Abstract
Background
Serum citrulline concentration is used as a biomarker of enterocyte mass and enteral tolerance, and low serum concentrations are correlated with bacteremia in immunosuppressed adults undergoing hematopoietic stem cell transplant. The authors sought to determine if citrulline was associated with the development of catheter-related bloodstream infections (CRBSIs) in children with intestinal failure.
Methods
Data were reviewed from 66 children treated in a multidisciplinary intestinal rehabilitation program, who had serum concentration citrulline measured between January 2007 and August 2009. All patients had a diagnosis of intestinal failure requiring parenteral nutrition (PN) support. Exclusion criteria included central venous catheter in situ <30 days, creatinine clearance <20 mL/ minute, or a history of organ transplant/immunosuppression.
Results
A total of 15 patients were excluded because of the above criteria. In this cohort of 51 patients, 26 (51%) developed CRBSIs. Both groups were similar in terms of gestational age, diagnosis, nutrition status, and biochemical liver function tests. The mean (± standard deviation [SD]) minimum serum citrulline concentration was significantly lower in patients who developed CRBSIs (6.7 ± 4.6 μmol/L) than in those who did not (11.3 ± 6.4 μmol/L, P = .004). Multivariate logistic regression analysis identified lower minimum serum citrulline concentration and longer central venous catheter duration as independently associated with CRBSI (P = .003 and P = .038, respectively).
Conclusions
Low serum citrulline concentration and longer central venous catheter time are independently associated with CRBSI in children with intestinal failure. Serum citrulline concentration may be a useful biomarker to identify patients with intestinal failure who are at high risk of developing a CRBSI.
Keywords: central venous catheter, citrulline, biomarker, catheter-related bloodstream infection
Introduction
Citrulline is a nonprotein amino acid that functions as an intermediate in the urea cycle, which eliminates nitrogenous waste by converting ammonia to urea.1 Synthesis of circulating citrulline occurs almost exclusively within enterocytes.2 Citrulline is synthesized primarily from glutamine, and conversion of ornithine and carbamoyl phosphate to citrulline by ornithine carbamoyltransferase is the ratelimiting step in citrulline production.2 Circulating serum citrulline is metabolized to arginine in the kidney by argininosuccinate synthetase.2,3
The integral role of the enterocyte in citrulline synthesis has led to citrulline being investigated as a biomarker of intestinal function in a variety of conditions. In children with intestinal failure, serum citrulline concentration is correlated with small bowel length,4 mucosal mass,5 and the ability to wean from parenteral nutrition (PN).4 Serum citrulline is also associated with intestinal mucosal injury due to radiation6,7 or human immunodeficiency virus infection,8 intestinal absorptive capacity independent of inflammation,5,9 and rejection of transplanted intestine.5,10-12
Serum citrulline concentration is correlated with intestinal mucosal injury following myeloablative therapy13,14 and chemotherapy.15 Recently serum citrulline has been evaluated as a marker of febrile mucositis16 and bacteremia in adults undergoing myeloablative therapy before hematopoietic stem cell transplant.17 The proposed mechanism of bacteremia was bacterial translocation secondary to impaired intestinal barrier function.
Many children with intestinal failure require long-term indwelling central venous access for PN. Sepsis resulting from catheter-related bloodstream infections (CRBSIs) is a leading cause of morbidity and mortality in patients on long-term PN.18 Children with intestinal failure typically have a low serum citrulline concentration because of prior intestinal resections, and some data have suggested that intestinal failure is marked by increased intestinal permeability.19 We hypothesized that low serum citrulline concentration in these patients may also reflect impaired intestinal barrier function, which contributes to the high incidence of CRBSIs. We investigated this hypothesis by evaluating the relationship between serum citrulline concentration and the development of laboratory-confirmed CRBSIs in children with intestinal failure.
Methods
Study Design
Following institutional review board approval, a retrospective medical record review of patients followed by the multidisciplinary Center for Advanced Intestinal Rehabilitation (CAIR) program at Children’s Hospital Boston between January 1, 2007, and August 20, 2009, was performed. Included were children aged 0 to 18 years, with a diagnosis of intestinal failure requiring PN via a central venous catheter (CVC), who had at least 1 serum citrulline concentration measured during the study period. Patients with end-stage renal disease (creatinine clearance <20 mL/minute) were excluded because citrulline metabolism is altered in renal disease.20 Patients with a history of organ transplant or those receiving immunosuppressive therapy were excluded from analysis because of increased infection risk from immunosuppressive regimens not associated with intestinal failure.21
Data Collection
Data collected included demographic factors (date of birth, gestational age), medical/surgical history (diagnoses, small bowel length, presence of ileocecal valve, presence of motility disorder, including gastroschisis and intestinal aganglionosis), and history of exposure to ethanollock therapy or ω-3 lipid emulsion. Catheter-specific information (number of catheter days, presence of tunneled CVC), laboratory test results (serum citrulline concentration, international normalized ratio [INR], direct bilirubin, alanine aminotransferase [ALT], albumin), nutrition status (weight and height z scores adjusted for age, percentage enteral nutrition [EN]), and CRBSI details (date of positive culture, organism[s]) were also obtained. Any serum citrulline concentration obtained within 5 days of infection was excluded because serum citrulline may drop acutely in this setting.22
Serum Citrulline Assay
Serum citrulline quantitation by high-performance liquid chromatography (HPLC) was performed using the Hitachi L-8800 Amino Acid Analyzer (Tokyo, Japan) at the Children’s Hospital Boston core laboratory. Nonheparinized plasma samples were deproteinized, and the amino acids were eluted using a cation exchange column. Serum citrulline concentration was reported as part of the amino acid panel.
CRBSI Guidelines
CRBSI were defined based on 2008 Centers for Disease Control and Prevention criteria.23 Diagnosis of a laboratory-confirmed bloodstream infection required at least 1 of the following criteria to be met: (1) a recognized pathogen cultured from at least 1 peripheral or central blood culture, and the organism cultured from the blood is not related to infection at another site; (2) at least 1 sign or symptom (eg, fever >38°C, chills, hypotension), which is unrelated to infection at another site, and a common skin contaminant is cultured from at least 2 blood cultures on separate occasions <2 days apart; or (3) a patient younger than 1 year, who has at least 1 sign or symptom (eg, fever >38°C rectal, hypothermia <37°C rectal, apnea, bradycardia), which is unrelated to infection at another site, and a common skin contaminant is cultured from at least 2 blood cultures on separate occasions <2 days apart.
Statistical Analysis
Statistical analyses were performed using SPSS (version 16.0; SPSS Inc, Chicago, IL). Summary statistics are reported as mean ± standard deviation (SD) or median (interquartile range [IQR]). P values for comparison summary statistics were obtained using Fisher exact test for categorical variables, and the Student t test or Mann-Whitney U test for continuous data, depending on normality of distribution as determined by the Kolmogorov-Smirnov test. Multivariate logistic regression was used to test minimum citrulline, catheter duration, and other covariates in differentiating the presence or absence of CRBSI with the likelihood ratio test used to assess significance. A 95% confidence interval (CI) was calculated for the mean difference in minimum citrulline between the 2 groups. Probability of CRBSI was derived for minimum serum citrulline concentration alone and in combination with catheter duration. Diagnostic accuracy of minimum citrulline for differentiating CRBSI from non-CRBSI groups was determined using area under the receiver operator characteristic (ROC) curve with 95% CI. The optimal relationship between sensitivity and specificity for serum citrulline as a biomarker for distinguishing the 2 groups was estimated using the Youden index in ROC analysis. All statistical tests were considered significant based on a P < .05 (2-tailed).
Results
A total of 66 children with intestinal failure followed by the CAIR program at Children’s Hospital Boston had at least 1 serum citrulline concentration drawn during the study period. The median number of citrulline measurements was 2 (range, 1–7). The following patients were excluded from analysis: 9 patients for CVC in situ <30 days, 5 patients for history of immunosuppression or transplant, and 1 patient for renal failure.
Summary data from the cohort of 51 children is shown in the first column of Table 1. A total of 17 (33%) patients were female. The mean ± SD gestational age was 33 ± 5 weeks. The median age at study start was 4 months (IQR 2, 9). The median percentage EN at study start was 0 (IQR 0, 15%). Common diagnoses were necrotizing enterocolitis (n = 20), intestinal atresia (n = 11), gastroschisis (n = 11), and volvulus (n = 9). In all, 18% of patients had multiple diagnoses. Fifteen patients (29%) had an intestinal motility disorder, including gastroschisis and intestinal aganglionosis. Thirty-nine patients (76%) had a history of exposure to ω-3 lipid emulsion. Twenty patients (39%) had a history of exposure to ethanol locks. The median CVC duration within the study period was 10.8 months (IQR 5, 18). Of the 46 patients with available catheter data, 34 patients had a tunneled CVC, 8 patients had a nontunneled CVC, and 4 patients had both catheter types during the study period. Patients had suboptimal growth, based on weight-for-age and height-for-age z scores. The median ALT was 67 U/L (IQR 35, 185). The median direct bilirubin was 5.0 mg/dL (IQR 0.4, 7.9).
Table 1.
Demographic and Clinical Characteristics of Pediatric Patients With Intestinal Failurea
| Characteristic | All Patients (n = 51) |
CRBSI (n = 26) |
No CRBSI (n = 25) |
P Value |
|---|---|---|---|---|
| Gender | ||||
| Male, n (%) | 34 (67) | 19 (73) | 15 (60) | .38 |
| Female, n (%) | 17 (33) | 7 (27) | 10 (40) | |
| Gestational age (weeks), mean ± SD | 33 ± 5 | 33 ± 5 | 32 ± 5 | .57 |
| Diagnosesb | ||||
| Necrotizing enterocolitis, n (%) | 20 (39) | 10 (39) | 10 (40) | .91 |
| Intestinal atresia, n (%) | 11 (22) | 5 (19) | 6 (24) | .74 |
| Gastroschisis, n (%) | 11 (22) | 7 (27) | 4 (16) | .50 |
| Volvulus, n (%) | 9 (18) | 6 (23) | 3 (12) | .47 |
| Other, n (%)c | 8 (16) | 3 (12) | 5 (20) | .47 |
| Motility disorder, n (%)d | 15 (29) | 8 (31) | 7 (28) | .83 |
| Small bowel length (cm), median (range), n = 32 | 46 (4–200) | 38 (4–105) | 30 (14 –200) | .94 |
| ICV, n (%) | 20 (39) | 9 (39) | 11 (46) | .77 |
| Age at study start (mo), median (IQR) | 4 (2, 9) | 7 (3, 11) | 4 (1, 7) | .17 |
| Values at study start, median (IQR) | ||||
| Percentage EN | 0 (0, 15) | 4 (0, 28) | 0 (0, 5) | .15 |
| Weight for age z score | −1.7 (−2.4, −0.7) | −1.6 (−2.3, −0.4) | −1.8 (−2.6, −0.9) | .30 |
| Height for age z score | −1.4 (−2.6, −0.6) | −1.4 (−2.5, −0.3) | −1.4 (−3.2, −0.8) | .48 |
| Albumin (g/dL) | 2.9 (2.5, 3.4) | 2.9 (2.5, 3.2) | 3.2 (2.5, 3.6) | .26 |
| ALT (U/L) | 67 (35, 185) | 72 (40, 196) | 66 (32, 140) | .42 |
| Direct bilirubin (mg/dL) | 5.0 (0.4, 7.9) | 3.5 (0.4, 8.7) | 5.9 (0.4, 7.9) | .95 |
| INR | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.2) | 1.1 (1.1, 1.2) | .94 |
| Catheter months, median (IQR) | 10.8 (5, 18) | 12.8 (8, 27) | 6.5 (3, 12) | .01 |
| Tunneled catheter only, n (%), n = 42 | 34 (81) | 13 (72) | 21 (88) | .26 |
| Minimum serum citrulline (μmol/L), mean ± SD | 9.0 ± 6.0 | 6.7 ± 4.6 | 11.3 ± 6.4 | .004 |
ALT, alanine aminotransferase; CRBSI, catheter-related bloodstream infection; EN, enteral nutrition; ICV, ileocecal valve; INR, international normalized ratio; IQR, interquartile range; SD, standard deviation.
Comparison is made between those patients with CRBSI and those without CRBSI.
Total greater than 100% because of patients with multiple diagnoses.
Intestinal aganglionosis (n = 4), congenital portoarterial fistula (n = 1), enteropathy (n = 1), microvillous inclusion disease (n = 1), ischemic bowel (n = 1).
Includes gastroschisis and intestinal aganglionosis.
In the cohort of 51 patients, 26 developed at least 1 CRBSI. Table 1 shows the demographic and clinical characteristics of the 2 groups. Using univariate analysis, only minimum citrulline concentration and CVC duration were significantly different between the 2 groups. The minimum citrulline concentration in patients who developed a CRBSI was significantly lower (mean, 6.7 ± 4.6 μmol/L) than in those patients who did not (mean, 11.3 ± 6.4 μmol/L, P = .004). The median CVC duration was 12.8 months (IQR 8, 27) in the group with CRBSI, and 6.5 months (IQR 3, 12) in the group without CRBSI (P = .013). No other demographic, surgical, laboratory, or nutrition variable differed significantly between the 2 groups.
Among the 26 patients who developed CRBSIs, there were a total of 60 infections, 30% of which were polymicrobial. The median number of infections was 1 (range, 1–13). Gram-negative rods and gram-positive cocci were the most frequently cultured microorganisms. Common intestinal flora (including Klebsiella, Enterococcus, Escherichia, Enterobacter, Bifidobacterium, Candida) comprised 65% of all organisms cultured (Table 2).
Table 2.
Frequency and Types of Organisms Identified Among Patients With CRBSI
| n (%)a | |
|---|---|
| Patients with CRBSI | 26 (51) |
| Number of CRBSIs per patient | |
| 1 | 16 (62) |
| 2 | 3 (11) |
| 3 | 1 (4) |
| 4 | 2 (8) |
| 5 | 3 (11) |
| 13 | 1 (4) |
| Type of infectionb | |
| Single organism | 42 (70) |
| Polymicrobial | 18 (30) |
| Type of organism, n Gram-negative rods |
|
| Klebsiella | 22 |
| Escherichia coli | 6 |
| Enterobacter | 5 |
| Pseudomonas | 1 |
| Gram-positive cocci | |
| Staphylococcus | 17 |
| Enterococcus | 15 |
| Streptococcus | 5 |
| Rhodococcus | 1 |
| Gram-positive rods | |
| Bifidobacterium | 1 |
| Bacillus | 1 |
| Yeast | |
| Candida | 5 |
CRBSI, cather-related bloodstream infection.
Except where specified.
Species unknown (n = 1).
Figure 1 shows the individual and mean minimum citrulline levels for patients with and without CRBSI. The mean difference in minimum serum citrulline between the 2 groups was 4.6 μmol/L (95% CI, 1.5–7.7). Eighteen of the 26 patients who developed CRBSI had a serum citrulline concentration drawn before the first infection. The median time between serum citrulline measurement and first CRBSI in these patients was 80 days. Subset analysis of these 18 patients found that the average minimum preinfection citrulline concentration was 6.6 ± 4.1 μmol/L. When compared with the minimum citrulline for patients who did not develop CRBSI (11.3 ± 6.4 μmol/L, n = 25), the minimum preinfection citrulline in patients who developed CRBSI was significantly lower (P = .009, Student t test), as shown in Figure 2. Multivariate logistic regression analysis revealed that minimum preinfection citrulline was significantly associated with CRBSI (P = .004). Despite the reduced power when only preinfection citrulline concentration was used, minimum serum citrulline concentration remained significantly associated with CRBSI.
Figure 1.
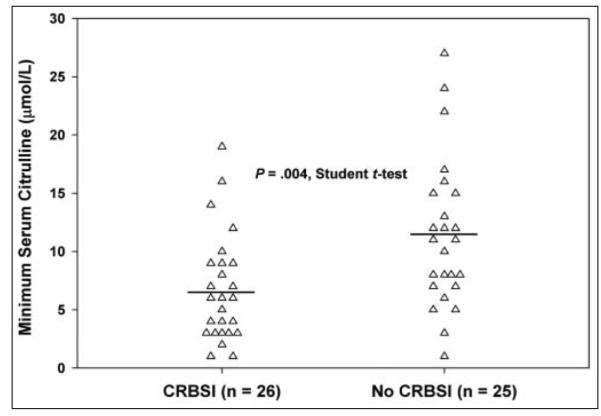
Minimum serum citrulline in patients with and without catheter-related bloodstream infections (CRBSI). Individual data points (triangles) and group means (horizontal lines) are shown. Citrulline was lower in patients with CRBSI (6.7 ± 4.6 μmol/L) than in those without (11.3 ± 6.4 μmol/L) (P = .004).
Figure 2.
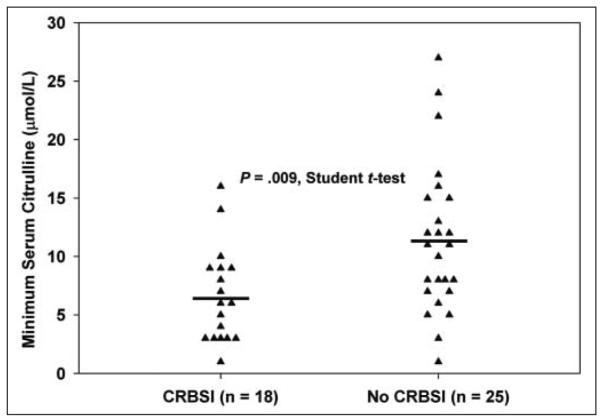
Minimum preinfection serum citrulline in patients with and without catheter-related bloodstream infections (CRBSIs). Individual data points (triangles) and group means (horizontal lines) are shown. Citrulline was lower in patients with CRBSI (6.6 ± 4.1 μmol/L) than in those without (11.3 ± 6.4 μmol/L) (P = .009).
Figure 3 is a histogram comparing minimum citrulline for the entire cohort of patients with and without CRBSI. The theoretical probability curve from logistic regression is superimposed. The probability of developing CRBSI was inversely related to minimum serum citrulline (P = .003). The optimal relationship between sensitivity and specificity for minimum serum citrulline in differentiating the groups with and without CRBSI was 10 μmol/L.
Figure 3.
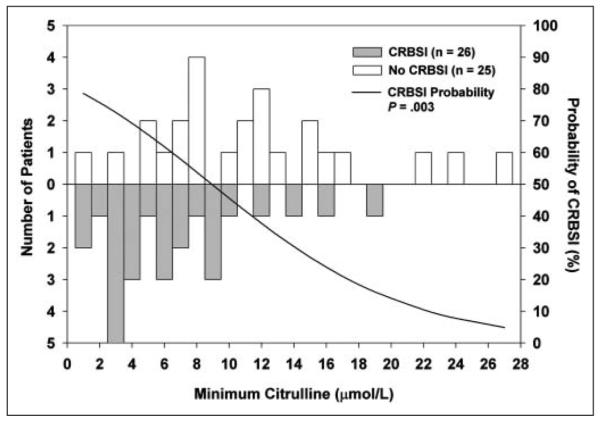
Histogram comparing minimum serum citrulline in patients with and without catheter-related bloodstream infections (CRBSIs), with superimposed theoretical probability curve from logistic regression. Patients who developed CRBSI (gray bars) had lower citrulline than those who did not (white bars). Probability of CRBSI (black curve) decreases with increasing citrulline (P = .003).
Multivariate logistic regression analysis identified minimum serum citrulline concentration and number of catheter days as independently associated with CRBSI (P = .003, P = .038, respectively). Area under the ROC curve was 0.733 (95% CI, 0.594–0.872) for minimum serum citrulline and CRBSI, and increased to 0.811 (95% CI, 0.686–0.935) when catheter duration was included with citrulline to predict CRBSI. Figure 4 illustrates the probability of CRBSI based on catheter duration and minimum serum citrulline. As catheter duration increases, the probability of CRBSI increases (P = .038) regardless of citrulline levels. As minimum serum citrulline level decreases, the probability of infection increases, independent of catheter duration (P = .003).
Figure 4.
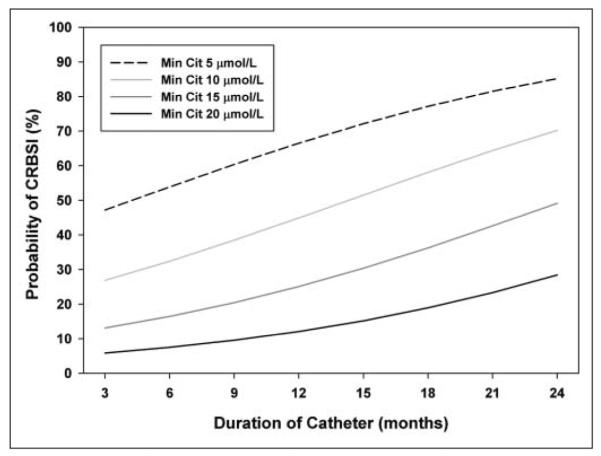
Probability of catheter-related bloodstream infections (CRBSI) based on catheter duration and minimum serum citrulline (Cit). Probability of CRBSI increases with increasing catheter duration (P = .038) and decreasing minimum serum citrulline (P = .003). Multivariate analysis confirmed both variables as significant predictors of CRBSI.
Discussion
CRBSI are a leading cause of morbidity and mortality in children with intestinal failure. In addition to immediate deleterious effects, bloodstream infections have also been associated with an increased rate of cholestasis associated with PN use.24,25 Identification of factors predisposing children with intestinal failure to CRBSI may lead to improved prevention strategies and reduce the potentially fatal consequences of catheter infections.
Previous studies have attempted to identify patients at particular risk for CRBSIs among the general population of patients with indwelling central venous access. Reported risk factors across various patient populations include the following: femoral insertion site,26 catheter type (CVC vs implanted port),27 administration of PN28,29 or blood products,30 underlying disease,31,32 and CVC duration.33,34 To our knowledge, this is the first study to evaluate clinical and demographic factors associated with CRBSI in pediatric intestinal failure patients.
In an attempt to identify variables associated with CRBSI in this cohort, a variety of demographic, medical, surgical, and nutrition risk factors were evaluated. Neither gender, age, prematurity, diagnosis, nutrition status (albumin, weight-for-age and height-for-age z scores, percentage EN received), nor biochemical liver function tests were associated with the development of CRBSI. However, lower minimum serum citrulline concentration was significantly associated with increased incidence of CRBSI. Minimum citrulline concentration remained independently associated with CRBSI, even when controlling for catheter duration.
Potential explanations for this association are suggested by the finding that 65% of organisms cultured were common gastrointestinal flora. Direct fecal contamination of the catheter or sample is possible, particularly when multiple microorganisms are isolated from a single culture. Because of the significantly lower citrulline concentration in the cohort that developed CRBSI, we hypothesize that bacterial translocation across an impaired mucosal barrier is the leading cause of CRBSI.
While our data suggest that citrulline may be a potential biomarker for children with intestinal failure at risk of CRBSI, it is important to point out some limitations. Serum citrulline measurements were not consistently obtained during the study period, which might lead to observational bias. The number of citrulline measurements per patient ranged from 1 to 7. Eight of the 26 patients with CRBSI did not have a serum citrulline concentration drawn before the first infection. However, results of the subset analysis from the 18 patients with a preinfection citrulline measurement confirmed that minimum citrulline level was associated with increased CRBSI (P = .009). A future study might prospectively collect data over a longer period of time in a larger group of patients to give additional insight into the validity of serum citrulline as a predictor of CRBSI. Finally, while the high number of enteric organisms cultured in these patients suggests a potential role for bacterial translocation in CRBSI, this retrospective study only measured associations and cannot deduce the mechanism of infection. Bacterial translocation has been well described in animal models,35,36 but because of limited data,37 the existence of bacterial translocation in humans has been questioned.38
Despite these limitations, this study has identified 2 important characteristics associated with CRBSI in children with intestinal failure: low serum citrulline concentration and increased catheter duration. This preliminary study suggests that minimum serum citrulline concentration may be a useful biomarker to identify children with intestinal failure at risk of developing CRBSI. These high-risk patients may benefit from aggressive preventive measures (such as ethanol-lock therapy39) to reduce the morbidity of CRBSI.
Clinical Relevancy Statement.
This study found that minimum serum citrulline was inversely correlated with the occurrence of catheterrelated bloodstream infections (CRBSI) in children with intestinal failure, independent of intestinal length. This retrospective study warrants further prospective investigation, but suggests that serum citrulline may be a useful biomarker to identify children at high risk for CRBSI who may benefit from aggressive preventive strategies.
Acknowledgments
We are indebted to Gail Potter Bynoe (Manager, Infection Control) for her assistance in compiling CRBSI data.
Footnotes
Financial disclosure: none declared.
References
- 1.Curis E, Nicolis I, Moinard C, et al. Almost all about citrulline in mammals. Amino Acids. 2005;29:177–205. doi: 10.1007/s00726-005-0235-4. [DOI] [PubMed] [Google Scholar]
- 2.Windmueller HG, Spaeth AE. Source and fate of circulating citrulline. Am J Physiol. 1981;241:E473–E480. doi: 10.1152/ajpendo.1981.241.6.E473. [DOI] [PubMed] [Google Scholar]
- 3.Levillain O, Hus-Citharel A, Morel F, Bankir L. Localization of arginine synthesis along rat nephron. Am J Physiol. 1990;259(6 Pt 2):F916–F923. doi: 10.1152/ajprenal.1990.259.6.F916. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgibbons S, Ching YA, Valim C, et al. Relationship between serum citrulline levels and progression to parenteral nutrition independence in children with short bowel syndrome. J Pediatr Surg. 2009;44:928–932. doi: 10.1016/j.jpedsurg.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27:328–339. doi: 10.1016/j.clnu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Lutgens LC, Deutz N, Granzier-Peeters M, et al. Plasma citrulline concentration: a surrogate end point for radiation-induced mucosal atrophy of the small bowel. A feasibility study in 23 patients. Int J Radiat Oncol Biol Phys. 2004;60:275–285. doi: 10.1016/j.ijrobp.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 7.Lutgens L, Lambin P. Biomarkers for radiation-induced small bowel epithelial damage: an emerging role for plasma Citrulline. World J Gastroenterol. 2007;13:3033–3042. doi: 10.3748/wjg.v13.i22.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crenn P, De Truchis P, Neveux N, Galperine T, Cynober L, Melchior JC. Plasma citrulline is a biomarker of enterocyte mass and an indicator of parenteral nutrition in HIV-infected patients. Am J Clin Nutr. 2009;90:587–594. doi: 10.3945/ajcn.2009.27448. [DOI] [PubMed] [Google Scholar]
- 9.Papadia C, Sherwood RA, Kalantzis C, et al. Plasma citrulline concentration: a reliable marker of small bowel absorptive capacity independent of intestinal inflammation. Am J Gastroenterol. 2007;102:1474–1482. doi: 10.1111/j.1572-0241.2007.01239.x. [DOI] [PubMed] [Google Scholar]
- 10.Pappas PA, Saudubray JM, Tzakis AG, et al. Serum citrulline as a marker of acute cellular rejection for intestinal transplantation. Transplant Proc. 2002;34:915–917. doi: 10.1016/s0041-1345(02)02668-4. [DOI] [PubMed] [Google Scholar]
- 11.Gondolesi G, Fishbein T, Chehade M, et al. Serum citrulline is a potential marker for rejection of intestinal allografts. Transplant Proc. 2002;34:918–920. doi: 10.1016/s0041-1345(02)02669-6. [DOI] [PubMed] [Google Scholar]
- 12.Gondolesi G, Ghirardo S, Raymond K, et al. The value of plasma citrulline to predict mucosal injury in intestinal allografts. Am J Transplant. 2006;6:2786–2790. doi: 10.1111/j.1600-6143.2006.01513.x. [DOI] [PubMed] [Google Scholar]
- 13.Blijlevens NM, Lutgens LC, Schattenberg AV, Donnelly JP. Citrulline: a potentially simple quantitative marker of intestinal epithelial damage following myeloablative therapy. Bone Marrow Transplant. 2004;34:193–196. doi: 10.1038/sj.bmt.1704563. [DOI] [PubMed] [Google Scholar]
- 14.Lutgens LC, Blijlevens NM, Deutz NE, Donnelly JP, Lambin P, de Pauw BE. Monitoring myeloablative therapy-induced small bowel toxicity by serum citrulline concentration: a comparison with sugar permeability tests. Cancer. 2005;103:191–199. doi: 10.1002/cncr.20733. [DOI] [PubMed] [Google Scholar]
- 15.van Vliet MJ, Tissing WJ, Rings EH, et al. Citrulline as a marker for chemotherapy induced mucosal barrier injury in pediatric patients. Pediatr Blood Cancer. 2009;53:1188–1194. doi: 10.1002/pbc.22210. [DOI] [PubMed] [Google Scholar]
- 16.van der Velden WJ, Blijlevens NM, Feuth T, Donnelly JP. Febrile mucositis in haematopoietic SCT recipients. Bone Marrow Transplant. 2009;43:55–60. doi: 10.1038/bmt.2008.270. [DOI] [PubMed] [Google Scholar]
- 17.Herbers AH, Blijlevens NM, Donnelly JP, de Witte TJ. Bacteraemia coincides with low citrulline concentrations after high-dose melphalan in autologous HSCT recipients. Bone Marrow Transplant. 2008;42:345–349. doi: 10.1038/bmt.2008.170. [DOI] [PubMed] [Google Scholar]
- 18.Raman M, Gramlich L, Whittaker S, Allard JP. Canadian home total parenteral nutrition registry: preliminary data on the patient population. Can J Gastroenterol. 2007;21:643–648. doi: 10.1155/2007/217897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Antiga L, Dhawan A, Davenport M, Mieli-Vergani G, Bjarnason I. Intestinal absorption and permeability in paediatric short-bowel syndrome: a pilot study. J Pediatr Gastroenterol Nutr. 1999;29:588–593. doi: 10.1097/00005176-199911000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Tizianello A, De Ferrari G, Garibotto G, Gurreri G, Robaudo C. Renal metabolism of amino acids and ammonia in subjects with normal renal function and in patients with chronic renal insufficiency. J Clin Invest. 1980;65:1162–1173. doi: 10.1172/JCI109771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viale P, Stefani S. Vascular catheter-associated infections: a microbiological and therapeutic update. J Chemother. 2006;18:235–249. doi: 10.1179/joc.2006.18.3.235. [DOI] [PubMed] [Google Scholar]
- 22.van Waardenburg DA, de Betue CT, Luiking YC, Engel M, Deutz NE. Plasma arginine and citrulline concentrations in critically ill children: strong relation with inflammation. Am J Clin Nutr. 2007;86:1438–1444. doi: 10.1093/ajcn/86.5.1438. [DOI] [PubMed] [Google Scholar]
- 23.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Sondheimer JM, Asturias E, Cadnapaphornchai M. Infection and cholestasis in neonates with intestinal resection and long-term parenteral nutrition. J Pediatr Gastroenterol Nutr. 1998;27:131–137. doi: 10.1097/00005176-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Hermans D, Talbotec C, Lacaille F, Goulet O, Ricour C, Colomb V. Early central catheter infections may contribute to hepatic fibrosis in children receiving long-term parenteral nutrition. J Pediatr Gastroenterol Nutr. 2007;44:459–463. doi: 10.1097/MPG.0b013e318031a5c7. [DOI] [PubMed] [Google Scholar]
- 26.Ishizuka M, Nagata H, Takagi K, Kubota K. Femoral venous catheterization is a major risk factor for central venous catheter-related bloodstream infection. J Invest Surg. 2009;22:16–21. doi: 10.1080/08941930802566698. [DOI] [PubMed] [Google Scholar]
- 27.Beckers MM, Ruven HJ, Seldenrijk CA, Prins MH, Biesma DH. Risk of thrombosis and infections of central venous catheters and totally implanted access ports in patients treated for cancer. Thromb Res. 2009;125:318–321. doi: 10.1016/j.thromres.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Beghetto MG, Victorino J, Teixeira L, de Azevedo MJ. Parenteral nutrition as a risk factor for central venous catheter-related infection. JPEN J Parenter Enteral Nutr. 2005;29:367–373. doi: 10.1177/0148607105029005367. [DOI] [PubMed] [Google Scholar]
- 29.Ishizuka M, Nagata H, Takagi K, Kubota K. Total parenteral nutrition is a major risk factor for central venous catheter-related bloodstream infection in colorectal cancer patients receiving postoperative chemotherapy. Eur Surg Res. 2008;41:341–345. doi: 10.1159/000160181. [DOI] [PubMed] [Google Scholar]
- 30.Hanna HA, Raad I. Blood products: a significant risk factor for long-term catheter-related bloodstream infections in cancer patients. Infect Control Hosp Epidemiol. 2001;22:165–166. doi: 10.1086/501885. [DOI] [PubMed] [Google Scholar]
- 31.Samaras P, Dold S, Braun J, et al. Infectious port complications are more frequent in younger patients with hematologic malignancies than in solid tumor patients. Oncology. 2008;74:237–244. doi: 10.1159/000151393. [DOI] [PubMed] [Google Scholar]
- 32.Hosoglu S, Akalin S, Kidir V, Suner A, Kayabas H, Geyik MF. Prospective surveillance study for risk factors of central venous catheter-related bloodstream infections. Am J Infect Control. 2004;32:131–134. doi: 10.1016/j.ajic.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Castelli GP, Pognani C, Stuani A, Cita M, Paladini R. Central venous catheter replacement in the ICU: new site versus guidewire exchange. Minerva Anestesiol. 2007;73:267–273. [PubMed] [Google Scholar]
- 34.van der Kooi TI, de Boer AS, Mannien J, et al. Incidence and risk factors of device-associated infections and associated mortality at the intensive care in the Dutch surveillance system. Intensive Care Med. 2007;33:271–278. doi: 10.1007/s00134-006-0464-3. [DOI] [PubMed] [Google Scholar]
- 35.Balzan S, Quadros C de Almeida, de Cleva R, Zilberstein B, Cecconello I. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol. 2007;22:464–471. doi: 10.1111/j.1440-1746.2007.04933.x. [DOI] [PubMed] [Google Scholar]
- 36.Sukhotnik I, Srugo I, Mogilner JG, Lurie M, Coran AG, Shaoul R. Effect of lactulose on bacterial translocation and intestinal adaptation in a rat model of short bowel syndrome. J Pediatr Gastroenterol Nutr. 2008;46:507–513. doi: 10.1097/MPG.0b013e31815faa88. [DOI] [PubMed] [Google Scholar]
- 37.O’Boyle CJ, MacFie J, Mitchell CJ, Johnstone D, Sagar PM, Sedman PC. Microbiology of bacterial translocation in humans. Gut. 1998;42:29–35. doi: 10.1136/gut.42.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lichtman SM. Bacterial [correction of baterial] translocation in humans. J Pediatr Gastroenterol Nutr. 2001;33:1–10. doi: 10.1097/00005176-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Sanders J, Pithie A, Ganly P, et al. A prospective double-blind randomized trial comparing intraluminal ethanol with heparinized saline for the prevention of catheter-associated bloodstream infection in immunosuppressed haematology patients. J Antimicrob Chemother. 2008;62:809–815. doi: 10.1093/jac/dkn284. [DOI] [PubMed] [Google Scholar]


