Abstract
The circadian clock protein Period 1 (Per1) contributes to the regulation of expression of the α subunit of the renal epithelial sodium channel (αENaC) at the basal level and in response to the mineralocorticoid hormone aldosterone. The goals of the present study were to define the role of Per1 in the regulation of additional renal sodium handling genes in cortical collecting duct cells and to evaluate BP in mice lacking functional Per1. To determine if Per1 regulates additional genes important in renal sodium handling, a candidate gene approach was employed. Immortalized collecting duct cells were transfected with a non-target siRNA or a Per1 specific siRNA. Expression of the genes for αENaC and Fxyd5, a positive regulator of Na, K-ATPase activity, decreased in response to Per1 knockdown. Conversely, mRNA expression of caveolin-1, Ube2e3 and ET-1, all negative effectors of ENaC, was induced following Per1 knockdown. These results led us to evaluate BP in Per1 KO mice. Mice lacking Per1 exhibit significantly reduced BP and elevated renal ET-1 levels compared to wild type animals. Given the established role of renal ET-1 in ENaC inhibition and blood pressure control, elevated renal ET-1 is one possible explanation for the lower blood pressure observed in Per1 KO mice. These data support a role for the circadian clock protein Per1 in the coordinate regulation of genes involved in renal sodium reabsorption. Importantly, the lower BP observed in Per1 KO mice compared to wild type suggests a role for Per1 in BP control as well.
Keywords: kidney, circadian rhythm, clock, collecting duct, gene regulation
Introduction
Approximately one-third of Americans are afflicted with hypertension, the leading risk factor for cardiovascular disease. The majority of these patients suffer from essential hypertension, for which there is no established etiology. Increasing evidence suggests a role for the circadian clock in the control of blood pressure (BP). A subset of hypertensive individuals do not experience the normal nighttime decrease in BP and are at greater risk for cardiovascular complications1. These so-called “non-dippers” are known to suffer from increased left ventricular hypertrophy, atherosclerosis, microalbuminuria, congestive heart failure, stroke and myocardial infarction. In addition to this non-dipping phenotype, BP abnormalities and cardiovascular disease are well known in night-shift workers2,3. Although these clinical correlations have been established, the underlying molecular mechanisms are poorly understood.
The core circadian clock consists of a positive and negative transcriptional feedback loop. In the positive loop, Bmal1 and Clock drive transcription of the Per (Per1, Per2 and Per3) and Cryptochrome (Cry1 and Cry2) genes. In the negative feedback loop, Per and Cry action inhibit the action of Bmal1 and Clock, thereby decreasing their own transcription4. Circadian clock proteins interact with E-box response elements in target gene promoters to affect transcriptional regulation. Although Per1 has been characterized as a transcriptional repressor, increasing evidence suggests that it may participate in transcriptional activation, perhaps in a gene or tissue specific manner5,6,7.
The circadian clock gene Per1 is an aldosterone target in renal collecting duct (CD) cells8. Per1 contributes to the basal and aldosterone-dependent transcription of the Scnn1a gene that encodes the α subunit of the epithelial sodium channel (αENaC)6. Scnn1a expression was reduced in the renal medulla of Per1 knockout (KO) mice. Further investigation into the regulation of αENaC by Per1 revealed that cortical αENaC mRNA was reduced in Per1 KO mice and Per1 knockdown resulted in reduced αENaC protein levels in immortalized murine renal cortical CD (CCD) mpkCCDc14 cells7. Given the critical role of ENaC in sodium transport and BP control, the results suggest that the clock contributes to circadian fluctuations in sodium excretion and BP.
Expression profiling experiments in different tissues have shown that 6-8% of the genes were subject to circadian control (reviewed in9). Temporal analysis of gene expression in the distal convoluted tubule and CCD showed that hundreds of transcripts were expressed in a circadian manner10. Given the known circadian oscillations in gene expression in these cell types, we used a model of the CCD to identify novel Per1 targets. The results suggest that Per1 coordinately regulates several genes encoding products that function in renal sodium reabsorption. Finally, we show for the first time that Per1 KO mice exhibited significantly lower BP compared to wild type (WT) mice.
Methods
Animals
Per1 KO mice (129/sv) were provided by Dr. David Weaver (University of Massachusetts11) and maintained by Animal Care Services at UF. WT 129/sv control mice were ordered from Charles River. Animals were maintained on a normal 12hr light:dark cycle and fed normal lab chow (Harlan #2018). Experiments were performed with the approval of UF and VA Medical Center IACUCs and in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Data Sciences International telemetry transmitters were surgically implanted through the left carotid artery, extending into the aortic arch (according to the method of12). Mice (18-20 weeks old) were allowed at least seven days to recover before recordings were made.
Cell culture and molecular biology
Detailed methods are available in the supplemental section, please see http://hyper.ahajournals.org.
Statistical Analysis
Statistical analyses were performed using the Student’s t-test in Excel. BP data and tissue ET-1 ELISA data were analyzed using two-way ANOVA (SigmaStat) with the Holm-Sidak test. P values less than 0.05 were considered significant.
Results
Per1 coordinately regulates expression of genes involved in sodium transport
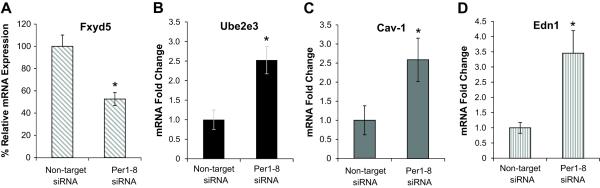
Given the demonstrated regulation of αENaC gene expression by Per16,7, we investigated the possibility that Per1 regulated additional genes encoding products that participate in sodium transport or the regulation of sodium transport. We used mpkCCDc14 cells as a model of the renal CCD because this cell line is well characterized13 and has been used extensively to study the regulation of ENaC14-18. In addition to αENaC, the sodium transport genes, Fyxd5, Ube2e3, Cav1 and Edn1 were identified as potential Per1 targets.
Fxyd5 is a positive effector of the Na, K-ATPase that mediates basolateral sodium transport to the blood stream19. Greater than 40% reduction in Fxyd5 mRNA was observed following Per1 knockdown in mpkCCDc14 cells (Figure 1A). In contrast to αENaC and Fxyd5, whose gene products function in sodium retention, the expression of three inhibitors of sodium reabsorption was induced following Per1 knockdown. Ube2e3 encodes an E3 ubiquitin ligase linked to ENaC turnover20, Caveolin-1 (Cav-1) is involved in endocytosis of ENaC21, and the endothelin-1 peptide (ET-1, encoded by the Edn1 gene) inhibits ENaC via a decrease in channel open probability22,23. Both Ube2e3 and Cav-1 mRNAs increased more than 2.5x in mpkCCDc14 cells following Per1 knockdown (Figure 1B, 1C). ET-1 mRNA was induced nearly 4x in the absence of Per1 (Figure 1D). Significant changes were not observed in expression of the Zinc transporter gene Slc39a14 following Per1 knockdown (Figure S1).
Figure 1. Altered expression of genes involved in sodium transport following Per1 knockdown in mpkCCDc14 Cells.
mpkCCDc14 cells were transfected with a non-target siRNA or Per1 specific siRNA. Under these conditions, Per1 mRNA levels were reduced by about 90%. QPCR was used to measure changes in gene expression following Per1 knockdown for A. Fxyd5, B. Ube2e3, C. Caveolin-1 and D. Edn1.
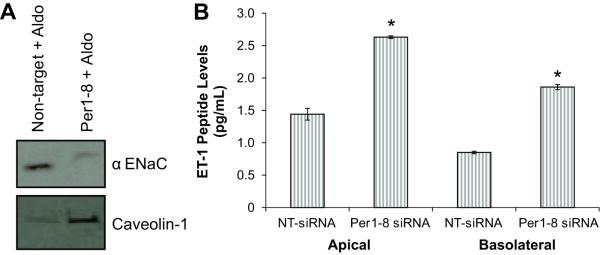
Since antibodies to Cav-1 and αENaC were readily available, we tested whether Per1-mediated regulation of these genes extended to the level of protein. Membrane protein levels of αENaC protein were reduced in Per1-8 siRNA-transfected cells as compared to the non-target siRNA control (Figure 2A, top panel). Cav-1 membrane protein levels were clearly induced following Per1 knockdown (Figure 2A, bottom panel). ET-1 is a secreted peptide hormone of 21 amino acids encoded by the Edn1 gene. Because the mpkCCDc14 cells were grown in transwell inserts, media was collected from the apical (lumen) and basolateral sides of the cultures. Media samples were collected from cells transfected with a control non-target siRNA or Per1-8 siRNA and evaluated for ET-1 peptide levels. ET-1 peptide levels were significantly greater in the media from mpkCCDc14 cells transfected with the Per1-8 siRNA compared to control (Figure 2B).
Figure 2. Protein expression levels are affected by Per1 knockdown in mpkCCDc14 cells.
mpkCCDc14 cells were transfected with a non-target siRNA or Per1 specific siRNA. Under these conditions, Per1 mRNA levels are reduced by 80%. A. Western blot analysis of membrane protein from aldosterone (Aldo)-treated, siRNA-transfected mpkCCDc14 cells was performed using an anti-αENaC antibody or an anti-Caveolin-1 antibody. B. ET-1 peptide levels were measured in media samples from cells grown in transwell dishes; top media corresponds to apical and bottom media to basolateral. *p<0.05, n=6. Data are representative of at least two independent experiments.
To test whether the regulation of these novel Per1 target genes occurred in a model of the inner medullary CD, mRNA expression levels of Fxyd5, Ube2e3, Cav-1 and ET-1 were evaluated in mIMCD-3 cells. Similar to the effect of Per1 knockdown in mpkCCDc14 cells, Fxyd5 mRNA levels were decreased by more than 50% following Per1 knockdown (Figure S2A). Similarly, Ube2e3, Cav-1 and ET-1 mRNA were significantly induced in the absence of Per1 (Figures S2B-D).
Temporal regulation of sodium transport genes
Regulation of Fxyd5, ET-1, Cav-1 and Ube2e3 by Per1 suggested that expression of these genes may be clock-controlled in vivo. Expression of αENaC and Per1 mRNA follows a circadian pattern of expression in the renal cortex with reduced mRNA levels at zeitgeiber time (ZT) 22 (active phase) versus ZT6 (sleep phase)6. Therefore, the levels of Fxyd5, ET-1, Cav-1 and Ube2e3 mRNA expression were tested in WT mice by measuring steady state mRNA expression levels during the light and dark cycles (Figure S3). Significant differences in mRNA levels between two time points are suggestive of clock-controlled regulation10,24. Like Per1 and αENaC6, Fxyd5 levels dramatically decreased at ZT22 relative to ZT6 (Figure S3A). Cav-1 and Ube2e3 expression levels were lower at ZT22 relative to ZT6 (Figures S3B, 3C). In contrast, the circadian pattern of ET-1 mRNA expression was inverted with an increase at ZT22 compared to ZT6 (Figure S3D). Given that Per1 mRNA expression is elevated at ZT6 when ET-1 mRNA levels are low and Per1 knockdown resulted in increased ET-1 mRNA, this result may reflect inhibitory action of Per1 on ET-1.
Figure 3. Per1 negatively regulates the expression of ET-1.
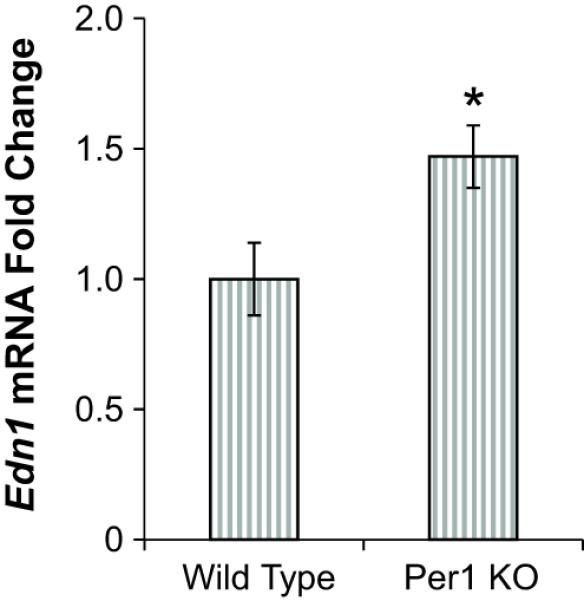
Edn1 mRNA levels are higher in the renal cortex of Per1 KO mice compared to WT mice. Cortex dissections were made from the kidneys of age-matched, male WT (129/sv) or Per1 KO mice. Gene expression was measured using QPCR, with values normalized to actin mRNA expression. Fold change values are relative to WT. *p<0.05, n=6 animals of each genotype.
Per1 negatively regulates ET-1 gene expression
Because renal ET-1 plays a critical role in BP control, the regulation of Edn1 gene expression by Per1 was evaluated in WT and Per1 KO mice. Consistent with an inhibitory action of Per1 on Edn1, levels of ET-1 mRNA were significantly elevated in the renal cortex of Per1 KO mice compared to WT control mice (Figure 3).
Regulation of ET-1 mRNA by Per1 suggested that Per1 might act directly on the ET-1 gene (Edn1). The Edn1 promoter was evaluated for putative E-box elements using TF Search (http://www.cbrc.jp/research/db/TFSEARCH.html). Of the predicted response elements, we focused on the E-boxes in the Edn1 promoter shown in Figure S4A, in part because of the proximity of these elements to the aldosterone response elements previously identified in the Edn1 promoter19. DNA affinity purification assays (DAPA) using mpkCCDc14 nuclear extracts were performed to investigate Per1 interaction with these putative E-boxes (Figure S4B). Per1 was detected only at E-box 2 located at position −680. Demonstrating the specificity of this interaction, Per1 bound poorly to a mutated E-box 2 probe (Figure S4C). Because Per1 does not contain an inherent DNA-binding domain, its interaction with E-box 2 is likely facilitated through additional clock proteins, as we have shown previously7.
To further confirm that ET-1 is a target of Per1, mpkCCDc14 cells were treated with the casein kinase I (CKI) δ/ε inhibitor PF670462. Phosphorylation by CKI δ/ε is required for Per1 entry into the nucleus25. PF670462 thereby blocks Per1 entry into the nucleus, resulting in phase delays in animal models26,27. Inhibition of CKI δ/ε in mpkCCDc14 cells resulted in a 4x increase in ET-1 mRNA levels (Figure S5).
Per1 KO mice exhibit a BP phenotype
The apparent coordinate regulation of sodium transport genes by Per1 led to the hypothesis that Per1 contributes to the positive regulation of sodium reabsorption and BP. Therefore, BP was evaluated in Per1 KO and WT 129/sv mice using an arterial radio-telemetry probe. Similar to previous findings28, this strain of WT mice displayed a mean arterial pressure (MAP) of 133 mm Hg under control conditions. However, Per1 KO mice exhibited a significantly lower BP compared to WT mice (115 mm Hg, p<0.05), and this was associated with decreases in both diastolic and systolic pressures (Table 1). No differences between WT and Per1 KO mice were observed in heart rate, pulse or activity (Table S1). Both WT and Per1 KO underwent a significant daytime reduction in BP (Figure 4), with Per1 KO MAP significantly lower during day and night.
Figure 4. Loss of the circadian clock protein Per1 results in decreased BP.
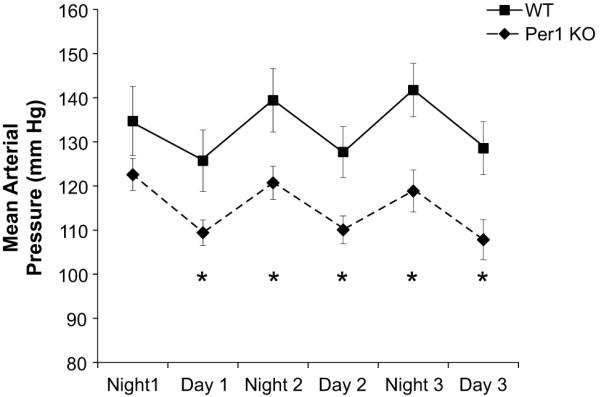
Radio-telemetry recordings were made in Per1 KO and WT mice. MAPs were averaged over each day or night period. *p<0.05 for Per1 KO compared to WT at each time point, n=6. Data were also analyzed by 2-way ANOVA (SigmaPlot). Genotype and time effects were significant but there was not a statistically significant interaction between genotype and time.
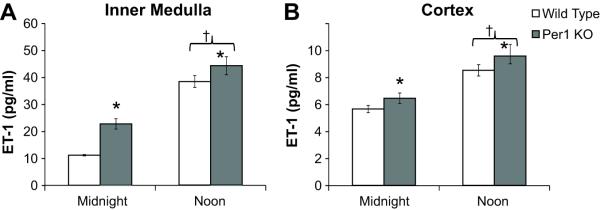
Per1 KO mice have increased renal ET-1
Because CD-localized ET-1 is known to regulate BP through an ENaC-dependent mechanism29,30, we examined renal ET-1 levels in Per1 KO versus WT mice. ET-1 levels were higher in the inner medulla and cortex of Per1 KO versus WT mice (Figure 5A and B). Interestingly, and consistent with the known role of renal ET-1 in the BP regulation, ET-1 levels were inversely correlated with day versus night BP values in both Per1 KO and WT mice (Figure S6).
Figure 5. Renal ET-1 peptide levels are elevated in Per1 KO mice.
ELISA was used to measure ET-1 peptide levels in the inner medulla (Panel A, n=4) and cortex (Panel B, n=6) of Per1 KO and WT mice at midnight or noon. Data were analyzed using 2-way ANOVA (SigmaPlot), with significant genotype (*P<0.05) and time (†P<0.05) effects. There was not a statistically significant interaction between genotype and time.
Discussion
The results of the present study demonstrate the novel finding that Per1 KO mice exhibit lower BP relative to WT mice, with significantly lower systolic and diastolic pressures. We previously reported that loss of Per1 reduced expression of αENaC and resulted in increased urinary sodium6. A candidate gene approach was used to identify additional Per1 target genes involved in the regulation of sodium transport. We show that mRNA levels of the Na, K-ATPase effector Fyxd5 were reduced in Per1 knockdown cells. Furthermore, several negative effectors of ENaC activity, Cav-1, Ube2e3 and ET-1, were induced in response to Per1 knockdown. Importantly, we show for the first time that renal ET-1 peptide levels were elevated in Per1 KO mice. These results are consistent with a role for the circadian clock in regulation of sodium homeostasis and BP.
Per1 KO animals exhibited an 18 mm Hg decrease in 24hr MAP relative to WT mice. These animals are on a 129/sv background, in which WT mice exhibit higher baseline BP than C57/BL6 WT mice28. Interestingly, Per1 KO mice maintained under normal light:dark conditions exhibited circadian variation in BP which suggests that, under these conditions, Per1 may contribute to the basal regulation of BP rather than temporal control. Reports of BP phenotypes in rodents with circadian clock disruption suggest that the clock is critical for cardiovascular function (reviewed in31). Whereas Clock KO mice maintained a normal 24hr rhythm of BP, the average MAP and mean systolic BP were significantly lower in Clock KO mice compared to WT10. Likewise, Bmal1 KO mice also exhibited lower BP but lacked circadian BP rhythmicity32. Elevated aldosterone levels and salt-sensitive hypertension were observed in Cry1/Cry2 KO mice33. Increased activity of Hsd3b6, an enzyme in the aldosterone synthesis pathway, was linked to this phenotype. When maintained on a standard 12hr light:dark cycle, Per2 mutant mice exhibited decreased 24hr diastolic BP, increased heart rate, and a decreased difference between day and night BP34. Under constant darkness, WT mice maintained normal 24hr rhythms in BP, activity and heart rate but Per2 mutant mice experienced a shortened circadian period. Per1 KO mice do not display arrhythmic behavior patterns unless they are placed in total darkness11. Whether or not total darkness disrupts the circadian pattern of BP in Per1 KO animals is unknown.
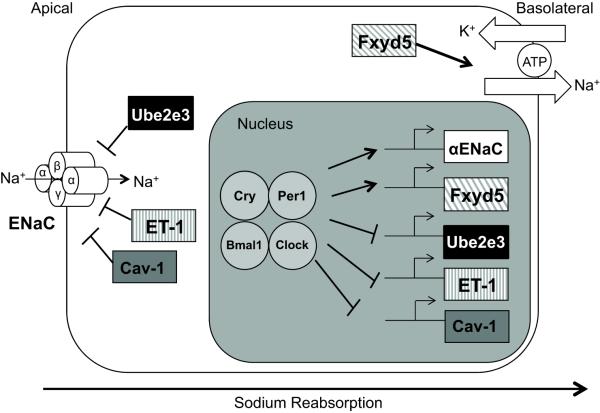
The present finding that Per1 KO mice display a lower BP than WT mice is consistent with our proposed role for Per1 in the stimulation of sodium reabsorption in the kidney (Figure 6). Moreover, the results demonstrate that Per1 acts as a coordinate regulator of genes encoding products that function in the regulation of sodium reabsorption. We have previously demonstrated that αENaC is positively regulated by Per16,7. The findings that Cav-1, ET-1, Fxyd5 and Ube2e3 appear to be Per1 targets suggest a model in which Per1 and other clock proteins coordinately regulate the expression of sodium transport genes (Figure 6). Importantly, these novel Per1 targets regulate sodium reabsorption at many levels, including ENaC open probability (ET-1), degradation and membrane recycling of ENaC (Ube2e3 and Cav-1), and positive regulation of Na, K ATPase activity (Fxyd5). Like Edn1, the promoters for Fxyd5, Ube2e3 and Cav-1 contain putative E-boxes (Figure S7). A direct link between the circadian clock and ET-1 in the kidney is a particularly intriguing result given that, in contrast to its role as a vasoconstrictor in the vasculature, ET-1 acts as a natriuretic and diuretic hormone in the kidney (reviewed in35,36). Kohan and colleagues demonstrated that renal CD-specific ET-1 KO mice exhibit salt-sensitive hypertension29. ET-1 represses renal sodium reabsorption, at least in part, via its inhibition of ENaC22,23 and this effect involves the ET-B receptor30. Our observation that inner medullary ET-1 is doubled in Per1 KO mice at midnight is especially intriguing given that the highlest levels of ET-1 in the body are found in the inner medulla35. Increased renal ET-1 in Per1 KO mice is one possible explanation for the lower BP observed in these animals.
Figure 6. A model for Per1 action on genes involved in sodium transport.
Gene expression studies in immortalized murine renal CD cells following Per1 knockdown indicate that Per1 positively regulates αENaC and Fxyd5 mRNA expression while it negatively regulates Ube2e3, ET-1 and Cav-1 mRNA expression. This coordinate regulation of sodium transport genes by Per1 suggests a role for Per1 in transepithelial sodium reabsorption.
Although the Per1 signaling mechanism identified here using a CCD model is consistent with the significantly lower BP observed in Per1 KO mice, extra-renal effects of Per1 cannot be ruled out because of global Per1 deletion in these animals. For example, the circadian clock regulates vascular function as well. Rudic and colleagues have demonstrated that Bmal1 KO mice and Clock mutant mice display endothelial dysfunction and vascular injury37. It is not yet clear if the aberrant vascular function observed in these mice contributes to the lower BP observed in both Bmal132 and Clock KO10 mice. Tissue-specific deletion of these clock genes may be needed to gain a more complete understanding of the mechanism through which the circadian clock contributes to regulation of cardiovascular function.
Perspectives
The present study demonstrates that Per1 KO mice display a significantly lower BP than WT mice and that Per1 represses the expression of ET-1, a known inhibitor of renal sodium reabsorption. The coordinate regulation of several key sodium transport genes by Per1 provides further support for a central role of the circadian clock in the regulation of renal function. That Per1 KO mice exhibit significantly lower BP suggests that loss of Per1 may be protective against hypertension. Future studies aimed at identifying how Per1 and the circadian clock regulate BP should shed significant light on the BP disorders so often observed in humans.
Supplementary Material
Novelty and Significance.
- What is New?
- Per1 contributes to the regulation of several genes encoding proteins that regulate renal sodium retention.
- Mice lacking functional Per1 have a significantly lower BP compared to control mice.
- What is Relevant?
- Because sodium handling by the kidney is an important determinant of BP, our observation that Per1 regulates genes involved in sodium transport demonstrates that Per1 may be an important player in the control of BP.
- Mice lacking Per1 have much lower BP than control mice, indicating that loss of Per1 may be protective against hypertension.
Summary: Per1 is an important regulator of gene expression in the kidney and likely plays a critical role in the regulation of BP.
Acknowledgments
Sources of Funding. This work was supported by NIH-DK085193 to MLG, American Heart Association postdoctoral fellowship to MLG, Department of Veteran Affairs Research Investment Fund to MLG, and NIH-DK082680 to BDC and CSW.
Footnotes
Disclosures. None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hermida RC, Ayala DE, Portaluppi F. Circadian variation of blood pressure: The basis for the chronotherapy of hypertension. Adv Drug Deliv Rev. 2007;59:904–922. doi: 10.1016/j.addr.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Biggi N, Consonni D, Galluzzo V, Sogliani M, Costa G. Metabolic syndrome in permanent night workers. Chronobiol Int. 2008;25:443–454. doi: 10.1080/07420520802114193. [DOI] [PubMed] [Google Scholar]
- 3.Su TC, Lin LY, Baker D, Schnall PL, Chen MF, Hwang WC, Chen CF, Wang JD. Elevated blood pressure, decreased heart rate variability and incomplete blood pressure recovery after a 12-hour night shift work. J Occup Health. 2008;50:380–386. doi: 10.1539/joh.l7056. [DOI] [PubMed] [Google Scholar]
- 4.Albrecht U. The mammalian circadian clock: A network of gene expression. Front Biosci. 2004;9:48–55. doi: 10.2741/1196. [DOI] [PubMed] [Google Scholar]
- 5.Bose S, Boockfor FR. Episodes of prolactin gene expression in gh3 cells are dependent on selective promoter binding of multiple circadian elements. Endocrinology. 2010;151:2287–2296. doi: 10.1210/en.2009-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest. 2009;119:2423–2434. doi: 10.1172/JCI36908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gumz ML, Cheng KY, Lynch IJ, Stow LR, Greenlee MM, Cain BD, Wingo CS. Regulation of alphaenac expression by the circadian clock protein period 1 in mpkccd(c14) cells. Biochim Biophys Acta. 2010;1799:622–629. doi: 10.1016/j.bbagrm.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol. 2003;285:F664–673. doi: 10.1152/ajprenal.00353.2002. [DOI] [PubMed] [Google Scholar]
- 9.Albrecht U, Eichele G. The mammalian circadian clock. Curr Opin Genet Dev. 2003;13:271–277. doi: 10.1016/s0959-437x(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 10.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A. 2009;106:16523–16528. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mper1, mper2, and mper3 in the scn circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 12.Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: A physiological genomics tool. Physiol Genomics. 2001;5:89–97. doi: 10.1152/physiolgenomics.2001.5.2.89. [DOI] [PubMed] [Google Scholar]
- 13.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol. 1999;10:923–934. doi: 10.1681/ASN.V105923. [DOI] [PubMed] [Google Scholar]
- 14.Chang CT, Wu MS, Tian YC, Chen KH, Yu CC, Liao CH, Hung CC, Yang CW. Enhancement of epithelial sodium channel expression in renal cortical collecting ducts cells by advanced glycation end products. Nephrol Dial Transplant. 2007;22:722–731. doi: 10.1093/ndt/gfl668. [DOI] [PubMed] [Google Scholar]
- 15.Fakitsas P, Adam G, Daidie D, van Bemmelen MX, Fouladkou F, Patrignani A, Wagner U, Warth R, Camargo SM, Staub O, Verrey F. Early aldosterone-induced gene product regulates the epithelial sodium channel by deubiquitylation. J Am Soc Nephrol. 2007;18:1084–1092. doi: 10.1681/ASN.2006080902. [DOI] [PubMed] [Google Scholar]
- 16.Hallows KR, Wang H, Edinger RS, Butterworth MB, Oyster NM, Li H, Buck J, Levin LR, Johnson JP, Pastor-Soler NM. Regulation of epithelial na+ transport by soluble adenylyl cyclase in kidney collecting duct cells. J Biol Chem. 2009;284:5774–5783. doi: 10.1074/jbc.M805501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert-Nicoud M, Flahaut M, Elalouf JM, Nicod M, Salinas M, Bens M, Doucet A, Wincker P, Artiguenave F, Horisberger JD, Vandewalle A, Rossier BC, Firsov D. Transcriptome of a mouse kidney cortical collecting duct cell line: Effects of aldosterone and vasopressin. Proc Natl Acad Sci U S A. 2001;98:2712–2716. doi: 10.1073/pnas.051603198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stow LR, Gumz ML, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Wingo CS. Aldosterone modulates steroid receptor binding to the endothelin-1 gene (edn1) J Biol Chem. 2009;284:30087–30096. doi: 10.1074/jbc.M109.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubarski I, Pihakaski-Maunsbach K, Karlish SJ, Maunsbach AB, Garty H. Interaction with the na,k-atpase and tissue distribution of fxyd5 (related to ion channel) J Biol Chem. 2005;280:37717–37724. doi: 10.1074/jbc.M506397200. [DOI] [PubMed] [Google Scholar]
- 20.Debonneville C, Staub O. Participation of the ubiquitin-conjugating enzyme ube2e3 in nedd4-2-dependent regulation of the epithelial na+ channel. Mol Cell Biol. 2004;24:2397–2409. doi: 10.1128/MCB.24.6.2397-2409.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee IH, Campbell CR, Song SH, Day ML, Kumar S, Cook DI, Dinudom A. The activity of the epithelial sodium channels is regulated by caveolin-1 via a nedd4-2-dependent mechanism. J Biol Chem. 2009;284:12663–12669. doi: 10.1074/jbc.M809737200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallego MS, Ling BN. Regulation of amiloride-sensitive na+ channels by endothelin-1 in distal nephron cells. Am J Physiol. 1996;271:F451–460. doi: 10.1152/ajprenal.1996.271.2.F451. [DOI] [PubMed] [Google Scholar]
- 23.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol. 2008;295:F1063–1070. doi: 10.1152/ajprenal.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatanaka F, Matsubara C, Myung J, Yoritaka T, Kamimura N, Tsutsumi S, Kanai A, Suzuki Y, Sassone-Corsi P, Aburatani H, Sugano S, Takumi T. Genome-wide profiling of the core clock protein bmal1 targets reveals a strict relationship with metabolism. Mol Cell Biol. 2010;30:5636–5648. doi: 10.1128/MCB.00781-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takano A, Isojima Y, Nagai K. Identification of mper1 phosphorylation sites responsible for the nuclear entry. J Biol Chem. 2004;279:32578–32585. doi: 10.1074/jbc.M403433200. [DOI] [PubMed] [Google Scholar]
- 26.Sprouse J, Reynolds L, Swanson TA, Engwall M. Inhibition of casein kinase i epsilon/delta produces phase shifts in the circadian rhythms of cynomolgus monkeys. Psychopharmacology (Berl) 2009;204:735–742. doi: 10.1007/s00213-009-1503-x. [DOI] [PubMed] [Google Scholar]
- 27.Badura L, Swanson T, Adamowicz W, Adams J, Cianfrogna J, Fisher K, Holland J, Kleiman R, Nelson F, Reynolds L, St Germain K, Schaeffer E, Tate B, Sprouse J. An inhibitor of casein kinase i epsilon induces phase delays in circadian rhythms under free-running and entrained conditions. J Pharmacol Exp Ther. 2007;322:730–738. doi: 10.1124/jpet.107.122846. [DOI] [PubMed] [Google Scholar]
- 28.Hartner A, Cordasic N, Klanke B, Veelken R, Hilgers KF. Strain differences in the development of hypertension and glomerular lesions induced by deoxycorticosterone acetate salt in mice. Nephrol Dial Transplant. 2003;18:1999–2004. doi: 10.1093/ndt/gfg299. [DOI] [PubMed] [Google Scholar]
- 29.Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest. 2004;114:504–511. doi: 10.1172/JCI21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bugaj V, Mironova E, Kohan DE, Stockand JD. Collecting duct-specific endothelin b receptor knockout increases enac activity. Am J Physiol Cell Physiol. 2012;302:C188–194. doi: 10.1152/ajpcell.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stow LR, Gumz ML. The circadian clock in the kidney. J Am Soc Nephrol. 2011;22:598–604. doi: 10.1681/ASN.2010080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, Ogawa O, Todo T, Tsutsui K, van der Horst GT, Okamura H. Salt-sensitive hypertension in circadian clock-deficient cry-null mice involves dysregulated adrenal hsd3b6. Nat Med. 2010;16:67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- 34.Vukolic A, Antic V, Van Vliet BN, Yang Z, Albrecht U, Montani JP. Role of mutation of the circadian clock gene per2 in cardiovascular circadian rhythms. Am J Physiol Regul Integr Comp Physiol. 2010;298:R627–634. doi: 10.1152/ajpregu.00404.2009. [DOI] [PubMed] [Google Scholar]
- 35.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev. 2011;91:1–77. doi: 10.1152/physrev.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stow LR, Jacobs ME, Wingo CS, Cain BD. Endothelin-1 gene regulation. FASEB J. 2011;25:16–28. doi: 10.1096/fj.10-161612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–1517. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.