Abstract
OBJECTIVE
The objective was to determine the safety and efficacy of a fish oil-based intravenous lipid emulsion (IFE) in the treatment of PNALD.
SUMMARY AND BACKGROUND DATA
Parenteral nutrition-associated liver disease (PNALD) can be a lethal complication in children with short bowel syndrome (SBS). IFE based on soybean oil administered with parenteral nutrition (PN) may contribute to its etiology.
METHODS
We performed an open-labeled trial of a fish-oil IFE in 42 infants with SBS who developed cholestasis (serum direct bilirubin > 2 mg/dL) while receiving soybean IFE. Safety and efficacy outcomes were compared with those from a contemporary cohort of 49 infants with SBS and cholestasis whose PN course included soybean IFE only. The primary efficacy end-point was time to reversal of cholestasis (direct bilirubin ≤2 mg/dL).
RESULTS
Three deaths and 1 liver transplantation occurred in the fish oil cohort, compared to 12 deaths and 6 transplants in the controls (P=0.005). Among survivors not transplanted during PN, cholestasis reversed while receiving PN in 19/38 patients in the fish oil cohort vs. 2/36 patients in the controls. Based on Cox models, subjects receiving fish oil-IFE experienced reversal of cholestasis 6 times faster (95% CI=2.0,37.3) than those receiving soybean IFE. The provision of fish oil IFE was not associated with hypertriglyceridemia, coagulopathy, essential fatty acid deficiency. Moreover, hypertriglyceridemic events and abnormal INR levels were more common among controls.
CONCLUSIONS
Fish oil IFE is safe, may be effective in treating PNALD, and may reduce mortality and organ transplantation rates in children with SBS.
BACKGROUND
Parenteral nutrition (PN) is a life-saving therapy for patients with intestinal failure syndromes, including patients with short bowel syndrome due to a variety of congenital or acquired gastrointestinal (GI) illnesses, as well as those with intractable GI motility problems. In 2002, the National Institute of Diabetes and Digestive and Kidney Diseases estimated that 20,000 individuals in the U.S. were sustained by PN for intestinal failure.1 Until the advent of routine use of PN, patients with these conditions commonly died of malnutrition.2–4
Parenteral nutrition contains both macronutrients (dextrose, fat, crystalline amino acids) , and micronutrients (vitamins and trace elements). Fat emulsions are administered to prevent essential fatty acid deficiency (EFAD) and to provide a calorically dense source of non-protein calories. Despite its life saving properties , PN is associated with hepatic dysfunction, including biochemical and histologic alterations.5 These abnormalities, which can range from mild elevations of hepatic transaminases to fibrosis and cirrhosis, are likely to progress unless PN is stopped and the patient is able to be maintained on enteral nutrition. The incidnece of this oftentimes fatal contdition is particularly prevalent in the pediatric population.6 In one center, 25% of children with short bowel syndrome went on to develop irreversible hepatic failure7
The etiology of PN associated liver disease (PNALD) is unclear and is likely multifactorial. Risk factors for PNALD include prolonged PN use, prematurity, frequent surgical procedures, lack of enteral intake, and sepsis. Cholestasis may be reversible once PN is discontinued and full enteral nutrition (EN) is established.8 Even partial EN may also be protective.9 However, treating cholestasis by discontinuing PN is difficult, as it may result in starvation if EN is not absorbed. Other treatment options, include reduction of lipid dose to less than 1 gram/kg/day and cyclic PN infusions, have had mixed results.10–13 Likewise, pharmacologic interventions such as treatment with metronidazole, ursodeoxycholic acid, or choline, have had moderate success.14 In refractory hepatic failure in the setting of intestinal failure, liver transplantation (with or without small bowel) remains the only option. Considering modern graft and patient 5 year survival rates for liver small bowel and small bowel alone transplants are in the range of 43–75% and 57–75% respectively, alternative medical and surgical strategies for short bowel syndrome and PNALD are still needed.15,16
Recent evidence suggests that one major contributing factor predisposes patients to PNALD may be the related to the composition of the intravenous fat emulsions. Currently in the U.S., fat emulsions are derived from either soybean or a combination of soybean and safflower oils (Table1). These oils are rich in omega 6 fatty acids and phytosterols which may contribute to liver injury.17 We have previously demonstrated in a murine model that fish oil derived lipid emulsions, in comparison to conventional soy-based products, were able to prevent hepatic steatosis.18,19 Unlike soy containing emulsions, fish oil-based emulsions contain the omega-3 fatty acids: docosahexaenoic acid (DHA) and eicosapentanoic acid (EPA), an eicosanoid precursor of the 5 series leukotrienes that are less inflammatory.20,21 Moreover these emulsions also contain arachidonic acid, an omega-6 fatty acid that is also not present in soy oil emulsions. In our previously published preliminary data, 2 and 18 infants with PNALD were treated with fish oil-based fat emulsions, and serum bilirubin levels normalized.22,23 In both studies, patients tolerated this therapy well and no adverse reactions attributed to its use were observed.
Table 1.
Comparison and characteristics of parenteral lipid emulsions (100 g fat/L)
| Product Manufacturer | IntralipidR Fresenius Kabi | Liposyn IIR Hospira | OmegavenR Fresenius Kabi |
|---|---|---|---|
| Oil source (g) | |||
| Soy bean | 100 | 50 | 0 |
| Safflower | 0 | 50 | 0 |
| MCT | 0 | 0 | 0 |
| Olive oil | 0 | 0 | 0 |
| Fish | 0 | 0 | 100 |
| Fat composition (%)* | |||
| Linoleic | 50 | 65 | 0.4 |
| α-Linolenic | 9 | 4 | <0.2 |
| EPA | 0 | 0 | 2.1 |
| DHA | 0 | 0 | 2.3 |
| Oleic | 26 | 17.7 | 1.0 |
| Palmitic | 10 | 8.8 | 0.6 |
| Stearic | 3.5 | 3.4 | 0.1 |
| Arachidonic | 0 | 0 | 0.3 |
This table shows the major components of 3 commercial lipid emulsions; Data has been provided by the manufacturer.
values in OmegavenR group represent means.
EPA: eicosapentaenoic acid; DHA: docosahexaenoica
Since the publication of these papers, we have further investigated the safety and efficacy of fish oil-based emulsions in the reversal of cholestasis. As part of an open label treatment protocol, PN-dependent children with cholestasis had their conventional soy-containing emulsions discontinued and replaced by fish oil-based emulsions. Outcomes were compared with contemporary patients from 1999 to 2006.
METHODS
Patients
Between August 2006 and November 2007 under a open label treatment protocol at Children’s Hospital Boston (CHB), 42 infants receiving PN with soybean emulsions who developed cholestasis were treated with fish oil emulsions and prospectively followed. Eligibility requirements included serum direct bilirubin ≥ 2 mg/dL and predicted PN duration of > 30 days due to congenital or acquired gastrointestinal disease. Children with other liver diseases (i.e. cystic fibrosis, inborn metabolic errors, hepatitis C) were excluded.
A comparison cohort of controls of 59 infants with short bowel syndrome (SBS), who were PN-dependent and treated at CHB from 1999–2005, was screened for eligibility. A total of 49 eligible subjects were identified with 2 consecutive direct bilirubin > 2 mg/dL while on PN, that could not be attributed to another cause of hepatic disease.
Study Treatment
In the fish oil cohort, the soybean-based emulsion (Intralipid®, Fresenius Kabi AG, Bad Homburg v.d.h., Germany) was discontinued and treatment with fish oil-based emulsion (OmegavenR, Fresenius Kabi AG, Bad Homburg v.d.h., Germany) was started. Patients received fish oil emulsions at a goal dose of 1 g/kg/day infused in conjunction with PN over 12 to 24 hours. The comparison group received Intralipid® (Fresenius Kabi AB) a soybean-based lipid emulsion. Patients in the comparison group received standard of care with fat doses ranging from 1–4 g/kg/day.
Study Outcomes
Safety outcomes included markers of fatty acid deficiency (defined as triene:tetraene (T/T) ratio > 0.2), dyslipidemia (defined as serum triglyceride levels > 400 mg/dL) and markers of coagulopathy, such as thrombocytopenia and an increase in international normalized ratio (INR) >2. Efficacy outcomes were based on trajectories of serum levels of direct bilirubin, alanine aminotransferase (ALT), and the rate of cholestasis reversal, defined as the time at which there was one of three consecutive serum direct bilirubin measurements ≤2 mg/dL. Additionally, we compared the risk of death or liver transplantation between the two study treatment groups. Laboratory values were prospectively measured at approximately weekly intervals. The soybean oil cohort had all available tests retrospectively recorded and measured approximately in weekly or bi-weekly intervals. With the exception of triglyceride and platelet levels,, safety outcomes were systematically recorded only in the experimental group.
Laboratory values were retrospectively recorded from baseline (week 0), defined as the date fish oil emulsion began for the fish oil cohort or as the date of the second of 2 consecutive direct bilirubin values > 2 mg/dL for the comparison group. If patients did not have results for the corresponding laboratory test at the baseline week, the closest previous value was used (up to 2 weeks before the baseline time). Patients were followed until two weeks after end of PN (so that they would have the opportunity to present with 3 consecutive direct bilirubin ≤2 mg/dL). Deaths or transplants that occurred a few months after cessation of PN were recorded.
Statistical Analysis
Statistical significance of differences of baseline characteristics and of frequency of morbidity outcomes (mortality and transplantation) between the two groups was assessed via t-tests when reporting means, Wilcoxon tests when reporting medians, and chi-square tests (or Fisher’s exact tests) when reporting proportions. Analysis of efficacy of fish oil emulsions was based on comparisons between the fish oil and the control cohorts of trends over time of direct bilirubin and ALT. Median values (equivalent to the exponential of the mean of the natural logarithm transformed tests) and interquartile ranges over two week periods of both tests were plotted from baseline until the time in which three or less children were still under observation. Statistical significance of differences in trends after baseline between the two groups was tested based on modeling the natural logarithm of the appropriate laboratory test through repeated measures models with generalized estimating equations (exchangeable working correlation matrix). In this paper we report comparisons adjusted for baseline values of the logarithm of the corresponding laboratory test. Although not shown, results based on crude analyses were comparable. Analyses comparing levels and trajectories of efficacy laboratory tests included all enrolled subjects. Efficacy was also based on differences between the two study groups in the time to reverse cholestasis estimated through the product limit estimator of Kaplan-Meier (KM) survival curves and compared through log-rank tests. In addition, crude and adjusted hazard ratios were estimated using proportional hazard models. In these last models, we considered adjustments for baseline covariates including duration of PN and baseline direct bilirubin. Although only baseline direct bilirubin was a statistically significant predictor, we reported the adjusted hazard ratio for both covariates. Results adjusted for direct bilirubin only were, however, comparable. For analysis of efficacy based on time to reverse cholestasis, subjects who were still on PN by the end of the study follow-up were censored, as were subjects who died or were transplanted while on PN. All survival analyses were performed including all subjects and excluding subjects who died or underwent transplantation while on PN.
Analysis of safety of fish oil was based on comparisons between the two study groups of the levels and trends over time of safety laboratory tests. To compare mean levels of safety tests, we fitted repeated measure models assuming that levels in the two groups were constant over time (i.e., including only a term for treatment group). Comparisons of trends over time of safety outcomes were performed similarly to comparisons of trends of efficacy outcomes described in the previous paragraph, i.e., by adding an interaction term between observation week and study treatment group in linear regression models adjusted for baseline of the logarithm of the corresponding laboratory tests. These models accounted for correlations within subjects through a generalized estimating equation approach. Additionally, Poisson rates (defined as number of events / number of tests) of triglycerides > 400 mg/dL, INR > 2, and T/T ratio > 0.2 were estimated and, when data was available in both study groups, differences between the two treatment groups were assessed via an overdispersed Poisson generalized linear model.
All p-values were two-sided. Analyses were performed in SAS 9.1 (SAS Institute, USA) and S-plus 8 (Insightful, USA).
As OmegavenR is not yet approved for use in the U.S., FDA approval was obtained. This study was also approved by the CHB institutional review board. Written informed consent was obtained for all patients receiving the intravenous fish oil emulsion.
RESULTS
Children in the fish oil group had younger gestational age than children in the control group (P=0.046) (Table 2). In addition, children in the fish oil group had been exposed to PN for a longer period prior to enrollment (P = 0.0008), were older (P = 0.0002), and presented with more advanced liver disease than children in the control cohort, as based on direct bilirubin (P = 0.0003) and ALT levels (P = 0.07), although difference in ALT levels were only borderline statistically significant. In summary, generally, the fish oil group seemed to have higher risk of liver disease than the control group.
Table 2.
Baseline characteristics of patients in the fish oil and soybean oil cohorts.*
| Variables | Fish Oil (N=42 ) | Soybean Oil (N=49 ) | P Value† |
|---|---|---|---|
| Demographic and Medical History | |||
| Gender (male), n (%) | 28 (67%) | 31 (63%) | 0.73 |
| Age (weeks), median (IQR) | 12 (8, 25) | 7 (5,12) | 0.0002 |
| Birth weight (kg), mean ± SD | 1.6 ± 0.86 | 1.8 ± 0.98 | 0.29 |
| Gestational age (weeks), mean ± SD | 30 ± 5 | 32 ± 5 | 0.046 |
| Duration of PN prior to enrollment (days), median (IQR) | 63 (48, 118) | 40 (21, 74) | 0.008 |
| Clinical Diagnosis | |||
| Gastroschisis, n (%) | 10 (24%) | 9 (18%) | 0.52 |
| Jejunal atresia, n (%) | 6 (14%) | 5 (10%) | 0.55 |
| Necrotizing enterocolitis, n (%) | 19 (45%) | 21 (43%) | 0.82 |
| Malrotation and/or midgut volvulus, n (%) | 5 (12%) | 6 (12%) | 0.96 |
| Omphalocele, n (%) | 1 (2%) | 3 (6%) | 0.62‡ |
| Hirschprung’s disease, n (%) | 2 (5%) | 2 (4%) | 1.0‡ |
| Ileal atresia, n (%) | 1 (2%) | 2 (4%) | 1.0‡ |
| Duodenal atresia, n (%) | 3 (7%) | 2 (4%) | 0.66‡ |
| Microvillus inclusion disease n (%) | 0 (0%) | 1 (2%) | 1.0‡ |
| Bowel obstruction, n (%) | 0 (0%) | 10 (20%) | 0.002‡ |
| Microgastria, n (%) | 1 (2%) | 0 (0%) | 0.46‡ |
| Esophageal atresia, n (%) | 0 (0%) | 1 (2%) | 1.0‡ |
| Other, n (%) | 3 (7%) | 15 (31%) | 0.005 |
| Laboratory | |||
| Direct bilirubin (mg/dL), median (IQR) | 5.5 (3.7, 8.5) | 3.3 (2.9, 4.4) | 0.0003 |
| ALT (mg/dL), median (IQR) | 88 (37, 174) | 44 (26, 117) | 0.07 |
| Platelets, median (IQR) | 160 (84, 249) | 251 (89, 391) | 0.22 |
| INR, median (IQR) | 1.1 (1.0, 1.2) | 1.2 (1.1, 1.3) | 0.55 |
| Triglycerides (mg/dL), median (IQR) | 156 (105, 213) | 123 (80, 176) | 0.046 |
| TT ratio, median (IQR) | 0.022 (0.016, 0.031) | - | - |
IQR= interquartile range; ALT = alanine aminotransferase ; INR = international normalized ratio; TT ratio = triene/tetraene ratio.
Baseline was defined as the date that treatment started for the fish oil cohort and the date of the second of 2 consecutive direct bilirubin > 2 mg/dL for the soybean oil l cohort. If a measurement was not available on that exact date, the first available measure up to one month before that date was used.
P-values for continuous variables were obtained via t-test, when reporting means, and Wilcoxon test, when reporting medians. P-values for differences of proportions were obtained via Pearson’s Chi-Square tests, except when marked with ‡, when Fisher’s exact test was used.
Patients were followed from baseline until the end of follow-up (due to reversal of cholestasis, cessation of PN, death or end of the observation period, whichever came first) for a median time of 7 weeks (IQR = 4, 14 weeks). Three (3) of 42 children in the fish oil cohort died whereas 12 of 49 children in the soybean oil cohort died; most deaths (3 in the fish oil and 7 in the control cohorts) occurred while on PN. All deaths occurred in children who never reversed liver dysfunction. A total of 6 children in the soybean oil cohort underwent liver transplantation, and 1 died. The risk of death or undergoing transplantation at any time (during or after cessation of PN) was 9.5% (4/42) and 34.7% (17/49) for the fish oil and control groups, respectively (P = 0.005). Among children who did not die or undergo transplantation while on PN, the KM median time to PN cessation was 20 weeks (IQR = 9, 29 weeks) in the fish oil cohort and 4 weeks (IQR = 3, 10 weeks) in the soybean oil cohort (P < 0.0001).
Efficacy of Fish Oil
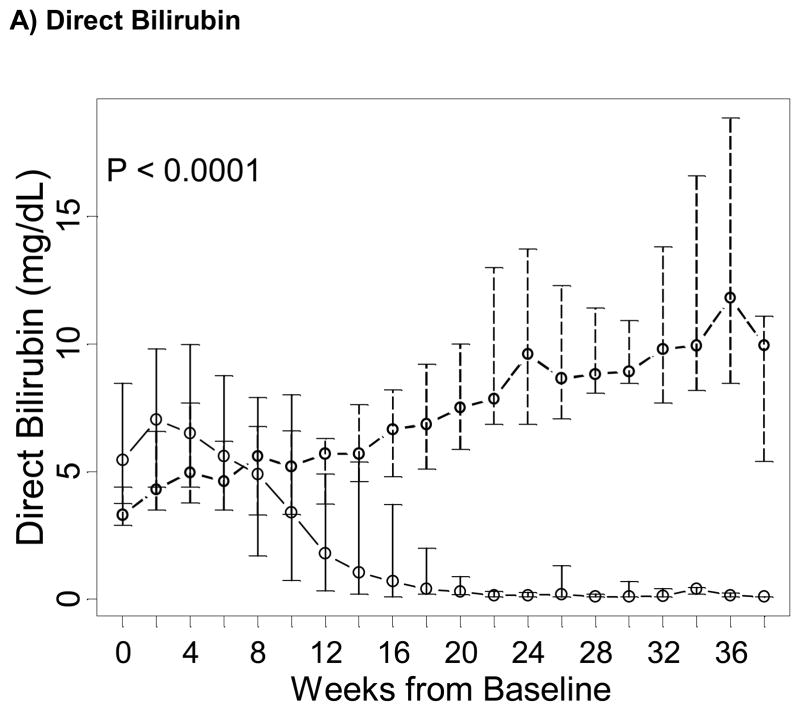
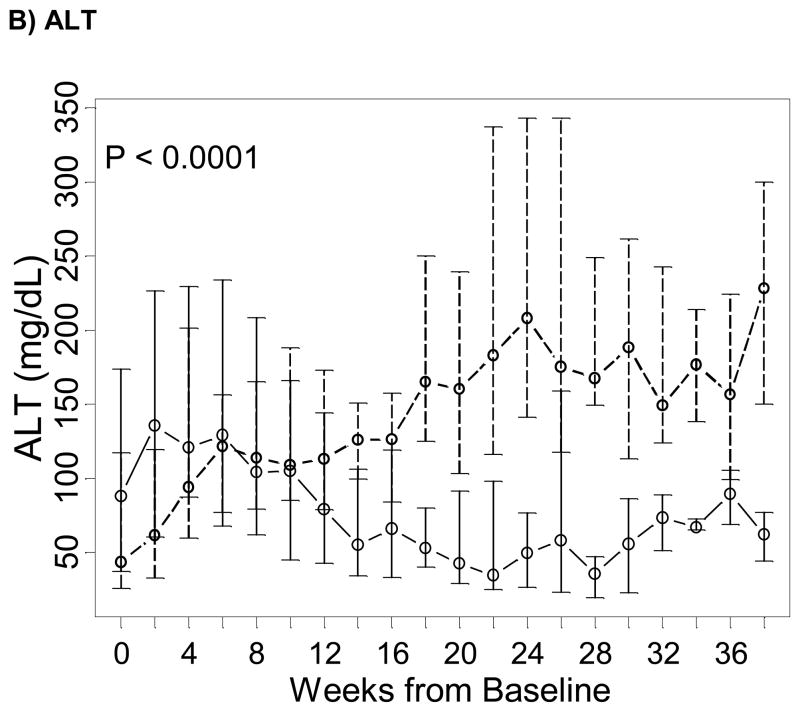
In all 91 subjects, while direct bilirubin and ALT markedly decreased over time in the fish oil cohort, they increased over time in the control cohort (P < 0.0001 for both tests; Figure 1). In 19/42 (45%) children in the fish oil cohort and 2/49 (4.1%) children in the control cohort, cholestasis reversed while on PN. Among children who did not die or were transplanted while on PN, 19/38 (50%) reversed cholestasis in the fish oil cohort and 2/36 (5.6%) reversed in the control cohort. Time to reverse cholestasis for the two patients in the control cohort were 3.1 and 9.7 weeks, and the Kaplan-Meier median time to reverse cholestasis in the fish oil cohort was 11.7 weeks (IQR = 7.7, 13.9 weeks). The difference of the distribution of time to reverse cholestasis between the two study groups was statistically significant (P < 0.001) whether analysis excluded patients who died or underwent transplantation while on PN, excluded patients who died or underwent transplantation at any time, or included all patients. Patients who did not die or underwent transplantation while on PN in the fish oil cohort had 8.6 (hazard ratio = 8.6; 95% CI = 2.0–37.3) times larger rate of reversing cholestasis than in the soybean oil cohort in crude analysis and 17.4 (95% CI = 3.7–83) times larger after adjusting for baseline direct bilirubin levels and duration of PN prior to enrollment.
Figure 1. Trends over time of markers of liver dysfunction in the fish oil (solid line) and in the soybean oil control cohorts (dashed line), from baseline in week 0 (starting admnistration of fish oil emulsion in the fish oil cohort and reaching the second direct bilirubin > 2 mg/dL in the soybean oil control cohort) until the end of follow-up of the majority of subjects.
Points represent medians (equivalent to the geometric mean) and vertical bars represent interquartile ranges. P represent p-values testing differences between the two study groups of linear trends over time in the natural logarithm of the corresponding test and were estimated based on a longitudinal model with a generalized estimating equation approach.
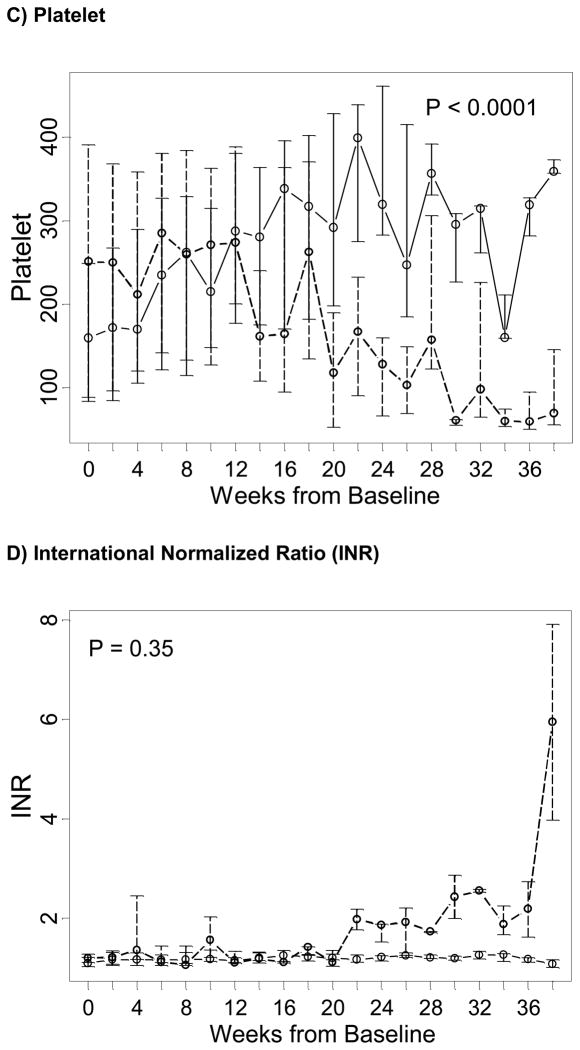
Safety of Fish Oil
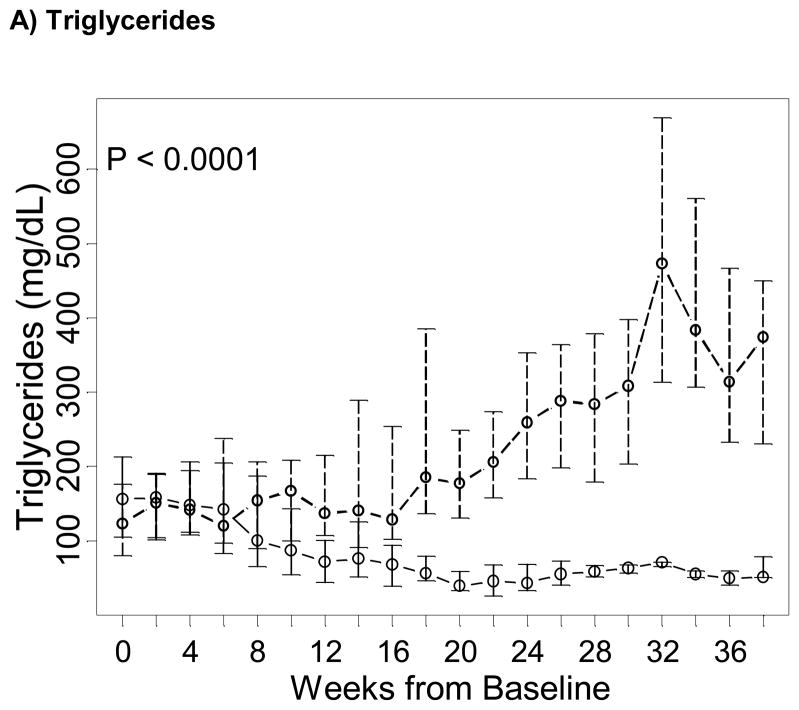
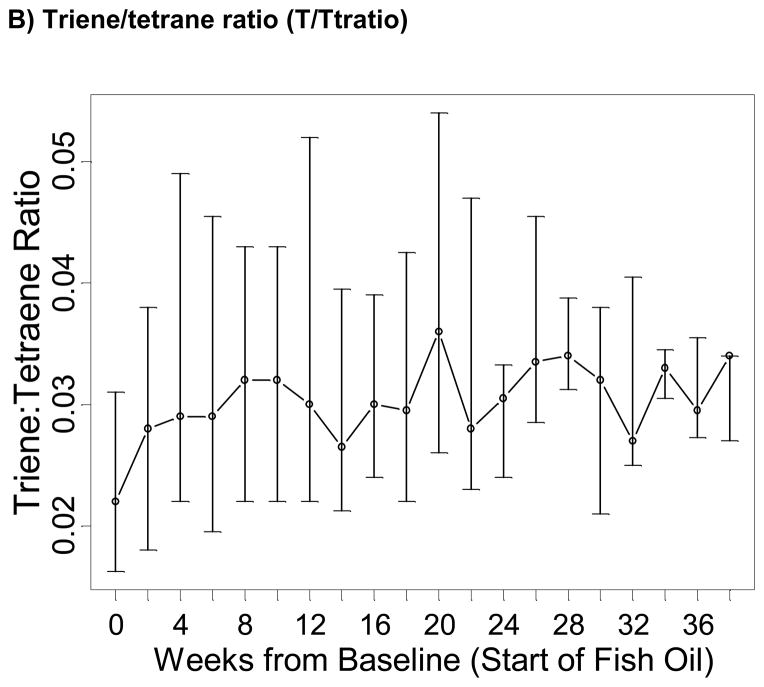
The occurrence of undesirable safety outcomes in children receiving fish oil-based emulsions was lower than in the control group. Based on triglyceride levels above 400mg/dL, the rate of hypertriglyceridemia in children receiving fish oil was lower than in children receiving soybean emulsions – a total of 2 episodes of hypertriglyceridemia occurred in 2 children after baseline in the fish oil group and of 56 events occurred in 12 children in the control group (Table 3). Also, triglyceride levels in the fish oil group were lower (P = 0.0002) and declined faster than in the control group, and differences in level and trends were statistically significant (P < 0.0001; Figure 2A). Based on T/T ratio, two subjects receiving fish oil displayed biochemical evidence of essential fatty acid deficiency (T/T ratio > 0.2) in three occasions. A review of those events showed the patients had had their doses held or interrupted due to access or fluid issues, further demonstrating the need to ensure that a minimum dose of 1g/kg/day is administered (data not shown). No comparisons in the incidence of EFAD between the fish oil and soybean oil cohorts could be performed, since T/T ratio was not measured in the soybean oil cohort.
Table 3.
Safety of fish oil from baseline until the end of follow-up.*
| Safety Marker | Fish Oil (N = 42) | Soybean Oil Controls (N = 49) | P |
|---|---|---|---|
| Triglycerides (mg/dL), mean ± SD§ | 105 | 166 | 0.0002 |
| Hypertriglyceridemia(>400), rate (weeks of observation)‡ | 0.3 | 8.8 | 0.0003 |
| INR, mean§ | 1.2 | 1.6 | 0.004 |
| INR > 2, rate (weeks of observation) ‡ | 1.5 | 3.6 | 0.08 |
| Platelets, mean§ | 192 | 174 | 0.44 |
| T/T ratio, mean§ | 0.032 | - | - |
| T/T ratio > 0.2, rate (weeks of observation)‡ | 0.5 | - | - |
INR = international normalized ratio; T/T ratio = triene/tetraene ratio.
Means and p-values for means were based on observations from baseline until observations performed at study week 38, since only 1 fish oil children was still being followed after this time.
Means represent geometric means, i.e., exponential of the mean of the natural logarithm of the corresponding laboratory test. P-values comparing means were obtained using a generalized estimating equation model in which the outcome was the natural logarithm of the corresponding laboratory test.
Rates represent overal poisson rates, i.e., total number of events/ total number of weeks under observation of each subject.
Figure 2. Trends over time of markers of essential fatty acid deficiency and coagulopathy in the fish oil (solid line) and in the soybean oil control cohorts (dashed line), from baseline in week 0 (starting admnistration of fish oil emulsion in the fish oil cohort and reaching the second direct bilirubin > 2 mg/dL in the control cohort) until the end of follow-up of the majority of subjects.
Points represent medians (equivalent to the geometric mean) and vertical bars represent interquartile ranges. P represent p-values testing differences between the two study groups of linear trends over time in the natural logarithm of the corresponding test and were estimated based on a longitudinal model with a generalized estimating equation approach.
With regards to coagulopathy markers, platelet counts also increased with time in the fish oil group in contrast with the control group, suggesting a possible resolution of the liver disease (P < 0.0001; Figure 2C). The mean INR trajectory was constant in the fish oil cohort, fluctuating between 1.10 and 1.25 at all times, while it seemed to increase in the soybean oil cohort fluctuating between 1.06 and 5.90 at all times. However, differences in trends over time between the two study groups were not statistically significant, possibly due to fact that INR levels between the two study treatment groups started to dissociate after week 20 (P = 0.35; Figure 2D).
DISCUSSION
Infants, particularly premature infants, may survive without a lifelong dependence on PN with as little as 11 cm of initial bowel length, due in part to rapidly growing bowel and adaptation during this time.24 However, in the PN dependent infant, hepatic injury frequently occurs before bowel adaptation and growth is complete and full EN intake has been achieved. Therefore, the goal is to develop an effective nutritional regimen that allows for bowel growth and adaptation before the onset of end stage liver disease. The significance of such a treatment strategy are considerable given the fact that PNALD has a mortality rate as high as 90% in those children unable to be weaned off PN within a year of diagnosis.25 Our results suggest that fish oil-based emulsions may reverse PNALD when used in place of standard soybean emulsions. The experimental group reversed cholestasis, as measured by decrease in direct bilirubin, more frequently and more rapidly than the comparison group. Previous experience suggests that in most instances, reversal of cholestasis could only occur after PN is discontinued and full EN has been established.
The etiology of PNALD may be due to use of soybean-based emulsions, secondary to pro-inflammatory metabolites of omega-6 fatty acids,26 coupled with a decreased hepatic clearance of the parenteral lipid.27 Unlike fish oil emulsions, soybean derived lipids contain phytosterols (eg stigmasterol, b-sitosterol, and campesterol) that are linked with impairment of biliary secretion.28 Past and very recent studies have suggested that phytosterols may be the "hepatoxic" or "cholestatic" component of soybean derived lipid emulsions This multitude of factors results in a cholestatic, steatotic liver that is particularly susceptible to inflammatory insults (e.g. bloodstream infections, surgery, hepatotoxic medications).29 In turn, repeated liver injury results in fibrosis, cirrhosis, and progresses to end stage liver disease. Fish oil-based emulsions address these problems on several fronts. Omega-3 fatty acid metabolites present in fish oil are less involved in the inflammatory response26 and animal models have shown that parenteral fish oil does not impair biliary secretion and may prevent steatosis.19,30,31
Comparisons based on a historical cohort could result in bias. Since previous medical records were less complete, more follow-up data was missing in this group. A delay in time to reverse cholestasis could result from missing bilirubin measurements and result in overestimating the effect of fish oil-based emulsions. To minimize these biases, bilirubin levels were imputed for certain data points. It is unlikely that worse outcomes in the soybean oil cohort were due to poorer management of care, resultant from historical trends. For example, mortality in the soybean oil cohort, uniformly recorded over time, did not increase and was consistent with experiences from other centers. Furthermore, the rate of increase of bilirubin in both groups when all patients were receiving soybean oil fat emulsions was similar. Underestimation of fish oil-induced reversal of cholestasis could result from enrollment under a compassionate protocol, because the patients were more severely ill and were higher risk due to several prognostic factors, including gestational age. The impact of these biases should be reduced when estimating adjusted effect in multiple regressions. Although clinical improvement in the fish oil cohort could theoretically be in part due to lower doses of 1g/kg/day than what is typically used in pediatric PN, several patients in the fish oil cohort developed cholestasis while receiving only 1g/kg/day of soybean oil. Although the fear of developing EFAD from the use of OmegavenR as monotherapy is real when doses are less than 1g/kg/day are used, adding back soybean oil at a dose of 1g/kg/day in combination with OmegavenR 1g/kg/day may actually hinder reversal of cholestasis as demonstrated in a recently published papers.32,33
In fact, in that case series, soybean oil emulsions were stopped when patients failed to improve.32,34 This practice of combining the 2 products may have even contributed to the progression of liver disease as evidenced by the need for 3/12 subjects to undergo a liver-intestine transplant. Although 9/12 subjects did experience complete and sustained resolution of their cholestasis, 5/9 patients had the IntralipidR stopped requiring rescue with OmegavenR given as monotherapy in order to see improvement. Only 4/12 patient’s cholestasis resolved on the combination therapy. Similar to our experience, no patients in that case series died due to PNALD. Moreover, it may be possible that even higher doses of OmegavenR could be used although we have not explored this possibility.33
In conclusion, we have demonstrated that utilization of fish oil-based emulsion in infants dependent on PN as a life-sustaining measure may reverse cholestasis and fatal liver disease. More importantly, we have not observed any deleterious side effects of treatment. These benefits may be due to the absence of soybean oil, the pharmacologic effects of fish oil, the reduction of lipid dose, the ability to increase enteral nutrition, or a combination of all these factors. Ideally, a prospective randomized controlled trial comparing fish oil emulsions to soybean emulsions in the treatment of established PNALD should be conducted, but this type of study would be difficult to conduct as some may consider it unethical to perform a study where children with preexisting PN liver injury could potentially be randomized to a treatment group in which they would continue to receive a soybean-oil based parenteral lipid emulsion. A prospective randomized trial is underway at our institiution to assess the efficacy of fish oil-based emulsions in the prevention of cholestasis in which subjects are randomized to either conventional soybean oil emulsion or fish oil emulsion at a goal dose of 1g/kg/day at the start of their PN course.
Acknowledgments
FUNDING:MP is funded by NIH grant DK069621-04, March of Dimes Foundation 6-FY06-332, FDA Office of Orphan Products Development FDA 1 R01 FD003460-01, FDA 1 R01 FD003436-02, the Children’s Hospital Translational Research Program Grant, and The Children’s Hospital Boston Surgical Foundation. HL was the recipient of the Joshua Ryan Rappaport Fellowship.
Special thanks to Jill Gambardella-Peterson, Danielle Arsenault , Loren Wolfe and the members of Center for Advanced Instestinal Rehabilitation (CAIR), Clinical Nutrition Service and Departments of Pharmacy and Nursing, all at Children’s Hospital Boston. These findings were also reviewed by the study’s data safety managment board.
References
- 1. [accessed January 20]; http://www.niddk.nih.gov/federal/ddicc/minutes6_25_02.pdf-
- 2.Dorney SF, Ament ME, Berquist WE, et al. Improved survival in very short small bowel of infancy with use of long-term parenteral nutrition. The Journal of pediatrics. 1985;107:521–5. doi: 10.1016/s0022-3476(85)80008-1. [DOI] [PubMed] [Google Scholar]
- 3.Dudrick S, Wilmore D, Vars H, et al. Long-term total parenteral nutrition with growth, development, and positive nitrogen balance. Surgery. 1968;64:134–42. [PubMed] [Google Scholar]
- 4.Norman K, Pichard C, Lochs H, et al. Prognostic impact of disease-related malnutrition. Clinical nutrition (Edinburgh, Scotland) 2008;27:5–15. doi: 10.1016/j.clnu.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Freund HR. Abnormalities of liver function and hepatic damage associated with total parenteral nutrition. Nutrition (Burbank, Los Angeles County, Calif. 1991;7:1–5. discussion -6. [PubMed] [Google Scholar]
- 6.Kubota A, Yonekura T, Hoki M, et al. Total parenteral nutrition-associated intrahepatic cholestasis in infants: 25 years' experience. Journal of pediatric surgery. 2000;35:1049–51. doi: 10.1053/jpsu.2000.7769. [DOI] [PubMed] [Google Scholar]
- 7.Diamond I, de Silva N, Pencharz P, et al. Neonatal short bowel syndrome outcomes after the establishment of the first Canadian multidisciplinary intestinal rehabilitation program: preliminaryexperience. J Pediatr Surg. 2007;42:806–11. doi: 10.1016/j.jpedsurg.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 8.Javid PJ, Collier S, Richardson D, et al. The role of enteral nutrition in the reversal of parenteral nutrition-associated liver dysfunction in infants. Journal of pediatric surgery. 2005;40:1015–8. doi: 10.1016/j.jpedsurg.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Zamir O, Nussbaum MS, Bhadra S, et al. Effect of enteral feeding on hepatic steatosis induced by total parenteral nutrition. Jpen. 1994;18:20–5. doi: 10.1177/014860719401800120. [DOI] [PubMed] [Google Scholar]
- 10.Colomb V, Jobert-Giraud A, Lacaille F, et al. Role of lipid emulsions in cholestasis associated with long-term parenteral nutrition in children. JPEN J Parenter Enteral Nutr. 2000;24:345–50. doi: 10.1177/0148607100024006345. [DOI] [PubMed] [Google Scholar]
- 11.Collier S, Crough J, Hendricks K, et al. Use of cyclic parenteral nutrition in infants less than 6 months of age. Nutr Clin Pract. 1994;9:65–8. doi: 10.1177/011542659400900265. [DOI] [PubMed] [Google Scholar]
- 12.Kumpf V. Parenteral nutrition-associated liver disease in adult and pediatric patients. Nutr Clin Pract. 2006;21:279–90. doi: 10.1177/0115426506021003279. [DOI] [PubMed] [Google Scholar]
- 13.Jensen A, Goldin A, Koopmeiners J, et al. The association of cyclic parenteral nutrition and decreased incidence of cholestatic liver disease in patients with gastroschisis. J Pediatr Surg. 2009;44:183–9. doi: 10.1016/j.jpedsurg.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 14.Buchman AL. Complications of long-term home total parenteral nutrition: their identification, prevention and treatment. Dig Dis Sci. 2001;46:1–18. doi: 10.1023/a:1005628121546. [DOI] [PubMed] [Google Scholar]
- 15.Jugie M, Canioni D, Le Bihan C, et al. Study of the impact of liver transplantation on the outcome of intestinal grafts in children. Transplantation. 2006;81:992–7. doi: 10.1097/01.tp.0000195899.32734.83. [DOI] [PubMed] [Google Scholar]
- 16.Vianna R, Mangus R, Tector A. Current status of small bowel and multivisceral transplantation. Adv Surg. 2008;42:129–50. doi: 10.1016/j.yasu.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Clayton P, Whitfield P, Iyer K. The role of phytosterols in the pathogenesis of liver complications of pediatric parenteral nutrition. Nutrition. 1998;14:158–64. doi: 10.1016/s0899-9007(97)00233-5. [DOI] [PubMed] [Google Scholar]
- 18.Javid PJ, Kim HB, Duggan CP, et al. Serial transverse enteroplasty is associated with successful short-term outcomes in infants with short bowel syndrome. Journal Of Pediatric Surgery. 2005;40:1019. doi: 10.1016/j.jpedsurg.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Alwayn IP, Gura K, Nose V, et al. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatric Research. 2005;57:445–52. doi: 10.1203/01.PDR.0000153672.43030.75. [DOI] [PubMed] [Google Scholar]
- 20.Prescott S. The effect of eicosapentaenoic acid on leukotriene B production by human neutrophils. J Biol Chem. 1984;259:7615. [PubMed] [Google Scholar]
- 21.Camandola S, Leonarduzzi G, Musso T, et al. Nuclear factor kB is activated by arachidonic acid but not by eicosapentaenoic acid. Biochem Biophys Res Commun. 1996;229:643–7. doi: 10.1006/bbrc.1996.1857. [DOI] [PubMed] [Google Scholar]
- 22.Gura KM, Duggan CP, Collier SB, et al. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;118:e197–201. doi: 10.1542/peds.2005-2662. [DOI] [PubMed] [Google Scholar]
- 23.Gura KM, Lee S, Valim C, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008;121:e678–86. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- 24.Wilmore DW. Factors correlating with a successful outcome following extensive intestinal resection in newborn infants. The Journal of pediatrics. 1972;80:88–95. doi: 10.1016/s0022-3476(72)80459-1. [DOI] [PubMed] [Google Scholar]
- 25.Wales PW, de Silva N, Kim JH, et al. Neonatal short bowel syndrome: a cohort study. Journal of pediatric surgery. 2005;40:755–62. doi: 10.1016/j.jpedsurg.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 26.Grimminger F, Wahn H, Mayer K, et al. Impact of arachidonic versus eicosapentaenoic acid on exotonin-induced lung vascular leakage: relation to 4-series versus 5-series leukotriene generation. Am J Respir Crit Care Med. 1997;155:513–9. doi: 10.1164/ajrccm.155.2.9032187. [DOI] [PubMed] [Google Scholar]
- 27.Zaman N, Tam YK, Jewell LD, et al. Effects of intravenous lipid as a source of energy in parenteral nutrition associated hepatic dysfunction and lidocaine elimination: a study using isolated rat liver perfusion. Biopharmaceutics & drug disposition. 1997;18:803–19. doi: 10.1002/(sici)1099-081x(199712)18:9<803::aid-bdd65>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Clayton PT, Whitfield P, Iyer K. The role of phytosterols in the pathogenesis of liver complications of pediatric parenteral nutrition. Nutrition (Burbank, Los Angeles County, Calif. 1998;14:158–64. doi: 10.1016/s0899-9007(97)00233-5. [DOI] [PubMed] [Google Scholar]
- 29.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–5. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 30.Araya J, Rodrigo R, Videla LA, et al. Increase in long-chain polyunsaturated fatty acid n - 6/n - 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 2004;106:635–43. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 31.Van Aerde JE, Duerksen DR, Gramlich L, et al. Intravenous fish oil emulsion attenuates total parenteral nutrition-induced cholestasis in newborn piglets. Pediatric research. 1999;45:202–8. doi: 10.1203/00006450-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Diamond I, Sterescu A, Pencharz P, et al. Changing the paradigm: omegaven for the treatment of liver failure in pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48:209–15. doi: 10.1097/MPG.0b013e318182c8f6. [DOI] [PubMed] [Google Scholar]
- 33.Ekema G, Falchetti D, Boroni G, et al. Reversal of severe parenteral nutrition-associated liver disease in an infant with short bowel syndrome using parenteral fish oil (Omega-3 fatty acids) J Pediatr Surg. 2008;43:1191–5. doi: 10.1016/j.jpedsurg.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Diamond I, Sterescu A, Pencharz P, et al. The rationale for the use of parenteral omega-3 lipids in children with short bowel syndrome and liver disease. Pediatr Surg Int. 2008;24:773–8. doi: 10.1007/s00383-008-2174-0. [DOI] [PubMed] [Google Scholar]