll Content
Chlorophyll content was estimated following method of [17]. Fresh leaves, from each pot, were cut into small pieces using a scissors and 200 mg of cut leaves were transferred into a plastic vial containing 20 mL of 80% acetone. The vial was quickly corked airtight and kept in the dark for 72 h. Absorbance of the solution was recorded at 645 and 663 nm spectrophotometrically (Model UV-3101PC, UV-VIS NIR). Chlorophyll content was estimated and expressed as mg g−1 of sample using the following formulae:
| (2) |
where A 645 and A 663 represent absorbance of solution at 645 and 663 nm, respectively, V: volume of the solution in mL, W: weight of fresh leaf sample in gram, 12.7, 2.69, 22.9, 4.86, 20.2, and 8.02 are absorption coefficients.
2.5. Determination of Relative Water Content
Relative water content (RWC) was determined as described by [18] on leaf tissues excised in the morning (around 9.00 am). Excised leaves from each pot (0.2 g) were measured for fresh weight (FW), and leaf samples were rehydrated in a water-filled petri dish for 4 h at room temperature. Turgor weight (TW) was measured by allowing full rehydration, removing all water from leaf surface, and weighing. Leaf dry weights were recorded after oven drying for one week at 60°C. The leaf relative water content was determined using the following formula:
| (3) |
2.6. Root Histology Using Scanning Electron Microscopy
Roots were sampled from two root zones (root tips at 0–50 mm from tip, and mature roots) and were cut into 5 mm portions with a sharp blade. The excised roots were placed in formalin acetic acid (FAA) and vacuumed for 1 h at 650 mm Hg. Specimens were postfixed in 1% osmium tetraoxide for 2 h, dehydrated for 30 min in each graded ethanol series at 30, 50, 70, 90, 95, and 100%, and dried in Baltec CPD 030 critical point dryer apparatus. The tissues were mounted on stubs, coated with gold using auto fine coater (JEOL JFC-1600, Japan) for 20 min, and viewed under a scanning electron microscope (JEOL JSM-5610LV, Japan), at high vacuum and acceleration voltage of 15 kV with a working distance of 23 mm.
2.7. Statistical Analysis
Data were analyzed statistically following randomized complete block design using ANOVA procedure in SAS statistical software (SAS). The treatment means were compared using protected least significant differences (LSD) at 5% level. Data of leaf firing was proportionate, so arcsine square root transformation was done.
3. Results
3.1. Leaf Firing
Interaction of salinity and species had a significant effect on leaf firing (Table 2). Leaf firing (%) increased with increasing salinity in all turfgrass species (Table 3). However, comparatively less salinity injury was recorded in P. vaginatum, Z. japonica, and Z. matrella compared to D. didactyla, C. dactylon “Tifdwarf,” and C. dactylon “Satiri” at all salinity levels. There was no injury (0%) recorded in all species up to 16 dS m−1 salinity, except for D. didactyla and C. dactylon “Tifdwarf” which showed light injury symptoms of 5 and 8%, respectively. At 24 dS m−1, the highest injury (25%) was recorded in D. didactyla, while the lowest injury of 5% was observed in P. vaginatum. At 32 dS m−1, leaf firing drastically increased to 79 and 75% in D. didactyla and C. dactylon “Tifdwarf,” respectively. At the highest salinity level of 48 dS m−1, the least leaf firing was observed in P. vaginatum (15%) followed by Z. japonica (25%) and Z. matrella (39%) compared to 80–100% leaf firing in D. didactyla, C. dactylon “Tifdwarf,” and C. dactylon “Satiri.” Overall, the highest leaf firing was recorded in D. didactyla, while the lowest in P. vaginatum.
Table 2.
Main effect and interaction effect on different variables by salinity and species.
| Variable | Salinity | Species | Salinity × species |
|---|---|---|---|
| Leaf firing | 1665.78*** | 513.16*** | 75.83*** |
| Shoot dry weight | 95.82*** | 1317.65*** | 4.01*** |
| Root dry weight | 79.83*** | 287.54*** | 1.15 ns |
| Proline | 2176.10*** | 585.87*** | 58.07*** |
| Relative water content | 78.07*** | 13.85*** | 1.45 ns |
| Chlorophyll-a | 30.03*** | 152.19*** | 0.89 ns |
| Chlorophyll-b | 67.91*** | 78.03*** | 4.20*** |
| Total chlorophyll | 65.86*** | 206.75*** | 2.13*** |
Numbers are F values significant at ***P < 0.0001, ns: not significant.
Table 3.
Effect of salinity on leaf firing of six turfgrass species.
| ECw
(dS m−1) |
Turfgrass species (% leaf firing) | ||||||
|---|---|---|---|---|---|---|---|
| P. vaginatum | Z. japonica | Z. matrella | D. didactyla | C. dactylon “Tifdwarf” | C. dactylon “Satiri” | LSD (0.05) | |
| 0 | 0 e (0.28) | 0 e (0.28) | 0 e (0.28) | 0 f (0.28) | 0 f (0.28) | 0 e (0.28) | 0.00 |
| 8 | 0 e (0.28) | 0 e (0.28) | 0 e (0.28) | 0 f (0.28) | 0 f (0.28) | 0 e (0.28) | 0.00 |
| 16 | 0 e (0.28) | 0 e (0.28) | 0 e (0.28) | 5 e (12.79) | 8 e (16.37) | 0 e (0.28) | 2.45 |
| 24 | 5 d (12.89) | 10 d (18.26) | 15 d (22.65) | 25 d (29.90) | 18 d (25.01) | 15 d (22.65) | 4.31 |
| 32 | 8 c (16.37) | 15 c (22.65) | 20 c (26.49) | 79 c (63.17) | 45 c (42.14) | 25 c (29.95) | 2.57 |
| 40 | 12 b (20.20) | 20 b (26.52) | 26 b (30.64) | 93 b (76.80) | 85 b (67.39) | 69 b (56.37) | 4.32 |
| 48 | 15 a (22.65) | 25 a (29.95) | 39 a (38.64) | 100 a (89.75) | 94 a (77.81) | 80 a (63.83) | 4.52 |
|
| |||||||
| LSD (0.05) | 2.31 | 2.34 | 2.30 | 6.19 | 4.36 | 5.11 | |
Means within columns followed by the same letter are not significantly different at P = 0.05 (LSD test).
Values in the parentheses indicate transformed by Arcsine square root.
3.2. Shoot Dry Weight
Interaction effect of salinity and species was significant (P < 0.05) on shoot dry weight (Table 2). Shoot dry weights (SDWs) of turfgrass species decreased as the level of salinity increased (Figure 1). Results showed that P. vaginatum was the most salt-tolerant species being statistically significant with others. At the highest salinity level (48 dS m−1), SDW reduction in P. vaginatum was only 23% relative to control treatment. Zoysia japonica followed a similar trend as P. vaginatum for salinities upto 24 dS m−1. At 48 dS m−1, significantly higher SDW reductions were observed in D. didactyla (51%), C. dactylon “Tifdwarf” (53%), and C. dactylon “Satiri” (44%).
Figure 1.
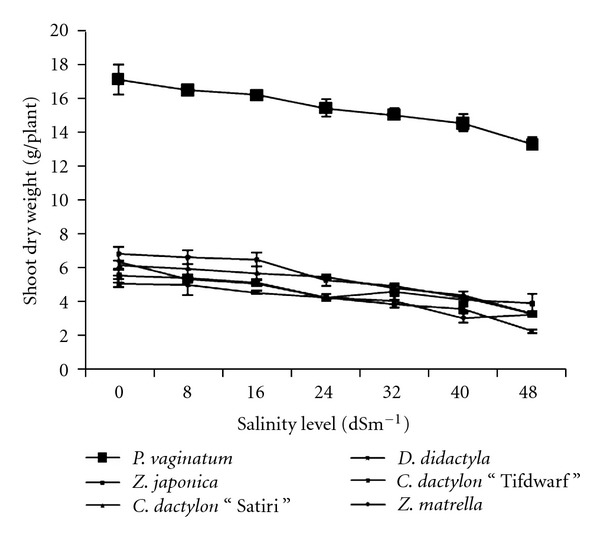
Shoot dry weight at different salinity levels of six turfgrass species.
3.3. Root Dry Weight
The results showed that root dry weight (RDW) significantly (P < 0.05) decreased with increasing salinity (Figure 2). At 16 dS m−1, a significant difference was noted among the species. However, P. vaginatum, C. dactylon “Tifdwarf,” Z. japonica, and Z. matrella produced greater RDW than the others at 24 dS m−1 salinity. At the highest salinity (48 dS m−1), RDW reduction was least in P. vaginatum (34%) followed by Z. japonica (46%); while highest in C. dactylon “Tifdwarf” (67%) followed by C. dactylon “Satiri” (54%), Z. matrella (53%), and D. didactyla (47%). However, there were nonsignificant effect on root dry matter yield when salinity and species were interacted (Table 2).
Figure 2.
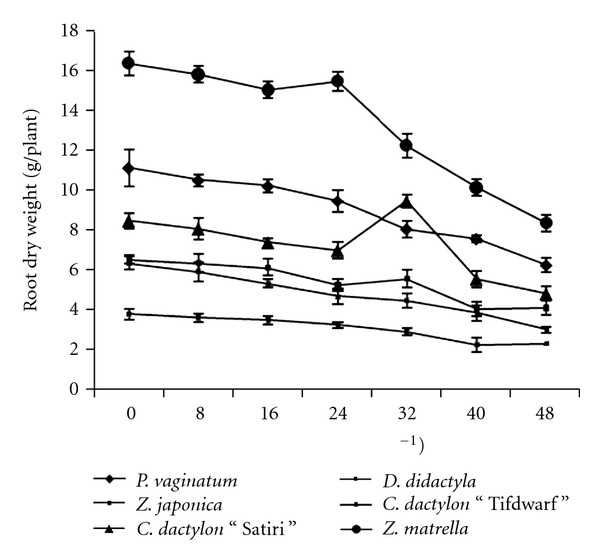
Root dry weight at different salinity levels of six turfgrass species.
3.4. Leaf Proline Content
Proline accumulation in the leaves of all turfgrass species increased with increasing salinity (Table 4). There were two distinct trends in proline accumulation among the species analyzed. In all turfgrass species (except C. dactylon “Satiri”), proline accumulation increased gradually up to 24 dS m−1 but increased abruptly at 32 and 48 dS m−1. At 48 dS m−1, a significantly higher (23.4-folds over the control) accumulation of proline was observed in P. vaginatum compared to in C. dactylon “Tifdwarf” (11.6-folds). There was a difference between the grasses with respect to proline accumulation patterns at 32 and 48 dS m−1. On the basis of proline accumulation ability, turfgrass species were ranked as P. vaginatum > Z. matrella > D. didactyla > Z. japonica > both of the C. dactylon entries. Interaction between salinity and species had also a significant (P < 0.001) effect on proline level (Table 7).
Table 4.
Effect of salinity on leaf proline content of six turfgrass species.
| ECw
(dS m−1) |
Turfgrass species (proline contents in mg g−1, fresh weight) | ||||||
|---|---|---|---|---|---|---|---|
| P. vaginatum | Z. japonica | Z. matrella | D. didactyla | C. dactylon “Tifdwarf” | C. dactylon “Satiri” | LSD (0.05) | |
| 0 | 3.33 f | 3.60 d | 3.67 f | 3.55 e | 5.60 f | 6.35 e | 0.96 |
| 8 | 4.60 ef (1.4) | 4.07 d (1.1) | 4.62 f (1.3) | 6.42 e (1.8) | 7.25 f (1.3) | 10.60 e (1.7) | 2.31 |
| 16 | 7.80 ed (2.3) | 6.50 d (1.8) | 6.02e (1.7) | 12.40 d (3.5) | 15.35 e (2.7) | 29.90 d (4.7) | 3.65 |
| 24 | 11.61 d (3.5) | 13.10 c (3.6) | 9.24 d (2.5) | 15.05 d (4.2) | 26.35 d (4.7) | 52.50 c (8.3) | 1.77 |
| 32 | 26.90 c (8.1) | 16.25 c (4.5) | 11.30 c (3.1) | 34.55 c (9.7) | 37.57 c (6.7) | 66.52 b (10.5) | 3.53 |
| 40 | 51.20 b (15.4) | 45.82 b (12.7) | 25.57 b (7.0) | 43.27 b (12.2) | 65.15 b (11.2) | 71.35 a (11.2) | 4.09 |
| 48 | 77.90 a (23.4) | 49.62 a (13.8) | 43.52 a (12.0) | 49.92 a (14.1) | 62.57 a (11.6) | 74.85 a (11.8) | 5.18 |
|
| |||||||
| LSD (0.05) | 4.45 | 3.26 | 1.26 | 3.49 | 1.93 | 4.43 | |
Means within columns followed by the same letter are not significantly different at P = 0.05 (LSD test).
Values in the parentheses indicate x-fold increase relative to the control.
Table 7.
Effect of salinity on chlorophyll-b concentration of six turfgrass species.
| ECw
(dS m−1) |
Turfgrass species (chlorophyll-b contents in mg g−1, fresh weight) | ||||||
|---|---|---|---|---|---|---|---|
| P. vaginatum | Z. japonica | Z. matrella | D. didactyla | C. dactylon “Tifdwarf” | C. dactylon “Satiri” | LSD (0.05) | |
| 0 | 0.14 a | 0.13 a | 0.12 a | 0.15 a | 0.20 a | 0.20 a | 0.031 |
| 8 | 0.13 ab | 0.13 a | 0.11 b | 0.13 ab | 0.19 ab | 0.19 ab | 0.022 |
| 16 | 0.12 ab | 0.10 b | 0.10 b | 0.13 ab | 0.18 b | 0.19 ab | 0.017 |
| 24 | 0.12 ab | 0.10 b | 0.10 bc | 0.12 ab | 0.18 b | 0.18 b | 0.019 |
| 32 | 0.11 b | 0.08 b | 0.09 cd | 0.10 bc | 0.12 c | 0.12 c | 0.023 |
| 40 | 0.11 b | 0.08 b | 0.08 de | 0.09 bc | 0.09 d | 0.11 cd | 0.020 |
| 48 | 0.12 ab | 0.08 b | 0.06 e | 0.08 c | 0.11 cd | 0.09 c | 0.019 |
|
| |||||||
| LSD (0.05) | 0.024 | 0.026 | 0.014 | 0.033 | 0.018 | 0.018 | |
Means within columns followed by the same letter are not significantly different at P = 0.05 (LSD test).
3.5. Leaf Relative Water Content (RWC)
Interaction effect of salinity and species was not significant for relative water content (Table 5). Relative water content (RWC) of all turfgrass species was significantly (P < 0.05) influenced by salinity. As salinity increased, RWC decreased. However, RWC for most of the species did not change up to 24 dS m−1 compared to the control (Table 5). Relative water content significantly decreased at 32 dS m−1 salinity level, except for C. dactylon “Satiri” and Z. matrella. According to reduction in RWC at 48 dS m−1 salinity level, species were ranked as D. didactyla (44.6%) > C. dactylon “Satiri” (42.7%) > C. dactylon “Tifdwarf” (37.5%) > Z. matrella (35.0%) > Z. japonica (33.7%) > P. vaginatum (21.3%).
Table 5.
Effect of salinity on leaf relative water content of six turfgrass species.
| ECw
(dS m−1) |
Turfgrass species (relative water contents in %, fresh weight) | ||||||
|---|---|---|---|---|---|---|---|
| P. vaginatum | Z. japonica | Z. matrella | D. didactyla | C. dactylon “Tifdwarf” | C. dactylon “Satiri” | LSD (0.05) | |
| 0 | 93.16 a | 89.48 a | 89.89 a | 87.33 a | 90.85 a | 90.18 a | 8.83 |
| 8 | 90.24 ab | 87.57 a | 90.97 a | 86.28 a | 90.14 a | 90.39 a | 6.29 |
| 16 | 90.23 ba | 85.22 a | 86.87 a | 84.78 a | 85.02 ba | 86.19 a | 6.64 |
| 24 | 87.84 ba | 84.92 a | 88.51 a | 82.42 a | 83.91 ba | 78.59 b | 9.92 |
| 32 | 86.04 b bc | 78.39 b | 84.09 a | 68.06 b | 78.70 b | 76.42 b | 9.97 |
| 40 | 79.77 dc | 72.28 c | 73.28 b | 63.46 b | 65.27 c | 64.85 b | 9.51 |
| 48 | 78.68 b | 66.30 d | 64.98 c | 55.35 c | 62.51 c | 57.30 c | 8.03 |
|
| |||||||
| LSD (0.05) | 6.49 | 5.94 | 8.05 | 5.85 | 8.76 | 6.97 | |
Means within columns followed by the same letter are not significantly different at P = 0.05 (LSD test).
3.6. Leaf Chlorophyll Content
Interaction effect of salinity and species was not significant for chlorophyll-a content (Table 7). Increasing salinity up to 24 dS m−1 did not affect chlorophyll-a content (Table 6). There were also no differences between 40 and 48 dS m−1 treatments on chlorophyll-b content, except for D. didactyla. In P. vaginatum, the chlorophyll-b content (0.11 mg g−1 FW) at 32 and 40 dS m−1 salinity levels was significantly different from other salinity levels (average 0.126 mg g−1 FW) (Table 7). In Z. japonica, a significant reduction in chlorophyll-b content was observed at 16 dS m−1, but there were no further reductions with increasing salinity.
Table 6.
Effect of salinity on chlorophyll-a concentration of six turfgrass species.
| ECw
(dS m−1) |
Turfgrass species (chlorophyll-a contents in mg g−1, fresh weight) | ||||||
|---|---|---|---|---|---|---|---|
| P. vaginatum | Z. japonica | Z. matrella | D. didactyla | C. dactylon “Tifdwarf” | C. dactylon “Satiri” | LSD (0.05) | |
| 0 | 0.49 a | 0.40 a | 0.36 a | 0.30 a | 0.49 a | 0.57 a | 0.070 |
| 8 | 0.47 a | 0.39 ab | 0.33 ab | 0.29 a | 0.48 a | 0.57 a | 0.059 |
| 16 | 0.46 ab | 0.39 ab | 0.31 abc | 0.27 a | 0.46 a | 0.56 a | 0.067 |
| 24 | 0.45 abc | 0.38 ab | 0.30 abc | 0.26 a | 0.45 ab | 0.55 a | 0.080 |
| 32 | 0.42 bc | 0.35 bc | 0.29 bc | 0.20 b | 0.40 bc | 0.53 a | 0.079 |
| 40 | 0.41 c | 0.33 cd | 0.26 c | 0.19 b | 0.35 cd | 0.45 b | 0.061 |
| 48 | 0.40 c | 0.30 d | 0.24 c | 0.12 c | 0.31 d | 0.41 b | 0.065 |
|
| |||||||
| LSD (0.05) | 0.051 | 0.042 | 0.066 | 0.063 | 0.052 | 0.077 | |
Means within columns followed by the same letter are not significantly different at P = 0.05 (LSD test).
Total chlorophyll content decreased under salt stress in different turfgrass species (Table 8). Interaction effect of salinity and species was significant (P < 0.05) for total chlorophyll (Table 7). Turf species with higher chlorophyll-a and chlorophyll-b contents, under control conditions, also had higher amounts of total chlorophyll. While C. dactylon “Satiri,” C. dactylon “Tifdwarf,” and D. didactyla had higher total chlorophyll under normal conditions, P. vaginatum, and Z. japonica maintained comparatively higher amounts of total chlorophyll under salt stress with marginal reductions compared to other turf species.
Table 8.
Effect of salinity on total chlorophyll concentration of six turfgrass species.
| ECw
(dS m−1) |
Turfgrass species (total chlorophyll contents in mg g−1, fresh weight) | ||||||
|---|---|---|---|---|---|---|---|
| P. vaginatum | Z. japonica | Z. matrella | D. didactyla | C. dactylon “Tifdwarf” | C. dactylon “Satiri” | LSD (0.05) | |
| 0 | 0.62 a | 0.53 a | 0.48 a | 0.45 a | 0.68 a | 0.77 a | 0.069 |
| 8 | 0.60 a | 0.52 a | 0.44 ab | 0.42 ab | 0.67 a | 0.76 a | 0.065 |
| 16 | 0.59 ab | 0.49 a | 0.41 b | 0.40 ab | 0.66 a | 0.74 a | 0.068 |
| 24 | 0.57 bac | 0.48 ab | 0.40 bc | 0.38 b | 0.63 ab | 0.73 a | 0.075 |
| 32 | 0.53 bc | 0.41 bc | 0.37 bc | 0.29 c | 0.52 bc | 0.65 b | 0.083 |
| 40 | 0.52 c | 0.43 dc | 0.34 cd | 0.29 c | 0.45 c | 0.54 c | 0.064 |
| 48 | 0.52 c | 0.38 d | 0.31 d | 0.20 d | 0.42 c | 0.52 c | 0.068 |
|
| |||||||
| LSD (0.05) | 0.060 | 0.050 | 0.079 | 0.068 | 0.100 | 0.074 | |
Means within columns followed by the same letter are not significantly different at P = 0.05 (LSD test).
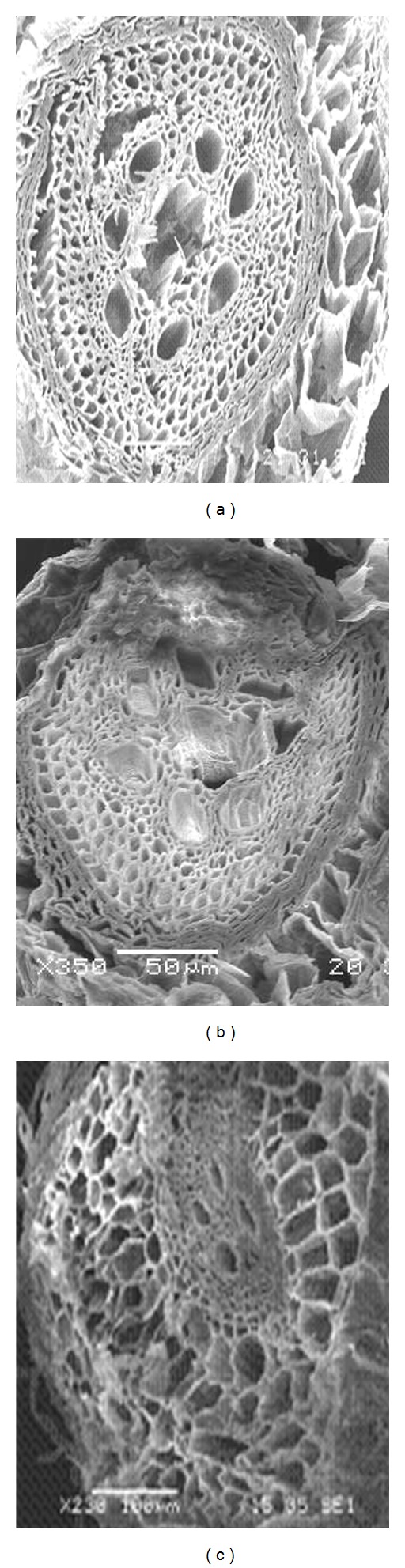
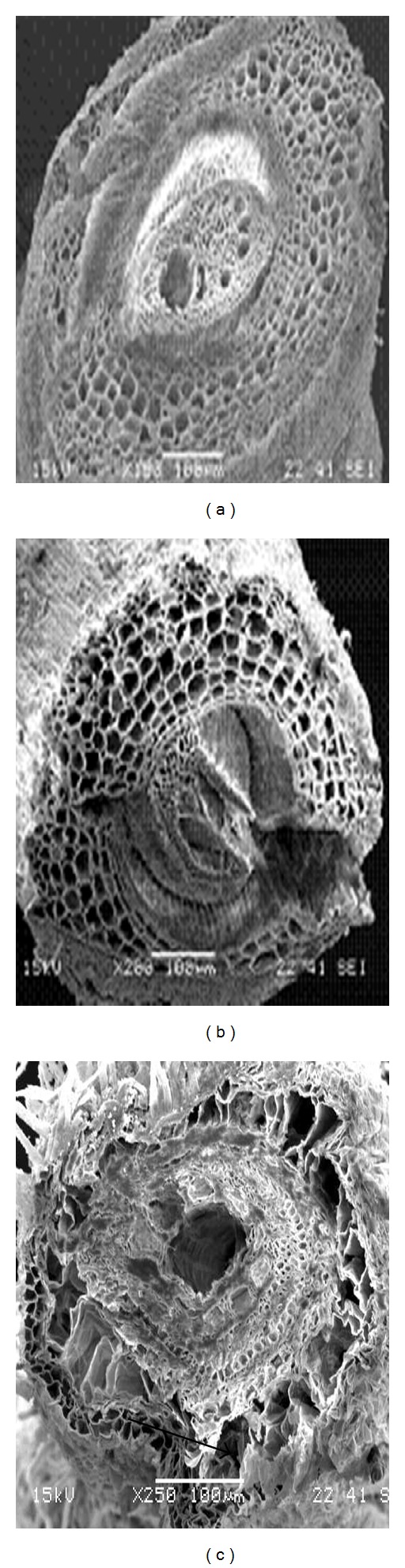
3.7. Root Cell Histology
Differences in cell damage to root cortex of turfgrass species were observed. The damage resulted from cell collapse due to salt stress. Cortical cell of P. vaginatum, and Z. japonica did not show cell collapse in 24 and 48 dS m−1 salinity treatments (Figures 3 and 4). Zoysia matrella showed less cell collapse at 48 dS m−1 salinity treatment (Figure 5). Digitaria didactyla, C. dactylon “Tifdwarf,” and C. dactylon “Satiri” showed severe cell collapse at the highest salinity level (48 dS m−1) compared to the control (Figures 6, 7, and 8).
Figure 3.
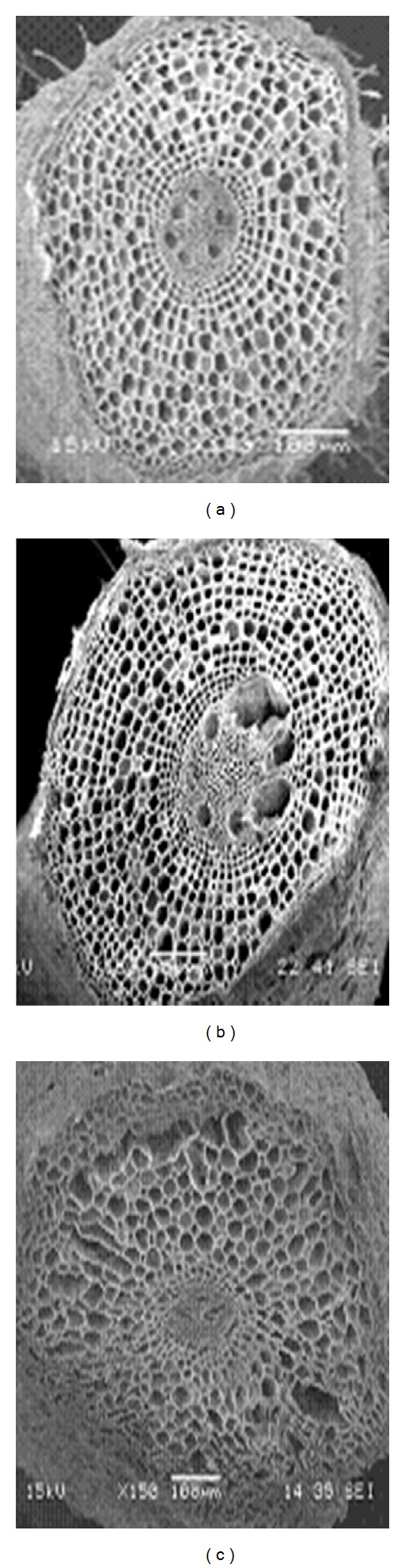
Scanning electron microscopy photographs showing root cortical tissue of Paspalum vaginatum under (a) 0, (b) 24, and (c) 48 dS m−1.
Figure 4.
Scanning electron microscopy photographs showing root cortical tissue of Zoysia japonica under (a) 0, (b) 24, and (c) 48 dS m−1.
Figure 5.
Scanning electron microscopy photographs showing root cortical tissue of Zoysia matrella under (a) 0, (b) 24, and (c) 48 dS m−1.
Figure 6.
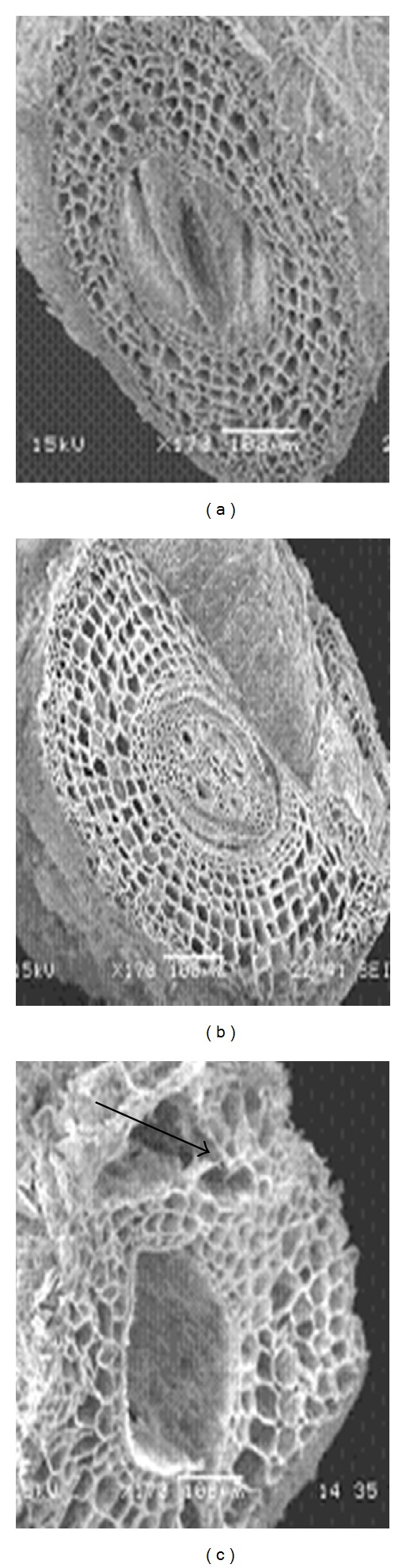
Scanning electron microscopy photographs showing root cortical tissue of C. dactylon “Tifdwarf” under (a) 0, (b) 24, and (c) 48 dS m−1. The arrow indicates cell damage (c) compared to control (a).
Figure 7.
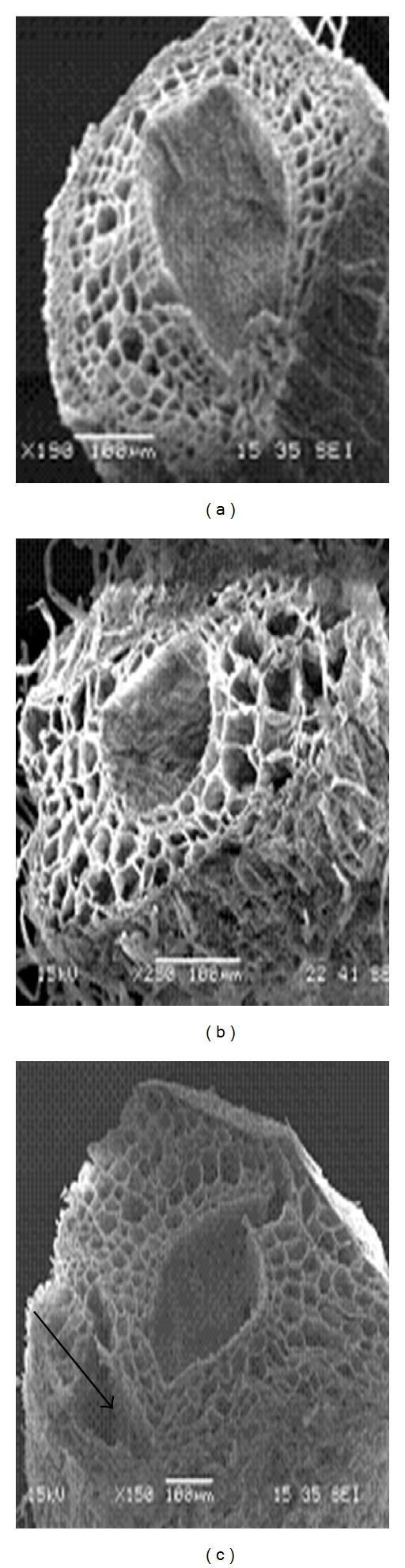
Scanning electron microscopy photographs showing root cortical tissue of C. dactylon “Satiri” under (a) 0, (b) 24, and (c) 48 dS m−1. The arrow indicates cell damage (c) compared to control (a).
Figure 8.
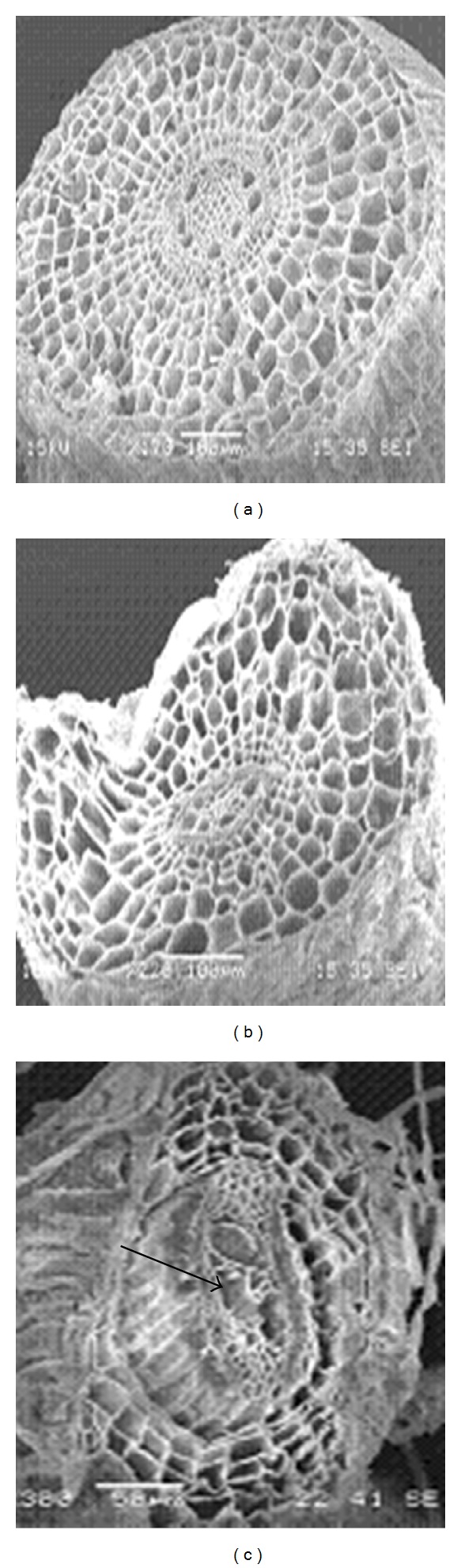
Scanning electron microscopy photographs showing root cortical tissue of Digitaria didactyla under (a) 0, (b) 24, and (c) 48 dS m−1. The arrow indicates cell damage (c) compared to control (a).
4. Discussion
The six turfgrass species in the present study exhibited a wide range in salinity tolerance in terms of dry matter production (Figures 1 and 2) and organic osmolyte accumulation (Table 4). In Malaysia, such type of research was not conducted ever before. Previously, we identified turfgrass species that were available in Malaysia and studied growth performance under salinity-stressed conditions [13, 14]. Throughout the globe, seashore paspalum exhibits a wide range of salinity tolerance among ecotypes [19–22]. A wide intraspecific variation in salinity tolerance has been reported to be as great as the interspecific variations [23]. Several researchers have reported that halophytes, which are ion includers, often adapt to low water potential by accumulation of inorganic solutes to maintain turgor pressure and total water potential [24–26].
Salinity stressed plants certainly face osmotic challenges. This is in agreement with several previous reports [5, 19, 20, 27], which concur that osmotic adjustment is the main mechanism for survival and growth of plants under salinity stress. The percentage relative water content (RWC) was determined as an indicator of osmotic status of turfgrass species studied (Table 5). Halophytes are often able to accumulate high charges of salts in their tissues for osmotic adjustment through the compartmentalization of ions in vacuoles and the production of compatible solutes, or osmotic, in the cytoplasm [27]. Some compatible solutes that show an increase in concentration under salinity stress may also play significant role in osmotic adjustment, and these include proline, glycine betaine, and sugars [28–31]. Glycine betaine and proline protect enzymes (proteins) from damages caused by salinity or dehydration stress [32, 33]. Interestingly, significant proline accumulation generally occurs only after exceeding a threshold of drought or salt stress [30]. In the current study, salinity triggered proline synthesis in response to salinity to turgor maintenance (Table 4). Osmotic adjustment through synthesis of organic compounds has been postulated to have a significant role in salt tolerance in P. vaginatum [34]. Our studies indicated that salinity damaged root structure as a result of cortical cell collapse in C. dactylon “Tifdwarf,” D. didactyla, and C. dactylon “Satiri.” The structural damage in cortical tissue would interrupt radial water movement in the roots, thus limiting water uptake [35].
Chlorophyll degradation is the primary cause of photosynthetic degeneration/leaf firing and a main biochemical factor for the observed growth reduction [36]. The NaCl-induced decrease in chlorophyll level is widely reported in both glycophytes and halophytes [37–39]. In the present study, the chlorophyll damage was not recorded until 24 dS m−1 salinity level and thereafter chlorophyll damage increased with increasing salinity (Tables 6, 7, and 8). The chlorophyll degradation is associated with leaf firing (Table 3). Salinity-induced chlorophyll reduction may be related either to Mg deficiency and/or chlorophyll oxidation since reactive oxygen species (ROS) generation is common in salinity stressed conditions [40]. The chlorophyll-a content of all species decreased much more with increasing salinity (Table 6). However, [41] observed that salinity decreased chlorophyll-b content much more than chlorophyll-a. Chlorophyll content of P. vaginatum and Z. japonica seem to be insensitive to salinity up to 48 dS m−1. This is consistent with the earlier reports for other monocots including rice, wheat and maize chlorophyll-a by [42–44], chlorophyll-b and total chlorophyll contents decreased with increasing salinity [45], and salt-sensitive rice cultivars had lower chlorophyll content than salt-tolerant rice cultivars [45]. Similar observations were made by [46, 47].
5. Conclusion
The development of turfgrass industry in the coastal areas of Malaysia is challenging due to scarcity of fresh water for irrigation and salt tolerant weed species infestation. Sea water irrigation is a new technology widely used to suppress weed and maintaining the turfgrass growth simultaneously. Appropriate, realistic physiological criteria are essential to define the salinity tolerance and growth responses of turfgrass species. In the present study, salinity tolerance was evaluated on the basis of leaf firing, shoot and root growth reduction, proline content, and relative water content. We observed that P. vaginatum was highly salt tolerant at 48 dS m−1 followed by Z. japonica and Z. matrella, while C. dactylon “Tifdwarf” was least salt tolerant followed by D. didactyla and C. dactylon “Satiri.” The conclusions are based on responses of six turfgrass species to salinity. Many of the principles can be employed to discuss issues related to development of better direct selection criteria for other turfgrass species.
Acknowledgments
Authors would like to acknowledge funding of this work by Malaysian Government Research Grant (Science Fund 05-01-04 SF0302) and University Putra Malaysia for Graduate Research Fellowship.
References
- 1.McCarty LB, Dudeck AE. Salinity effects on bentgrass germination. HortScience. 1993;28(1):15–17. [Google Scholar]
- 2.Murdoch CL. Water the limiting factor for golf course development in Hawaii. U.S.G.A. Green Section Record. 1987;25:11–13. [Google Scholar]
- 3.Harivandi A, Bulter JD, Wu L. Salinity and turfgrass culture. In: Waddington DV, Carrow RN, Shearman RC, editors. Turfgrass. Madison, Wis, USA: ASA. CSSA and SSSA; 1992. pp. 208–230. (Agronomy Monograph). [Google Scholar]
- 4.Hixson AC, Crow WT, McSorley R, Trenholm LE. Saline irrigation affects Belonolaimus longicaudatus and Hoplolaimus galeatus on seashore paspalum. Journal of Nematology. 2005;37(1):37–44. [PMC free article] [PubMed] [Google Scholar]
- 5.Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 6.Marcum KB. Use of saline and non-potable water in the turfgrass industry: constraints and developments. Agricultural Water Management. 2006;80(1–3):132–146. [Google Scholar]
- 7.Rhodes D, Nadolska-Orczyk A, et al. Salinity, osmolytes and compatible solutes. In: Lauchli A, Luttge U, editors. Salinity: Environment-Plants-Molecules. Boston, Mass, USA: Kluwer Academic; 2002. [Google Scholar]
- 8.Borsani O, Valpuesta V, Botella MA. Developing salt tolerant plants in a new century: a molecular biology approach. Plant Cell, Tissue and Organ Culture. 2003;73(2):101–115. [Google Scholar]
- 9.Sairam RK, Tyagi A, Chinnusamy V. Salinity tolerance: cellular mechanisms and gene regulation. In: Huang B, editor. Plant–Environment Interactions. 3rd edition. Boca Raton, Fla, USA: CRC Press; 2006. [Google Scholar]
- 10.Jacoby B. Mechanism involved in salt tolerance of plants. In: Pessarakli M, editor. Handbook of Plant and Crop Stress. New York, NY, USA: Marcel Dekker, Inc.; 1999. pp. 97–124. [Google Scholar]
- 11.Kamal-Uddin MK, Juraimi AS, Begum M, Ismail MR, Rahim AA, Othman R. Floristic composition of weed community in turf grass area of West Peninsular Malaysia. International Journal of Agriculture and Biology. 2009;11(1):13–20. [Google Scholar]
- 12.Uddin MK, Juraimi AS, Ismail MR, Brosnan JT. Characterizing weed populations in different turfgrass sites throughout the Klang Valley of Western Peninsular Malaysia. Weed Technology. 2010;24(2):173–181. [Google Scholar]
- 13.Uddin MK, Juraimi AS, Ismail MR, Othman R, Rahim AA. Growth response of eight tropical turfgrass species to salinity. African Journal of Biotechnology. 2009;8(21):5799–5806. [Google Scholar]
- 14.Uddin MK, Juraimi AS, Ismail MR, Othman R, Rahim AA. Relative salinity tolerance of warm season turfgrass species. Journal of Environmental Biology. 2011;32(3):309–312. [PubMed] [Google Scholar]
- 15.Alshammary SF, Qian YL, Wallner SJ. Growth response of four turfgrass species to salinity. Agricultural Water Management. 2004;66(2):97–111. [Google Scholar]
- 16.Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant and Soil. 1973;39(1):205–207. [Google Scholar]
- 17.Witham FH, Blaydes DF, Devlin RM. Exercises in Plant Physiology. 2nd edition. Boston, Mass, USA: PWS; 1986. [Google Scholar]
- 18.Whetherly PE. Studies in the water relation of cotton plants. The field measurement of water deficit in leaves. New Phytology. 1950;49:81–87. [Google Scholar]
- 19.Lee G, Carrow RN, Duncan RR. Photosynthetic responses to salinity stress of halophytic seashore paspalum ecotypes. Plant Science. 2004;166(6):1417–1425. [Google Scholar]
- 20.Lee G, Carrow RN, Duncan RR. Salinity tolerance of seashore paspalum ecotypes: shoot growth responses and criteria. HortScience. 2004;39(5):1138–1142. [Google Scholar]
- 21.Lee G, Carrow RN, Duncan RR. Criteria for assessing salinity tolerance of the halophytic turfgrass seashore paspalum. Crop Science. 2005;45(1):251–258. [Google Scholar]
- 22.Lee G, Carrow RN, Duncan RR. Growth and water relation responses to salinity stress in halophytic seashore paspalum ecotypes. Scientia Horticulturae. 2005;104(2):221–236. [Google Scholar]
- 23.Hester MW, Mendelssohn IA, McKee KL. Species and population variation to salinity stress in Panicum hemito-mon, Spartina patens, and Spartina alterniflora: morphological and physiological constraints. Environmental and Experimental Botany. 2001;46(3):277–297. [Google Scholar]
- 24.Glenn EP. Relationship between cations accumulation and water content of salt-tolerant grasses and a sedg. Plant Cell Environment. 1987;10:205–212. [Google Scholar]
- 25.Flowers TJ, Flowers SA, Hajibagheri MA, Yeo AR. Salt tolerance in the halophytic wild rice, Porteresia coantata . New Phytology. 1990;114:675–684. [Google Scholar]
- 26.Glenn EP, Watson MC, O’Leary JW, Axelson RD. Comparison of salt tolerance and osmotic adjustment of low-sodium and high-sodium subspecies of the C4 halophytes, Atriplex canescens . Plant Cell Environment. 1992;15(6):711–718. [Google Scholar]
- 27.Gorham J, Jones RGW, McDonnell E. Some mechanisms of salt tolerance in crop plants. Plant and Soil. 1985;89(1–3):15–40. [Google Scholar]
- 28.Storey RG, Jones RW. Response of Atriplex spongiosa and Suaeda monoica to salinity. Plant Physiology. 1979;63(1):156–162. doi: 10.1104/pp.63.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briens M, Larher F. Osmoregulation in halophytic higher plants: a comparative study of soluble carbohydrates, polyols, betaines and free proline. Plant Cell Environment. 1982;5(4):287–292. [Google Scholar]
- 30.Cavalieri AJ, Huang AHC. Evaluation of proline accumulation in the adaptation of diverse species of marsh halophytes to the saline environment. American Journal of Botany. 1979;66(3):307–312. [Google Scholar]
- 31.Cavalieri AJ, Huang AHC. Accumulation of proline and glycinebetaine in Spartina alterniflora Loisel. in response to NaCl and nitrogen in the marsh. Oecologia. 1981;49(2):224–228. doi: 10.1007/BF00349192. [DOI] [PubMed] [Google Scholar]
- 32.Paleg LG, Stewart GR, Bradbeer JW. Proline and glycine betaine influence protein salvation. Plant Physiology. 1984;75(4):974–978. doi: 10.1104/pp.75.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smirnoff N, Cumbes QJ. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28(4):1057–1060. [Google Scholar]
- 34.Marcum KB, Murdoch CL. Salinity tolerance mechanisms of six C4 turfgrasses. Journal of the American Society for Horticultural Science. 1994;119(4):779–784. [Google Scholar]
- 35.Huck MG, Klepper BL, Taylor HM. Diurnal variations in root diameter. Plant Physiology. 1970;45(4):529–530. doi: 10.1104/pp.45.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Everard JD, Gucci R, Kann SC, Flore JA, Loescher WH. Gas exchange and carbon partitioning in the leaves of celery (Apium graveolens L.) at various levels of root zone salinity. Plant Physiology. 1994;106(1):281–292. doi: 10.1104/pp.106.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdullah Z, Khan MA, Flowers TJ. Causes of sterility in seed set of rice under salinity stress. Journal of Agronomy and Crop Science. 2001;187(1):25–32. [Google Scholar]
- 38.Kaya C, Tuna AL, Ashraf M, Altunlu H. Improved salt tolerance of melon (Cucumis melo L.) by the addition of proline and potassium nitrate. Environmental and Experimental Botany. 2007;60(3):397–403. [Google Scholar]
- 39.Shabala L, Cuin TA, Newman IA, Shabala S. Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants . Planta. 2005;222(6):1041–1050. doi: 10.1007/s00425-005-0074-2. [DOI] [PubMed] [Google Scholar]
- 40.Moradi F, Ismail AM. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Annals of Botany. 2007;99(6):1161–1173. doi: 10.1093/aob/mcm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Islam MS. Morpho-physiology of blackgram and mungbean as influenced by salinity. Department of Agronomy, BSMRAU, Salna, Gazipur; 2001. M.S. thesis. [Google Scholar]
- 42.Lutts S, Kinet JM, Bouharmont J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Annals of Botany. 1996;78(3):389–398. [Google Scholar]
- 43.Krishna S, Raj BT, Mawson EC, Yeung T, Thorpe A. Utilization of induction and quenching kinetics of chlorophyll a fluorescence for in vivo salinity screening studies in wheat (Triticum aestivum vars. Kharchia-65 and Fielder) Canadian Journal of Botany. 1993;71(1):87–92. [Google Scholar]
- 44.Shabala SN, Shabala SI, Martynenko AI, Babourina O, Newman IA. Salinity effect on bioelectric activity, growth, Na+ accumulation and chlorophyll fluorescence of maize leaves: a comparative survey and prospects for screening. Australian Journal of Plant Physiology. 1998;25(5):609–616. [Google Scholar]
- 45.Pushpam R, Rangasamy SRS. In vivo response of rice cultivars to salt stress. Journal of Ecobiology. 2002;14:177–182. [Google Scholar]
- 46.Mohan MM, Narayanan SL, Ibrahim SM. Chlorophyll stability index (CSI): its impact on salt tolerance in rice. International Rice Research Notes. 2000;25(2):38–39. [Google Scholar]
- 47.Mandal MP, Singh RA. Impact of salt stress on chlorophyll content in rice genotypes. Journal Research Birsa Agriculture University India. 2001;13:61–63. [Google Scholar]


