Abstract
Objective
We examined the relationships between exclusive breast-feeding and the risks of respiratory, diarrhoea and nutritional morbidities during the first 2 years of life among children born to women infected with HIV-1.
Design
We prospectively determined the incidence of respiratory illnesses, diarrhoea, fever, hospitalizations, outpatient visits and nutritional morbidities. Generalized estimating equations were used to estimate the relative risks for morbidity episodes and Cox proportional hazards models to estimate the incidence rate ratios of nutritional morbidities.
Setting
Dar es Salaam, Tanzania.
Subjects
The sample consisted of 666 children born to HIV-infected women.
Results
The 666 children were followed for 2 years. Exclusive breast-feeding was associated with lower risk for cough (rate ratio (RR) = 0·49, 95 % CI 0·41, 0·60, P < 0·0001), cough and fever (RR = 0·44, 95 % CI 0·32, 0·60, P < 0·0001) and cough and difficulty breathing or refusal to feed (RR = 0·31, 95 % CI 0·18, 0·55, P < 0·0001). Exclusive breast-feeding was also associated with lower risk of acute diarrhoea, watery diarrhoea, dysentery, fever and outpatient visits during the first 6 months of life, but showed no effect at 6–24 months of life. Exclusive breast-feeding did not significantly reduce the risks of nutritional morbidities during the first 2 years of life.
Conclusions
Exclusive breast-feeding is strongly associated with reductions in the risk of respiratory and diarrhoea morbidities during the first 6 months of life among children born to HIV-infected women.
Keywords: Breast-feeding, Morbidity, Tanzania
According to WHO, breast-feeding is the best way of providing young infants with the nutrients they need for healthy growth and development(1). Infant health benefits of breast-feeding include provision of nutritional, immunological and developmental benefits(2). Over the past several years, the health advantages of breast-feeding and recommendations for practice have been strengthened. The health benefits of breast-feeding are influenced by both the duration and intensity of breast-feeding and by age at which the child receives complementary foods(3–6).
Exclusive breast-feeding (EBF) is defined as feeding a child with breast milk only and no other liquids or solids with the exception of drops or syrups consisting of vitamins, mineral supplements or medicines(7). Several studies have shown that breast-feeding and specifically EBF for 3 to 6 months is associated with reduced risk of diarrhoea and respiratory illnesses among children in both developed(4,5,8,9) and developing countries(6,10–13). Enhanced child physical growth has also reported in both settings(9,14). Prolonged and exclusive breast-feeding has also been shown to have protective effect against long-term chronic conditions and diseases such as type 1 diabetes mellitus(15), obesity(16), Crohn’s disease(17) and lymphoma(18).
The recommended practice of exclusively breast-feeding infants up to 6 months of age has been emphasized for all mothers and was revisited by WHO/UNICEF/United Nations Joint Programme on HIV/AIDS following the spread of HIV/AIDS in developing countries, to take account of new scientific and epidemiological information with the overall objective of preventing HIV transmission through breast-feeding while continuing to protect, promote and support breast-feeding for HIV-negative women and those of unknown status(19). Recent evidence on HIV transmission through breast-feeding indicates that EBF for up to 6 months was associated with a three- to fourfold decreased risk of transmission of HIV compared with non-EBF in three large cohort studies conducted in Côte d’Ivoire, South Africa and Zimbabwe(20). In addition, early cessation of breast-feeding (before 6 months) has been associated with an increased risk of infant morbidity (particularly diarrhoea) and mortality in HIV-exposed children(20).
Although EBF is recommended up to 6 months of life, programmes lag behind because of a lack of clarity on the importance of exclusive v. partial breast-feeding from 4 to 6 months, which motivates the need for studies to examine the costs of partial breast-feeding (PBF) during this part of the first 6 months of life. Also difficulty in comparing results from various studies addressing the health benefits of breast-feeding often occurs due to differences in the timing of exposure measurement; for example, EBF rates based on surveys may use 24 h recall and definitions of breast-feeding indicators may differ by setting. Furthermore, only a few studies have examined whether such health benefits – especially reduction in respiratory illness, diarrhoea and growth faltering – are observed among children born to HIV-infected women.
We prospectively followed a cohort of children born to HIV-infected women who participated in a randomized controlled trial of multivitamins and vitamin A in Dar es Salaam, Tanzania. We examined the associations between EBF, infectious and other morbidities and growth in the first 2 years of life. Endpoints included diarrhoea, respiratory illness, hospitalizations, outpatient clinic visits, fever, and underweight, stunting and wasting.
Experimental methods
Study design and population
HIV-infected pregnant women (between 12 and 27 weeks of gestation) who were residents of Dar es Salaam, Tanzania, were enrolled in a trial of multivitamins. The trial aimed to examine the effects of oral supplementation of multivitamins on HIV-disease progression among women, maternal-to-child transmission of HIV-1, and morbidity and mortality outcomes among women and children. Detailed descriptions of the trial design and follow-up procedures have been published elsewhere(21–23). Between April 1995 and July 1997, clinical and other health-care services for pregnant women and children were provided during monthly visits to a study clinic according to the Tanzania Ministry of Health guidelines.
At each visit, data were collected on child feeding practices in the previous month or since the last clinic visit, including breast-feeding status, age at weaning and age at introduction of specific foods (cow’s milk/milk powder, porridge, fruits/fruit juice, solids). We defined EBF as feeding a child with breast milk only without additional foods. Unfortunately no data were collected on water, teas or other non-nutritive liquids, and these were not considered in our definition of EBF. Duration of EBF was defined as the time in months from birth to the time when a woman first reported having given the child other foods in addition to breast milk.
Sociodemographic characteristics were collected at enrolment, including maternal and paternal age, occupation and education, marital status, income, number of living children and pregnancy history. During the monthly visits, a study physician performed a clinical examination of the women and their children. Research nurses measured maternal and child anthropometry including weight, height and mid upper-arm circumference (MUAC) using standardized procedures(24) and calibrated instruments.
Maternal laboratory blood parameters measured at baseline include Hb, plasma vitamin A and E concentrations, erythrocyte sedimentation rate (ESR), absolute counts of T-cell subsets (CD4+ and CD8+), total lymphocytes and viral load. Child CD4+ T-cell counts were measured at birth. HIV status in children was measured at birth, 6 weeks and at 3-month intervals thereafter, as described in detail elsewhere(23,25). A child was considered HIV-infected when a peripheral blood mononuclear cell specimen tested positive by the PCR technique at any point before 18 months of age. For children aged 18 months and above, HIV serostatus was determined by ELISA and confirmed by Western blot test when ELISA results were discordant.
Child morbidity and anthropometric status were ascertained at monthly clinic visits. Women were asked to report occurrence of any respiratory illness, diarrhoea and fever on the day of clinic visit and during the previous month. Body temperature was recorded on the day of clinic visit using a digital thermometer to the nearest 0·1°C. Fever was considered when the body temperature recorded was above 36·7°C. Information on child hospitalizations and unscheduled outpatient visits during the previous month was also collected. We collected information on respiratory symptoms including cough, difficult breathing and refusal to eat, breast-feed or drink liquids. Respiratory rate was measured for 1 min using a stopwatch, and we defined rapid respiratory rate as ≥50 breaths/min for infants and ≥40 breaths/min for children over 12 months. The number of days that the child had diarrhoea in the previous month was recorded. Diarrhoea was defined to the mother as presence of three or more watery stools in the prior 24 h. Inquiry was made whether the stool contained mucus or blood during any episode of diarrhoea. Respiratory endpoints were defined as follows: the occurrence of cough alone; cough and fever; cough plus one or more of these events (difficult breathing, chest retractions and refusal to eat, drink or breast-feed); or cough with rapid respiratory rate on the day of clinic visit. We divided diarrhoea episodes into four clinical types, each of which reflects the basic underlying pathology and changes in physiology, as recommended by WHO(24) and as reported in previous studies in the same setting(23,25). An acute diarrhoea episode was considered to have occurred when a child had loose or watery stools for one or more days but less than 14 d. Acute diarrhoea was further classified as dysentery (diarrhoea with visible blood or mucus) or watery diarrhoea (episodes with neither mucus nor blood). Persistent diarrhoea was considered when a child had loose or watery stools for 14 d or more, but few children had this endpoint and so it is not examined herein.
We defined moderate and severe undernutrition (underweight, stunting or wasting) by comparing child anthropometry with the reference population growth charts by age from the National Center for Health Statistics(26,27). We computed standardized Z-scores for weight-for-age (WAZ), height-for-age (HAZ) and weight-for-height (WHZ) from the measurements of weight, length and child age in months during the first 2 years of life. A child was considered to be moderately underweight, stunted or wasted when WAZ, HAZ or WHZ respectively was below –2. Severe underweight, stunting or wasting was considered when WAZ, HAZ or WHZ respectively was below –3.
Data analyses
A total of 939 singleton children were born alive to HIV-infected women in the study. Of the 939 singletons, one never breast-fed, 213 had insufficient data on breast-feeding to be included in the present analysis and fiftynine did not have data in the relevant time period of 3 months to 2 years, leaving 666 children available for analysis. We compared the 666 children with the 273 who were not in these analyses, and found that children who were not in the analyses were comparable to those in the analyses based on baseline maternal education, marital status, occupation, gestational age at enrolment and at birth, CD4+ and CD8+ T-cell counts, ESR and Hb levels. Mothers of children who were excluded from the analysis had lower MUAC and vitamin E concentrations, and were in HIV disease stage II, III or IV at baseline.
Examination of associations of EBF with the incidence of infectious episodes and other morbidities was carried out using generalizing estimating equations (GEE) in the GENMOD procedure of the Statistical Analysis Systems statistical software package version 9·1 (SAS Institute Inc., Cary, NC, USA)(28). The binomial distribution with the log link function and a working exchangeable covariance structure was specified for repeated morbidity episodes. We used Cox proportional hazards regression methods(29) to analyse the relationship between EBF and time to first episode of adverse physical growth outcomes. A data structure with one record per child-visit was utilized to facilitate the handling of time-varying covariates. EBF was treated as a time-varying binary (yes/no) variable during the 0–6 months period and as a continuous variable thereafter (EBF duration), since no child was exclusively breast-fed after 6 months of life. Children were censored at the end of 24 months if they were event-free, or at their last visit. Effect estimates are incidence (hazard) rate ratios (RR) and their corresponding 95 % confidence intervals.
GEE and Cox proportional hazards models were run for the entire study period and for these periods: 0–6, 6–12 and 12–24 months of age. For inclusion in the models, we considered the potential confounders and independent risk factors for the outcomes from a list of candidate variables. Furthermore, variables were included in multivariate models if the estimated RR for EBF changed by 10 % or more compared with the saturated main effects model(30). The confounders used in the multivariate model for respiratory illnesses were identified in the model for cough and rapid respiratory rate; for diarrhoea outcomes, the confounders were identified in the model for acute diarrhoea; and a different group of confounders was identified for hospitalization and for fever. The adjusted confounders were then applied to all other outcomes in the respective categories.
The Research and Publications Committee of Muhimbili University of Health and Allied Sciences, the Ethical Committee of the National AIDS Control Programme of the Tanzanian Ministry of Health, and the Institutional Review Board of the Harvard School of Public Health approved the study protocol.
Results
Six hundred and sixty-six children were followed-up for up to 2 years in the present study. The distribution of maternal and paternal sociodemographic characteristics, maternal indicators of nutritional and immunological status, and child HIV and nutritional status is given in Table 1. The majority of the mothers were 20–29 years old (71 %), had at least 8 years of formal education (89 %) and worked at home (housewife, 73 %). The majority of women’s partners had less than 9 years of formal education (65 %). At baseline, the majority of women had favourable nutritional and immunological status (normal BMI (18·50–24·99 kg/m2), 74 %; not anaemic, 75 %; WHO HIV disease stage I, 81 %; viral load <50 000 copies/ml, 54 %). However, most women had a CD4+ T-cell count of <500/μl (69 %). The majority of children were HIV-negative at 3 months of age (81 %) and were likely to have MUAC ≥13 cm or greater at 6 weeks or the latest value before 3 months of age.
Table 1.
Sociodemographic and clinical characteristics among HIV-infected mothers* and children born to HIV-infected mothers, Dar es Salaam, Tanzania
| Characteristic | n | %† |
|---|---|---|
| Maternal age (years) | ||
| <20 | 78 | 12 |
| 20–24 | 260 | 39 |
| 25–29 | 216 | 32 |
| ≥30 | 112 | 17 |
| Maternal education (years) | ||
| 0–8 | 596 | 89 |
| ≥9 | 70 | 11 |
| Maternal occupation | ||
| Housewife | 487 | 73 |
| Other | 179 | 27 |
| Partner’s education (years) | ||
| 0–8 | 383 | 65 |
| ≥9 | 206 | 35 |
| Maternal BMI 6 weeks after delivery (kg/m2) | ||
| <18·50 | 52 | 9 |
| 18·50–24·99 | 433 | 74 |
| ≥25·00 | 102 | 17 |
| Maternal baseline Hb (g/dl) | ||
| <8·5 | 167 | 25 |
| ≥8·5 | 491 | 75 |
| Maternal WHO HIV disease stage | ||
| I | 539 | 81 |
| II or above | 126 | 19 |
| Maternal CD4+ T-cell count (cells/μl) | ||
| 0–199 | 66 | 10 |
| 200–499 | 369 | 58 |
| ≥500 | 199 | 31 |
| Maternal viral load (copies/ml) | ||
| <50 000 | 166 | 54 |
| ≥50 000 | 142 | 46 |
| Child HIV status at 3 months | ||
| Not infected | 533 | 81 |
| Infected | 128 | 19 |
| Child MUAC at age <3 months (cm) | ||
| <13 | 137 | 33 |
| ≥13 | 278 | 67 |
The women were at 12–27 weeks of gestation at enrolment.
Percentages may not total 100 because of rounding.
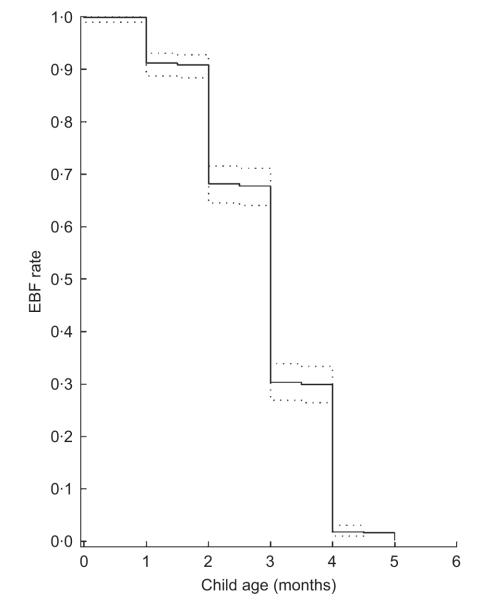
The rate of EBF by child age is shown in Fig. 1. The rate fell rapidly after 1 month of life and no child was exclusively breast-fed after 5 months. The univariate- and multivariate-adjusted results on the association of EBF and subsequent risks of infectious and other morbidity episodes during the first 6 months of life are presented in Table 2. We examined the relationship of EBF with various definitions of acute respiratory infections. In multivariate analysis, EBF was associated with a 51 % decreased risk of cough (P< 0·0001), a 56 % reduced risk of cough and fever (P< 0·0001) and a 69 % reduced risk of cough and difficulty breathing or chest retraction or refusal to feed (P< 0·0001). However, EBF was not significantly associated with the risk of developing cough and rapid respiratory rate (P = 0·54).
Fig. 1.
Rates of exclusive breast-feeding (EBF) by child age among children born to HIV-infected mothers, Dar es Salaam, Tanzania (…, 95 % confidence bounds)
Table 2.
Prospective association of exclusive breast-feeding with subsequent risk of morbidity episodes among children during 0–6 months of life, Dar es Salaam, Tanzania
| Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|
| Outcome | Events | RR | 95 % CI | P | RR | 95 % CI | P |
| Respiratory infection | |||||||
| Cough* | 993 | 0·52 | 0·43, 0·62 | <0·0001 | 0·49 | 0·40, 0·60 | <0·0001 |
| Cough and fever† | 300 | 0·47 | 0·35, 0·63 | <0·0001 | 0·44 | 0·32, 0·60 | <0·0001 |
| Cough and difficulty breathing or chest retraction or refusal to feed† |
119 | 0·33 | 0·19, 0·58 | 0·0001 | 0·31 | 0·18, 0·55 | <0·0001 |
| Cough and rapid respiratory rate† | 44 | 0·90 | 0·46, 1·75 | 0·75 | 0·80 | 0·40, 1·62 | 0·54 |
| Diarrhoea‡ | |||||||
| Acute | 223 | 0·33 | 0·22, 0·49 | <0·0001 | 0·29 | 0·19, 0·44 | <0·0001 |
| Watery | 88 | 0·33 | 0·18, 0·62 | 0·0006 | 0·29 | 0·15, 0·59 | 0·0005 |
| Dysentery | 135 | 0·34 | 0·21, 0·57 | <0·0001 | 0·30 | 0·18, 0·52 | <0·0001 |
| Fever and hospitalizations§ | |||||||
| Fever | 515 | 0·51 | 0·41, 0·65 | <0·0001 | 0·49 | 0·39, 0·64 | <0·0001 |
| Hospitalization | 48 | 0·71 | 0·37, 1·35 | 0·30 | 0·51 | 0·25, 1·06 | 0·07 |
| Outpatient visit | 370 | 0·45 | 0·34, 0·59 | <0·0001 | 0·43 | 0·32, 0·57 | <0·0001 |
RR, rate ratio; MUAC, mid upper-arm circumference; HAZ, height-for-age Z-score; WAZ, weight-for-age Z-score; WHZ, weight-for-height Z-score.
Adjusted for child HIV status, MUAC, HAZ, WAZ, WHZ, gender, birth weight and birth height; and maternal viral load, CD4 count, Hb, BMI, MUAC, age, education, occupation, social support, clinical depression, partner education, number of living children, calendar year of delivery and type of delivery.
Adjusted for child HIV status and MUAC; and maternal occupation, MUAC and partner education.
Adjusted for child MUAC; and maternal social support, clinical depression score, CD4 count, Hb, year of delivery and number of living children.
Adjusted for child birth weight, HIV status, WAZ and WHZ; and maternal clinical depression score, BMI and year of delivery.
We further assessed the association of EBF and diarrhoeal diseases and found that EBF was associated with a 71 % reduced risk of acute diarrhoea (P< 0·0001), a 71 % reduced risk of watery diarrhoea (P = 0·0005) and a 70 % reduced risk of dysentery (P< 0·0001). EBF was also found to be associated with a 51 % reduced risk of fever (P< 0·0001) and a 57 % reduced risk of outpatient visit (P< 0·0001) during the first 6 months of life. EBF was not associated with the risk of hospitalization (P = 0·07) during the first 6 months of life.
The multivariate-adjusted results for infectious and other morbidity episodes during the 6–12 and 12–24 months periods are shown in Table 3. The strongly beneficial effects of EBF on infectious and other morbidity episodes did not persist except for a 10 % reduction in the risk of cough (RR = 0·90, 95 % CI 0·82, 0·99, P = 0·03) during the 6–12 months period. In addition, we found that EBF was not associated with risk of developing moderate or severe underweight, stunting or wasting during the first 6 months of life (Table 4) or after 6 months of life (Table 5), compared with PBF.
Table 3.
Prospective association of exclusive breast-feeding with subsequent risk of morbidity episodes among children during 6–24 months of life, Dar es Salaam, Tanzania
| 6–12 months |
12–24 months |
|||||
|---|---|---|---|---|---|---|
| Outcome | RR | 95 % CI | P | RR | 95 % CI | P |
| Respiratory infection | ||||||
| Cough* | 0·90 | 0·82, 0·99 | 0·03 | 0·92 | 0·84, 1·00 | 0·05 |
| Cough and fever† | 0·93 | 0·83, 1·04 | 0·19 | 0·96 | 0·85, 1·09 | 0·52 |
| Cough and difficulty breathing or chest retraction or refusal to feed† |
1·01 | 0·85, 1·20 | 0·94 | 1·06 | 0·90, 1·24 | 0·48 |
| Cough and rapid respiratory rat† | 1·20 | 0·77, 1·87 | 0·42 | 1·07 | 0·84, 1·36 | 0·60 |
| Diarrhoea‡ | ||||||
| Acute | 0·96 | 0·85, 1·08 | 0·50 | 0·99 | 0·88, 1·10 | 0·80 |
| Watery | 0·95 | 0·82, 1·11 | 0·52 | 1·00 | 0·86, 1·16 | 0·95 |
| Dysentery | 0·97 | 0·84, 1·13 | 0·71 | 0·97 | 0·85, 1·11 | 0·65 |
| Fever and hospitalizations§ | ||||||
| Fever | 0·95 | 0·86, 1·04 | 0·29 | 0·98 | 0·89, 1·09 | 0·74 |
| Hospitalization | 1·10 | 0·86, 1·41 | 0·45 | 1·12 | 0·89, 1·43 | 0·33 |
| Outpatient visit | 0·98 | 0·88, 1·10 | 0·77 | 1·05 | 0·95, 1·17 | 0·33 |
RR, rate ratio; MUAC, mid upper-arm circumference; HAZ, height-for-age Z-score; WAZ, weight-for-age Z-score; WHZ, weight-for-height Z-score.
Adjusted for child HIV status, MUAC, HAZ, WAZ, WHZ, gender, birth weight and birth height; and maternal viral load, CD4 count, Hb, BMI, MUAC, age, education, occupation, social support, clinical depression, partner education, number of living children, calendar year of delivery and type of delivery.
Adjusted for child HIV status and MUAC; and maternal occupation, MUAC and partner education.
Adjusted for child MUAC; and maternal social support, clinical depression score, CD4 count, Hb, year of delivery and number of living children.
Adjusted for child birth weight, HIV status, WAZ and WHZ; and maternal clinical depression score, BMI and year of delivery.
Table 4.
Prospective association of exclusive breast-feeding with subsequent risk of first episode of growth faltering among children during 0–6 months of life, Dar es Salaam, Tanzania
| Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|
| Outcome | Events | RR | 95 % CI | P | RR | 95 % CI | P |
| Z-score (<–22 v. ≥–22) | |||||||
| WAZ (underweight)* | 80 | 1·28 | 0·77, 2·14 | 0·34 | 1·29 | 0·75, 2·21 | 0·36 |
| HAZ (stunted)‡ | 103 | 1·28 | 0·79, 2·07 | 0·32 | 1·34 | 0·82, 2·20 | 0·25 |
| WHZ (wasted)‡ | 32 | 0·88 | 0·36, 2·11 | 0·77 | 0·77 | 0·31, 1·92 | 0·58 |
| Z-score (<–23 v. ≥–23) | |||||||
| WAZ (underweight)§ | 22 | 0·64 | 0·23, 1·80 | 0·40 | 0·54 | 0·17, 1·66 | 0·28 |
| HAZ (stunted)∥ | 30 | 1·43 | 0·60, 3·39 | 0·41 | 1·27 | 0·50, 3·22 | 0·61 |
| WAZ (wasted)¶ | 7 | – | – | – | – | ||
RR, rate ratio; WAZ, weight-for-age Z-score; HAZ, height-for-age Z-score; WHZ, weight-for-height Z-score; MUAC, mid upper-arm circumference.
Adjusted for child gender and HIV status; and maternal age, viral load, MUAC and social support.
Adjusted for child gender, birth height and HIV status; and maternal age, partner education, number of living children and type of delivery.
Adjusted for child birth height; and maternal social support, CD4 count, Hb, MUAC, BMI and type of delivery.
Adjusted for child HIV status; and maternal partner education and CD4 count.
Adjusted for child gender, birth weight and HIV status; and maternal social support and partner education.
Adjusted for child HIV status; and maternal social support, clinical depression score and type of delivery.
Table 5.
Prospective association of exclusive breast-feeding with subsequent risk of first episode of growth faltering among children during 6–24 months of life, Dar es Salaam, Tanzania
| 6–12 months |
12–24 months |
|||||
|---|---|---|---|---|---|---|
| Outcome | RR | 95 % CI | P | RR | 95 % CI | P |
| Z-score (<–22 v. ≥–22) | ||||||
| WAZ (underweight)* | 1·07 | 0·85, 1·36 | 0·56 | 1·09 | 0·76, 1·55 | 0·66 |
| HAZ (stunted)† | 1·04 | 0·84, 1·30 | 0·71 | 1·07 | 0·76, 1·50 | 0·71 |
| WHZ (wasted)‡ | 0·91 | 0·64, 1·29 | 0·60 | 1·19 | 0·89, 1·59 | 0·24 |
| Z-score (<–23 v. ≥–23) | ||||||
| WAZ (underweight)§ | 1·06 | 0·74, 1·52 | 0·76 | 0·96 | 0·61, 1·52 | 0·87 |
| HAZ (stunted)∥ | 1·05 | 0·69, 1·59 | 0·82 | 1·03 | 0·69, 1·53 | 0·88 |
| WAZ (wasted)¶ | 1·11 | 0·47, 2·66 | 0·81 | 1·67 | 0·93, 3·00 | 0·09 |
RR, rate ratio; WAZ, weight-for-age Z-score; HAZ, height-for-age Z-score; WHZ, weight-for-height Z-score; MUAC, mid upper-arm circumference.
Adjusted for child gender and HIV status; and maternal age, viral load, MUAC and social support.
Adjusted for child gender, birth height and HIV status; and maternal age, partner education, number of living children and type of delivery.
Adjusted for child birth height; and maternal social support, CD4 count, Hb, MUAC, BMI and type of delivery.
Adjusted for child HIV status; and maternal partner education and CD4 count.
Adjusted for child gender, birth weight and HIV status; and maternal social support and partner education.
Adjusted for child HIV status; and maternal social support, clinical depression score and type of delivery.
Discussion
Breast-feeding provides nutritional, immunological and developmental benefits for human infants. In the present 2-year follow-up study, EBF among children born to HIV-infected women was strongly associated with reductions in risk of cough, cough and fever, cough and difficulty breathing or chest retraction or refusal to feed, acute diarrhoea, watery diarrhoea, dysentery, fever and outpatient visit during the first 6 months of life. The observed beneficial effect of EBF for cough continued in the first 12 months of life. The importance of these findings lies in the demonstration of health benefits of EBF, especially reduction in respiratory and diarrhoea morbidity among children born to HIV-infected women. These results are similar to those reported from other studies that examined the associations of EBF and child morbidity outcomes(12,31,32); however, the previous studies were done in different settings and populations. In a cohort study from India, the risk of diarrhoea among 6–11-month-old infants was threefold higher among those who had PBF at or below 3 months compared with infants who had EBF beyond 3 months(31). In another longitudinal study in Peru, the prevalence of diarrhoea among children who were partially breast-fed was double that among children who were exclusively breast-fed (15 % v. 7 %) during the first 6 months of life(12). In that study children who were par tially breast-fed at 3–5 months had a threefold higher risk of diarrhoea using the prevalence data and had 1·8-fold insignificant risk of diarrhoea using the incidence data compared with children who were exclusively breast-fed. In the Philippines, the risk of diarrhoea comparing partially breast-fed children at 4 months with those who were exclusively breast-fed beyond 4 months was found to be 12·9 in the urban and 6·3 in the rural sample(32).
The advantages of EBF include provision of nutrients and reduced exposure to pathogens in contaminated complementary foods, thereby decreasing the risk of gastrointestinal infections(33). Potential mechanisms whereby EBF would reduce the risk of infectious diarrhoea are through provision of immune factors in the gut that may inhibit pathogens and by enhancing the integrity of infant gut mucosa. The presence of immune factors and adequate nutrients in breast milk could also enhance infant systemic immune function and nutritional status, thus lowering the risk of infectious morbidity such as respiratory illnesses. Secretory IgA (sIgA) antibodies for instance are the main component of humoral factors(8), and protect against viral(34,35) and bacterial infections(35–37). Non-specific factors such as lactoferrin, lysozymes, nucleotides, oligosaccharides and cytokines in breast milk further aid the sIgA functions(2). Breast milk has growth factors that enhance infant gastrointestinal maturity and function and help maintain gut mucosa integrity.
Inconsistent results on the relationship between EBF and child growth have been reported in a few studies. No difference in weight gain and length gain at 4–6 months was found among children fully breast-fed (defined as no other nutritive foods or liquids) compared with partially breast-fed infants in the Philippines(38). Also a study in Sudan that compared EBF and PBF showed no significant difference in weight gain, but higher length gain in the EBF group during 20–24 weeks of age(39). In a cohort study from Belarus, EBF for 3 months was associated with significantly greater weight and length gain during 3 to 6 months compared with children exclusively breast-fed for 6 months or more(9). Findings from Brazil showed that EBF was associated with reduced diarrhoea episodes and better WAZ overall(40). Our study did not find any benefit or disadvantage in exclusive v. partial breast-feeding in relation to growth faltering.
The major strength of our study is the prospective nature in which data were collected, allowing for a proper temporal relationship to be assessed between EBF and infectious and other morbidities. However, the study had several limitations. It is possible that methodological limitations may have accounted for the lack of association between EBF and growth faltering, for instance, since much of the morbidity data were based on 1-month recalls by mothers and it is possible that mild symptoms such as cough and diarrhoea may not have been recalled well for that long, which may have led to misclassification of these endpoints. On the other hand, such misclassification is most likely non-differential with respect to EBF status and might have led to null associations. We also conducted analysis combining both HIV-infected and non-infected children primarily because there were only a few HIV-infected children during the first 2 years of follow up. Furthermore, the results of the present study may not be generalizable to children born to mothers with a later HIV disease stage since only 20 % of the mothers in our study were stage II or higher at enrolment.
In conclusion, we observed a strong association between EBF and significant reduction in the risks of respiratory and diarrhoeal morbidities during the first 6 months of life among children born to early-stage HIV-infected women in Tanzania. These findings support the recommendation of promoting EBF from 4 to 6 months among HIV-infected women who opt to breast-feed. Therefore, women should be supported in their choice of infant feeding practices and educated on the advantages and disadvantages of exclusive v. partial breast-feeding.
Acknowledgements
R.S.M. received support from the Informatics Training for Global Health (ITGH) Program (NIH D43TW007013-02) and the FIC/Ellison Clinical Research Training Supplement (NIH D43TW007013-04S1). There are no conflicts of interests. R.S.M. interpreted the data and wrote the manuscript. D.S. provided statistical guidance in data analysis. E.L. analysed the data. K.P. and C.D. assisted with data interpretation. S.A. contributed to the study implementation in the field, in the laboratory and reviewed the manuscript. G.M. and W.W.F. contributed to the study design of the original trial of vitamins study, the present study being a secondary analysis of data collected in the trial of vitamins. All authors contributed to the manuscript revisions. We thank the mothers and children for their participation; the nursing and technical staff in Dar es Salaam, Tanzania; and the various scientists at Harvard School of Public Health and Muhimbili University of Health and Allied Sciences for their collaboration and assistance throughout. We greatly appreciate the input of Dr Annamaria Kiure in the initial draft of this study.
References
- 1.World Health Organization [accessed December 2009];Breastfeeding. 2009 http://www.who.int/topics/breastfeeding/en/
- 2.Goldman AS. The immune system of human milk: antimicrobial, antiinflammatory and immunomodulating properties. Pediatr Infect Dis J. 1993;12:664–671. doi: 10.1097/00006454-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Horta BL, Bahl R, Martninez JC, et al. Evidence on the Long-term Effects of Breastfeeding: Systematic Reviews and Meta-analysis. WHO; Geneva: 2007. [Google Scholar]
- 4.Beaudry M, Dufour R, Marcoux S. Relation between infant feeding and infections during the first six months of life. J Pediatr. 1995;126:191–197. doi: 10.1016/s0022-3476(95)70544-9. [DOI] [PubMed] [Google Scholar]
- 5.Dewey KG, Heinig MJ, Nommsen-Rivers LA. Differences in morbidity between breastfed and formulafed infants. J Pediatr. 1995;126:696–702. doi: 10.1016/s0022-3476(95)70395-0. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham AS, Jelliffe DB, Jelliffe EF. Breast-feeding and health in the 1980s: a global epidemiologic review. J Pediatr. 1991;118:659–666. doi: 10.1016/s0022-3476(05)80023-x. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . Indicators for Assessing Breastfeeding Practices. WHO; Geneva: 1991. WHO/CDD/SER/91.14. [Google Scholar]
- 8.Howie PW, Forsyth JS, Ogston SA, et al. Protective effect of breast feeding against infection. BMJ. 1990;300:11–16. doi: 10.1136/bmj.300.6716.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer MS, Guo T, Platt RW, et al. Infant growth and health outcomes associated with 3 compared with 6 mo of exclusive breastfeeding. Am J Clin Nutr. 2003;78:291–295. doi: 10.1093/ajcn/78.2.291. [DOI] [PubMed] [Google Scholar]
- 10.Clemens JD, Stanton B, Stoll B, et al. Breast feeding as a determinant of severity in shigellosis. Evidence for protection throughout the first three years of life in Bangladeshi children. Am J Epidemiol. 1986;123:710–720. doi: 10.1093/oxfordjournals.aje.a114291. [DOI] [PubMed] [Google Scholar]
- 11.Briend A, Wojtyniak B, Rowland MG. Breast feeding, nutritional state, and child survival in rural Bangladesh. Br Med J (Clin Res Ed) 1988;296:879–882. doi: 10.1136/bmj.296.6626.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown KH, Black RE, de Romana G Lopez, et al. Infant-feeding practices and their relationship with diarrheal and other diseases in Huascar (Lima), Peru. Pediatrics. 1989;83:31–40. [PubMed] [Google Scholar]
- 13.Victora CG, Barros FC, Halpem R, et al. Longitudinal study of the mother and child population in an urban region of southern Brazil, 1993: methodological aspects and preliminary results. Rev Saude Publica. 1996;30:34–45. doi: 10.1590/s0034-89101996000100005. [DOI] [PubMed] [Google Scholar]
- 14.Eckhardt CL, Rivera J, Adair LS, et al. Full breast-feeding for at least four months has differential effects on growth before and after six months of age among children in a Mexican community. J Nutr. 2001;131:2304–2309. doi: 10.1093/jn/131.9.2304. [DOI] [PubMed] [Google Scholar]
- 15.Gerstein H. Cow’s-milk exposure and type-I diabetes mellitus: a critical review of the clinical literature. Diabetes Care. 1994;17:13–19. doi: 10.2337/diacare.17.1.13. [DOI] [PubMed] [Google Scholar]
- 16.Gillman M, Rifas-Shiman S, Camargo C, et al. Risk of overweight among adolescents who were breastfed as infants. JAMA. 2001;285:2461–2467. doi: 10.1001/jama.285.19.2461. [DOI] [PubMed] [Google Scholar]
- 17.Koletzko S, Sherman P, Corey M, et al. Role of infant feeding practices in development of Crohn’s disease in childhood. BMJ. 1989;298:1617–1618. doi: 10.1136/bmj.298.6688.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis M. Review of the evidence for an association between infant feeding and childhood cancer. Int J Cancer Suppl. 1998;11:29–33. [PubMed] [Google Scholar]
- 19.United Nations Joint Programme on HIV/AIDS . HIV and Infant Feeding: Guidelines for Decision-makers. UNICEF/UNAIDS/WHO; Geneva: 1998. [Google Scholar]
- 20.World Health Organization . WHO HIV and Infant Feeding Technical Consultation Held on behalf of the Inter-agency Task Team (IATT) on Prevention of HIV Infections in Pregnant Women, Mothers and their Infants. Geneva, October 25–27, 2006. WHO Consensus Statement. WHO; Geneva: 2006. [Google Scholar]
- 21.Fawzi WW, Msamanga GI, Spiegelman D, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet. 1998;351:1477–1482. doi: 10.1016/s0140-6736(98)04197-x. [DOI] [PubMed] [Google Scholar]
- 22.Fawzi WW, Msamanga GI, Spiegelman D, et al. Rationale and design of the Tanzania Vitamin and HIV Infection Trial. Control Clin Trials. 1999;20:75–90. doi: 10.1016/s0197-2456(98)00045-2. [DOI] [PubMed] [Google Scholar]
- 23.Antelman G, Msamanga GI, Spiegelman D, et al. Nutritional factors and infectious disease contribute to anemia among pregnant women with human immunodeficiency virus in Tanzania. J Nutr. 2000;130:1950–1957. doi: 10.1093/jn/130.8.1950. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization . Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series no. 854. WHO; Geneva: 1995. [PubMed] [Google Scholar]
- 25.Fawzi WW, Msamanga GI, Wei R, et al. Effect of providing vitamin supplements to human immunodeficiency virus-infected, lactating mothers on the child’s morbidity and CD4+ cell counts. Clin Infect Dis. 2003;36:1053–1062. doi: 10.1086/374223. [DOI] [PubMed] [Google Scholar]
- 26.Dibley MJ, Staehling N, Nieburg P, et al. Interpretation of Z-score anthropometric indicators derived from the international growth reference. Am J Clin Nutr. 1987;46:749–762. doi: 10.1093/ajcn/46.5.749. [DOI] [PubMed] [Google Scholar]
- 27.Dibley MJ, Goldsby JB, Staehling NW, et al. Development of normalized curves for the international growth reference: historical and technical considerations. Am J Clin Nutr. 1987;46:736–748. doi: 10.1093/ajcn/46.5.736. [DOI] [PubMed] [Google Scholar]
- 28.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford Science Publications; New York: 1994. pp. 183–188. [Google Scholar]
- 29.Cox DR. Regression models and life tables. J R Stat Soc Ser B. 1972;34:187–220. [Google Scholar]
- 30.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–349. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mondal SK, Gupta PG, Gupta DN, et al. Occurrence of diarrhoeal diseases in relation to infant feeding practices in a rural community in West Bengal, India. Acta Paediatr. 1996;85:1159–1162. doi: 10.1111/j.1651-2227.1996.tb18221.x. [DOI] [PubMed] [Google Scholar]
- 32.Popkin BM, Adair L, Akin JS, et al. Breast-feeding and diarrheal morbidity. Pediatrics. 1990;86:874–882. [PubMed] [Google Scholar]
- 33.World Health Organization/UNICEF . Complementary Feeding of Young Children in Developing Countries: A Review of Current Scientific Knowledge. WHO; Geneva: 1998. [Google Scholar]
- 34.Hanson LA, Ahlstedt S, Andersson B, et al. Protective factors in milk and the development of the immune system. Pediatrics. 1985;75:172–176. [PubMed] [Google Scholar]
- 35.Hanson LA. Breastfeeding provides passive and likely long-lasting active immunity. Ann Allergy Asthma Immunol. 1998;81:523–533. doi: 10.1016/S1081-1206(10)62704-4. [DOI] [PubMed] [Google Scholar]
- 36.Glass RI, Svennerholm AM, Stoll BJ, et al. Protection against cholera in breast-fed children by antibodies in breast milk. N Engl J Med. 1983;308:1389–1392. doi: 10.1056/NEJM198306093082304. [DOI] [PubMed] [Google Scholar]
- 37.Cruz JR, Gil L, Cano F, et al. Breast milk anti-Escherichia coli heat-labile toxin IgA antibodies protect against toxin-induced infantile diarrhea. Acta Paediatr Scand. 1988;77:658–662. doi: 10.1111/j.1651-2227.1988.tb10726.x. [DOI] [PubMed] [Google Scholar]
- 38.Adair L, Popkin BM, VanDerslice J, et al. Growth dynamics during the first two years of life: a prospective study in the Philippines. Eur J Clin Nutr. 1993;47:42–51. [PubMed] [Google Scholar]
- 39.Zumrawi FY, Dimond H, Waterlow JC. Faltering in infant growth in Khartoum province, Sudan. Hum Nutr Clin Nutr. 1987;41:383–395. [PubMed] [Google Scholar]
- 40.Barros FC, Semer TC, Filho S Tonioli, et al. The impact of lactation centres on breastfeeding patterns, morbidity and growth: a birth cohort study. Acta Paediatr. 1995;84:1221–1226. doi: 10.1111/j.1651-2227.1995.tb13537.x. [DOI] [PubMed] [Google Scholar]