SYNOPSIS
Objectives.
In June 2006, the District of Columbia (DC) Department of Health launched a citywide rapid HIV screening campaign. Goals included raising HIV awareness, routinizing rapid HIV screening, identifying previously unrecognized infections, and linking positives to care. We describe findings from this seminal campaign and identify lessons learned.
Methods.
We applied a mixed-methods approach using quantitative analysis of client data forms (CDFs) and qualitative evaluation of focus groups with DC residents. We measured characteristics and factors associated with client demographics, test results, and community perceptions regarding the campaign.
Results.
Data were available on 38,586 participants tested from July 2006 to September 2007. Of those, 68% had previously tested for HIV (44% within the last 12 months) and 23% would not have sought testing had it not been offered. Overall, 662 (1.7%) participants screened positive on the OraQuick® Advance™ rapid HIV test, with non-Hispanic black people, transgenders, and first-time testers being significantly more likely to screen positive for HIV than white people, males, and those tested within the last year, respectively. Of those screening positive for HIV, 47% had documented referrals for HIV care and treatment services. Focus groups reported continued stigma regarding HIV and minimal community saturation of the campaign.
Conclusions.
This widespread campaign tested thousands of people and identified hundreds of HIV-infected individuals; however, referrals to care were lower than anticipated, and awareness of the campaign was limited. Lessons learned through this scale-up of population-based HIV screening resulted in establishing citywide HIV testing processes that laid the foundation for the implementation of test-and-treat activities in DC.
District of Columbia (DC) surveillance data indicate that the city has a severe and generalized human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) epidemic, with an HIV/AIDS prevalence of 3.2% and the highest AIDS case rate in the United States (148.1 cases per 100,000 population in DC compared with 12.5 cases per 100,000 population in the U.S.).1,2
In September 2006, the Centers for Disease Control and Prevention (CDC) issued revised HIV testing recommendations for health-care settings. These guidelines recommended routine screening of people in all health-care settings and screening of those at high risk for infection on an annual basis.3
In an effort to increase testing in advance of the release of the revised CDC recommendations, in June 2006 the DC Department of Health HIV/AIDS Administration (DCDOH/HAA) launched a citywide rapid HIV screening campaign called “Come Together DC—Get Screened for HIV”. During the previous year, 2005, DCDOH funded a limited number of community-based, HIV counseling and testing, and clinical sites to conduct targeted rapid HIV testing using Orasure®, fingersticks, and venipuncture. Approximately 20,000 publicly funded HIV tests were conducted in 2005 (Personal communication, Nestor Rocha, DCDOH, October 2008).
As one of the first cities in the U.S. to attempt to establish processes and systems to implement routine testing citywide, DC launched its campaign with three primary goals for raising awareness regarding DC's HIV epidemic: (1) reinvigorating the city's response and reaching out to the entire population to stop the spread of HIV, (2) routinizing HIV screening in both medical and community health-care settings, and (3) reducing HIV transmission by identifying and linking to care and treatment newly diagnosed HIV-positive people and encouraging people living with HIV to make healthy decisions regarding their behaviors.4
Through a public health-academic partnership between The George Washington University (GWU) Department of Epidemiology and Biostatistics and the DCDOH/HAA, data collected on people tested through the campaign were analyzed. The objectives of the analysis were to describe people being tested through the campaign, identify potential factors associated with screening HIV-positive, and determine the level of community saturation regarding the campaign among DC residents.
METHODS
The DCDOH conducted outreach to public, private, and community-based organizations, including both medical and nonmedical providers, to engage them in the use of rapid HIV testing. Social marketing for the campaign included street outreach, media advertisements, and several public events that aimed to publicize the campaign. OraQuick® ADVANCE™ rapid HIV tests (OraSure Technologies, Bethlehem, Pennsylvania) were procured through CDC and extensively distributed free of charge to organizations previously conducting HIV screening, as well as those new to conducting HIV screening. The OraQuick ADVANCE rapid HIV test is a Food and Drug Administration-approved, Clinical Laboratory Improvement Amendments-waived rapid HIV screening test that screens for HIV-1 and HIV-2 in oral fluid, blood, and plasma and has a specificity of 99.8%.5–8
All sites receiving test kits were trained by OraSure Technologies, free of charge, on how to properly administer and interpret the test. Participating testing sites were encouraged to implement the CDC recommendations including removing the requirement for pretest counseling and using the opt-out testing approach. For all people screening HIV-positive, referrals for confirmatory testing and referrals for HIV care and treatment and/or prevention were to be made by staff at the testing site. Furthermore, in addition to already existing HIV testing and reporting requirements, participating sites were asked to collect data on each person tested through the campaign through an anonymous standard client data form (CDF).
A mixed-methods approach using quantitative and qualitative data-collection methodologies was employed to assess the extent to which the campaign's goals had been achieved. Data collection for campaign participants involved completion of CDFs by testing staff while participants awaited their rapid HIV test results. The form collected participant demographic information, testing venue, previous testing history, reason for testing, and test result. Data for each test administered were collected from July 2006 through September 2007 and entered into a Microsoft® Access database. Analysis of quantitative data was conducted using univariate, bivariate, and multivariable statistical analyses in SAS® version 8.0.9 We conducted logistic regression to identify factors potentially associated with testing preliminary positive (PP). We controlled for possible confounders including testing site, gender, race/ethnicity, age group, previous HIV testing, time of last HIV test, request for test had it not been offered, and reason for testing. We considered a p-value of ≤0.05 to be statistically significant.
Qualitative methods involved assessing community perceptions of the campaign, including saturation of the social marketing campaign. Data were obtained through three focus groups of DC residents; one session was with females, another was with males, and one was a mixed group of males and females. Each group of participants convened for two sessions. Participants were recruited from venues through flyers, community outreach workers, and general street recruitment and provided informed consent prior to participation. The focus groups were conducted in a sequential fashion and designed to take advantage of participants' networks and engagement in the community, thereby obtaining perspectives of others in their networks and communities. We developed a focus group guide that elicited information about general health knowledge, HIV knowledge, knowledge regarding HIV testing, and, specifically, the testing campaign. After the initial focus group, participants were given instructions asking them to discuss HIV in general and the testing campaign specifically with friends, family members, and associates and to then report back on these conversations during the subsequent focus group. Focus groups were tape-recorded, transcribed, and entered into ATLAS.ti qualitative data analysis software for thematic coding and analysis.10
RESULTS
Client data forms
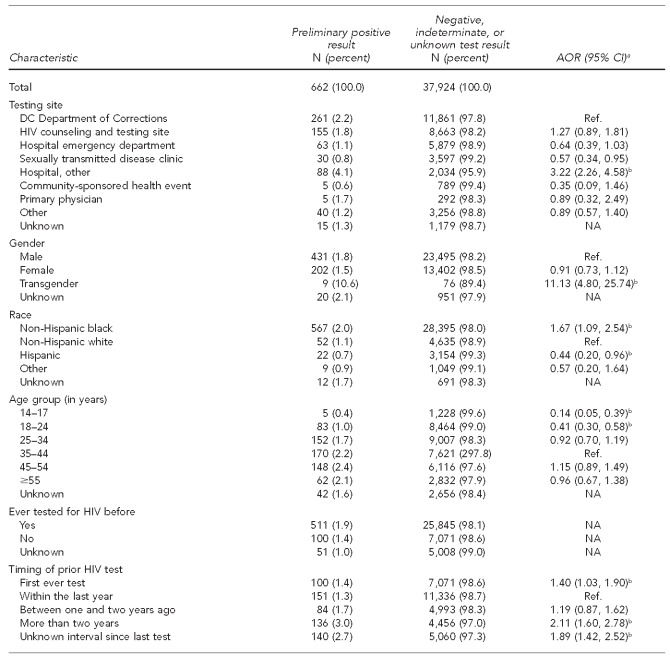
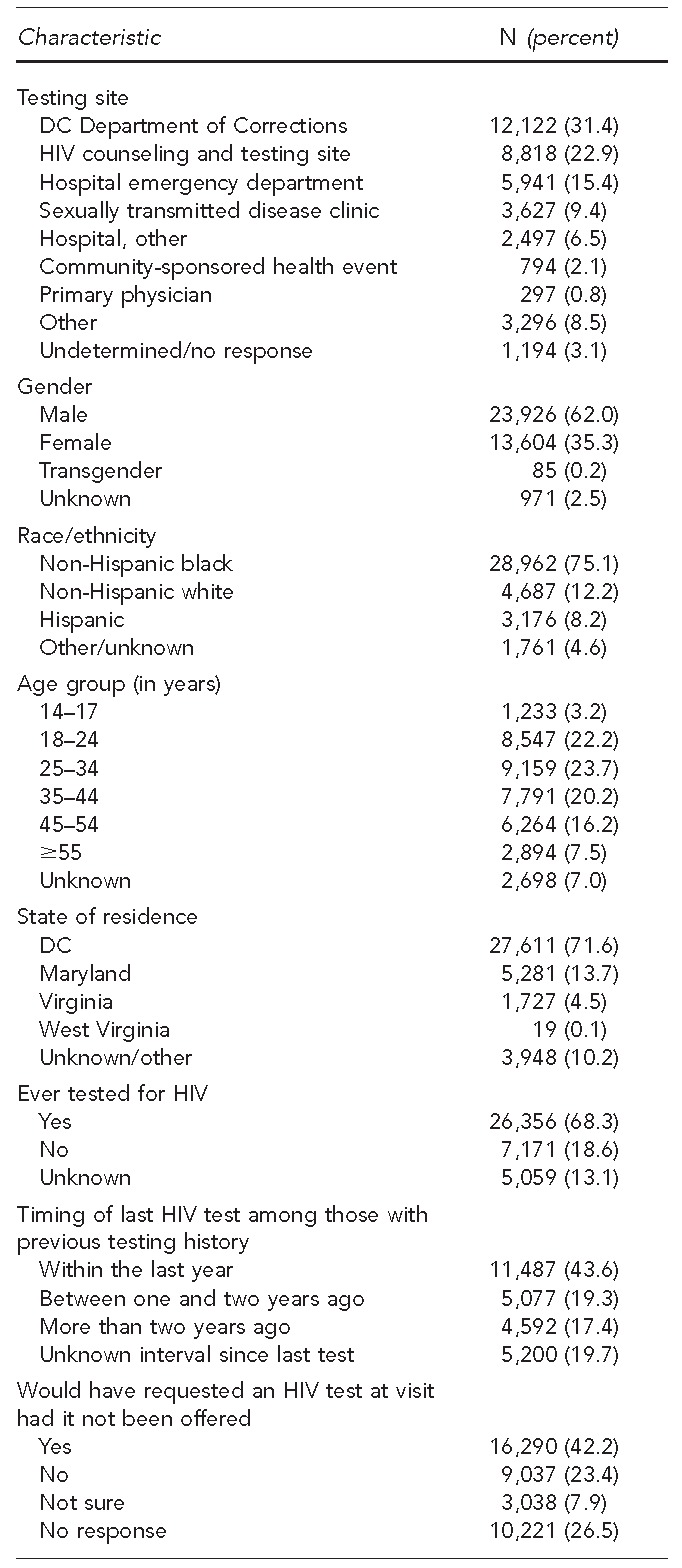
From July 2006 to September 2007, HAA received 38,586 completed CDFs. Although 38,586 CDFs were analyzed, they may have represented people who were tested more than once during this time period. However, as this could not be determined due to the anonymous nature of the data collection, we referred to characteristics documented on each CDF form as being attributed to a “participant.” Based on the receipt of CDFs, the highest proportion of tests (31.4%) was conducted through the DC Department of Corrections (DCDOC) Central Detention Facility (Table 1). Of completed CDFs, 75.1% were non-Hispanic black, 62.0% were male, and the mean age was 34.9 years (standard deviation [SD] = 12.97; median = 35.0 years; range: 14–84) (data not shown).
Table 1.
Characteristics of HIV testing campaign participants: Washington, DC, July 2006–September 2007 (n=38,586)a
aOf those completing a client data form
bParticipants were able to indicate more than one reason. Percentages are calculated out of total number of reasons given (n=39,759).
HIV = human immunodeficiency virus
DC = District of Columbia
When asked about previous HIV testing, 68.3% of participants reported having been previously tested for HIV prior to the test that was conducted through the campaign. Of the 26,356 participants reporting a history of prior HIV testing, 43.6% had been tested within the last 12 months (Table 1). Among those who had tested previously, 1.0% stated that they were already aware of their HIV-positive status (data not shown).
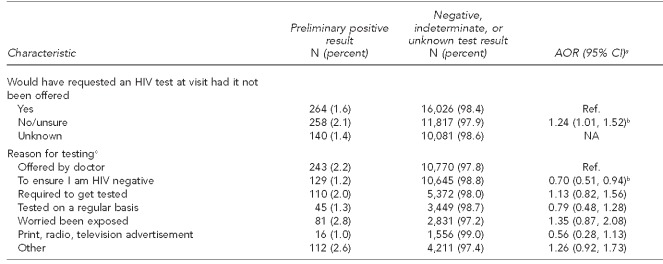
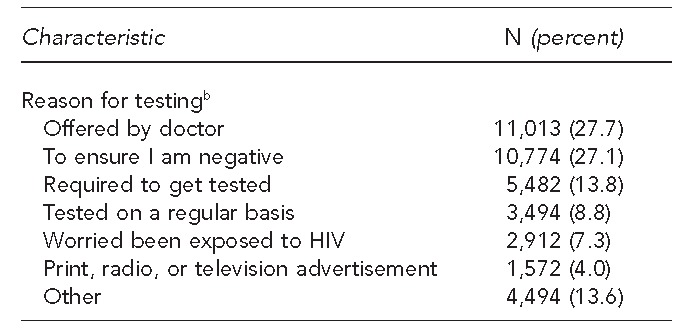
Almost one-third of participants (31.3%) indicated that they would not have requested or were unsure if they would have had an HIV test had it not been offered at the visit. The most commonly reported reasons for obtaining an HIV test were because it was “offered by doctor” (27.7%) and “to ensure I am negative” (27.1%). Almost 14% of participants stated that they were tested because they perceived it was required by insurance, military, court order, or another agency. Of people who indicated this perception as their primary reason for testing, 92.1% were tested as part of the DCDOC policy to provide opt-out screening of inmates upon entry and release from the DCDOC Central Detention Facility (data not shown).
Preliminary positive participants
Of the 38,586 CDFs collected, 98.3% of HIV tests (n=37,924) were negative, indeterminate, or did not indicate a result on the form; 1.7% (n=662) of tests were PP for HIV (Table 2). Based on self-reported information from the CDF, 173 (26.1%) of these participants were previously aware of their HIV infection, resulting in 489 (73.9%) potentially newly identified HIV infections (data not shown). Due to the inability to confirm participants' self-reported HIV status, the results presented include all 662 PP tests. The proportion of those screening positive was highest at the DCDOC (2.2%), all hospital sites (i.e., emergency department and other hospital sites) (5.2%); and HIV counseling, testing, and referral (CTR) sites (1.8%), with hospitals being three times more likely to identify PP participants compared with the DCDOC (Table 2). Collectively, DCDOC, HIV CTR, emergency department, and other hospital sites accounted for 85.6% of all PP participants identified. Eighty-six percent of PP participants were non-Hispanic black, 65.1% were male, 48.6% were aged 25–44 years, and 74.2% were DC residents.
Table 2.
Characteristics of HIV testing campaign participants (n=38,586), by test result and adjusted odds of testing preliminary positive: Washington, DC, July 2006–September 2007
aAdjusted for all other variables in the model. Due to the large number of missing/unknown responses, these were excluded in the model as indicated by NA. Due to strong correlation between the “ever HIV tested” and “timing of prior HIV test” categories, the “ever HIV tested” category was also excluded from the adjusted model.
bp≤0.05
cParticipants were able to indicate more than one reason for getting tested.
HIV = human immunodeficiency virus
DC = District of Columbia
AOR = adjusted odds ratio
CI = confidence interval
Ref. = reference group
NA = not applicable
As shown in Table 2, in the adjusted analysis, transgender participants were more than 11 times more likely than males to test PP (adjusted odds ratio [AOR] = 11.13, 95% confidence interval [CI] 4.80, 25.74), non-Hispanic black people were 67% more likely than white people to test PP (AOR=1.67, 95% CI 1.09, 2.54), and Hispanic people were 56% less likely than non-Hispanic white people to test PP (AOR=0.44, 95% CI 0.20, 0.96). Those aged 14–24 years were significantly less likely to test PP than those who were aged 35–44 years (AOR for those aged 14–17 years = 0.14, 95% CI 0.05, 0.39; AOR for those aged 18–24 years = 0.41, 95% CI 0.30, 0.58).
Among the 662 PP participants, 100 (15.1%) reported that this was their first ever HIV test, while 511 (77.2%) reported having previously been tested for HIV. Among those who knew when they had last been tested, 151 (40.7%) were tested within the past year. Approximately 37% of participants reported their reason for testing was because the test was offered by a doctor or other health-care provider, and 39.0% of those testing PP responded that they either would not have been tested or were unsure that they would have requested a test had it not been offered by the provider. After adjusting for all other variables, those who tested PP were 40% more likely than those tested within the last year to report that this was their first ever HIV test (AOR=1.40, 95% CI 1.03, 1.90) and two times more likely to have been tested more than two years prior (AOR=2.11, 95% CI 1.60, 2.78). PP participants were 24% more likely to have not requested an HIV test during the visit had it not been offered (AOR=1.24, 95% CI 1.01, 1.52) than those who would have requested an HIV test. PP participants were 30% less likely to have been tested to ensure that they were HIV-negative (AOR=0.70, 95% CI 0.51, 0.94) than those who only tested because the test was offered by a doctor (Table 2).
As part of the DCDOH memorandum of understanding, participating organizations were required to refer PP participants to follow-up care for confirmatory testing and to make appropriate care and treatment and/or prevention recommendations. Among the 83% (n=551) of participants testing PP for whom referral information was available, 47.0% (n=259) were documented as having received a referral for care and treatment, 21.1% (n=116) documented a referral for prevention services, and 11.6% (n=64) documented that a referral was made for all three services. For 20.3% (n=112) of PP participants, no referral was made; however, reasons for the lack of referrals were not documented on the CDFs. Referral rates to care, treatment, and/or prevention were highest among hospitals (93.2%) and primary care physicians (100%) and lowest among the sexually transmitted disease (STD) clinic (6.7%), emergency departments (39.7%), community health events (40.0%), and the DCDOC (59.4%) (data not shown).
Qualitative findings
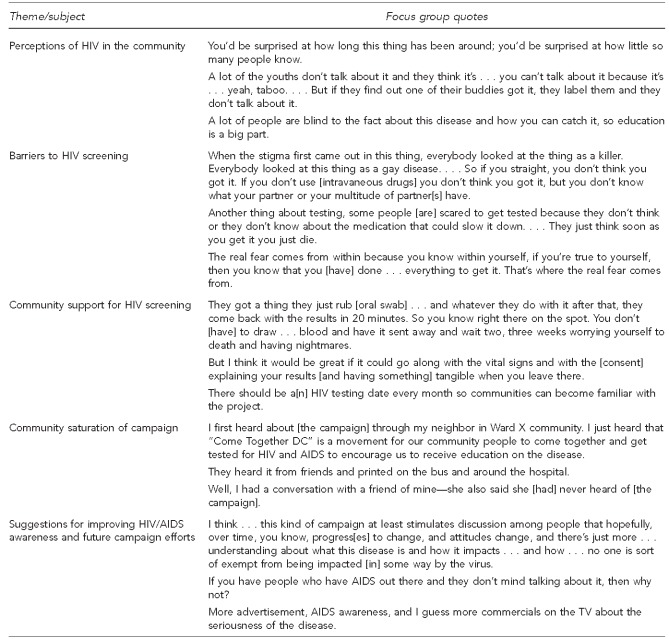
To assess community perceptions and saturation of the campaign, three focus groups were held: a male focus group of 13 participants, a female group of 10 participants, and a mixed-gender group of 14 participants. Among the 37 participants, 36 were non-Hispanic black, 17 had graduated high school or had a general equivalency diploma, two had graduated college, the mean age was 41.8 years (SD=12.4, range: 18–66), and 33 had been tested for HIV. The second focus group meeting for each group was held within two weeks of the initial group meeting, and 94% of participants attended the second meeting. Sample quotes from participants are shown in the Figure.
Figure.
Key focus group findings and sample quotes regarding the Washington, DC, routine HIV testing campaign: July 2006–September 2007
DC = District of Columbia
HIV = human immunodeficiency virus
AIDS = acquired immunodeficiency syndrome
Focus group participants recognized HIV as a significant problem in DC. Thematic analysis demonstrated that although participants were aware of high-risk behaviors that place people at increased risk for HIV, they believed the community was not sufficiently talking about HIV. There was also discussion concerning the continued stigma and lack of education about the disease.
Participants had mixed perspectives regarding the barriers to HIV screening in DC. Fear of testing positive was the one issue that emerged during each focus group discussion as a primary obstacle for HIV screening and was identified as a barrier among all demographic groups. Other barriers mentioned included perceptions about not being at risk for HIV; lack of money, transportation, and education; inadequate or no health insurance; and other cultural as well as structural barriers.
Efforts to encourage and support screening in the community were also explored. Participants identified the ease of rapid testing and quick turnaround for obtaining results as incentives for testing. Participants also felt that HIV testing should be a part of any medical examination, similar to getting one's vital signs checked.
Focus group participants were asked to discuss HIV and the DC testing campaign when they returned to their communities. Participants' responses suggested there was minimal community saturation about the campaign. Among the few that had heard about the campaign, they reported seeing advertisements on buses and billboards, and hearing about it on the radio, local news, and from neighbors and community-based organizations. However, most of the participants and the people they spoke with in the community had not heard about the campaign.
The participants demonstrated awareness of DC's HIV/AIDS epidemic and had suggestions for -improving awareness overall and for future campaign efforts. Suggestions included identifying people to serve as role models (e.g., those living with HIV/AIDS) and increasing advertisements and providing statistics about HIV/AIDS in the community. Participants felt that the current campaign efforts would raise awareness, stimulate discussions about HIV, and help people realize that their communities were directly affected by the illness.
DISCUSSION
The results of this evaluation demonstrate that the “Come Together DC—Get Screened for HIV” campaign resulted in HIV screening and data collection for almost 40,000 people from throughout the DC metropolitan area, and potentially identified hundreds of individuals with previously undiagnosed HIV infection. These data suggest that the campaign's goals—to raise awareness regarding the HIV epidemic, to routinize screening, and to identify previously unrecognized infections—were in part achieved.
Implementation of the CDF allowed essential information to be collected about those getting screened, testing behaviors, motivations for testing, and referral patterns among campaign participants. A screening positivity rate of approximately 2%, with the majority of positives identified among non-Hispanic black males and females, was consistent with rates observed through other screening initiatives.11–14 In addition, the high prevalence observed at the DCDOC Central Detention Facility, at a local emergency department, and at CTR sites underscores the importance of conducting screening in both medical and nonmedical settings.15 Furthermore, implementation of testing in an environment such as the DCDOC, where people's movements in and out of the system are more controlled, allowed for the identification of many infected people.
Results from this analysis reinforce the CDC approach of non-risk-based opt-out screening, as the DC citywide testing campaign did not collect risk information and almost one-third of PP participants would not have sought testing had it not been offered by a health-care provider.3 Although participants reported high rates of annual HIV testing, given the high prevalence rates, non-risk-based opt-out testing, in conjunction with routine targeted risk-based screening, may assist in identifying those people who are hesitant to seek testing, perceive themselves at low risk, or are perceived as low risk by health-care providers and who may otherwise go undiagnosed.16–18
Although the campaign tested thousands of people, documented referrals were lower than expected, with slightly fewer than half of those screening PP having documentation of a referral for care and treatment. This low rate of linkage, particularly among new HIV testing partners, represents a significant missed opportunity to engage infected people in care and secondary prevention. Previous studies have shown that linkages to confirmatory testing and entry into care present a distinct challenge when rapid testing is employed in nontraditional settings.16,19–21
Qualitative data revealed that although HIV was recognized by community members as a serious health threat in DC, there was persistent stigma associated with the disease. Despite several public events to launch and promote the campaign, these data also showed that there was minimal community saturation and awareness of the campaign, despite overall acceptability of routine HIV testing among DC residents. This qualitative approach provides an important addition to the quantitative data in its ability to characterize knowledge, attitudes, and perceptions that are difficult to elicit through close-ended questions. Importantly, these qualitative findings highlighted community buy-in regarding routine screening and the need for individuals to take personal responsibility to advocate for screening.
Through lessons learned from this analysis, the DCDOH has modified its organizational processes and logistics and tailored messages to the patient and provider communities concerning the importance of routine HIV testing. Changes to routine testing approaches include use of a unique identifier to collect data so that analysis of testing rates and characteristics of testers can occur at the individual level; identifying community HIV providers who are willing to conduct the initial HIV care evaluation inclusive of confirmatory testing within 24–72 hours of testing PP, known as the Red Carpet Entry Program; establishing navigator programs to assist in linking people to HIV care and treatment; and requiring documentation of linkage into care, including confirmatory testing among those screening positive, with confirmation of such through laboratory reporting and surveillance data.
In an effort to increase awareness of routine testing, social-marketing initiatives have been designed by the DCDOH to encourage residents to know their status, know their partner's status, and begin the dialogue regarding reducing HIV risk behaviors (e.g., with consistent condom use). Public media campaigns focus on empowering patients to ask for the test at medical visits and encourage providers to conduct routine opt-out testing for all patients. For those people initially refusing an HIV test, providers are encouraged to revisit testing during the clinical examination. In addition, if a person is identified as HIV-infected, identification and testing of sexual partners is also routinely offered.
Limitations
There were several limitations to the approaches used in this analysis. First, because the CDF was anonymous, it is likely that data were collected on participants who were tested more than once through the campaign and would therefore have been counted multiple times in the analysis. Second, although the OraQuick ADVANCE rapid test is a highly sensitive and specific screening tool for HIV detection, its use alone is not diagnostic of infection.5,22,23 Thus, all individuals testing PP on the rapid test should have been referred for confirmatory testing and, if confirmed positive, referred for care, treatment, and prevention. Lastly, although we accounted for it in our quantitative analysis, we had a substantial amount of missing data that may have limited the interpretation and generalizability of our findings. However, our overall findings are consistent with the epidemiologic profile of HIV in DC.
CONCLUSIONS
The DC campaign, “Come Together DC—Get Screened for HIV”, was one of the first in the U.S. to attempt to systematize and implement routine testing at a citywide level, as recommended by CDC in 2006. Our results suggest that centrally supported rapid HIV screening activities at the DCDOH, coupled with acceptability at the facility level and demonstrated client acceptance, can galvanize communities to fight HIV. Since 2006, other cities including the Bronx (New York), Miami (Florida), and Oakland (California) have also implemented citywide HIV testing initiatives.24–26 Serving as the foundation for the scale-up of routine population-based HIV screening in DC, the campaign has resulted in the selection of DC as one of the Testing and Linkage to Care Plus study intervention communities. The study includes implementation of community-level routine testing, as well as enhanced linkage to care and treatment.27 Lessons learned from this campaign may provide useful information as other cities expand their routine rapid testing initiatives, while taking into consideration establishment of community partnerships, adequacy of referral systems, and the balance between targeted and routine screening.
Acknowledgments
The authors acknowledge current and former District of Columbia Department of Health (DCDOH)/HIV/AIDS Administration (HAA) staff Michael Kharfen, Paul Cunningham, Leo Rennie, and Donald Hitchcock for their assistance and expertise throughout the analysis. The authors also thank The George Washington University (GWU) research staff: Sarah Willis, Anthony Rawls, Luz Montanez, and Montina Befus; and former research assistants through the DCDOH/HAA-GWU Partnership: Arit Amana, Jennifer Beal, Kathryn Cape, and Vienna Mbagaya. The authors also acknowledge the staff of local organizations, health-care facilities, and agencies; DCDOH/HAA staff; and the residents of Washington, DC, without whom this analysis would not have been possible.
Footnotes
This analysis was conducted as a part of the Public Health-Academic Partnership between the DCDOH, HIV/AIDS, Hepatitis, STD, TB Administration and the GWU School of Public Health and Health Services, Department of Epidemiology and Biostatistics, Contract #POHC-2006-C-0030.
Portions of these data were presented in preliminary format at the following conferences: American Public Health Association Annual Meeting (Washington, DC, 2007); Centers for Disease Control and Prevention HIV Prevention Conference (Atlanta, Georgia, 2007); and the Conference on Retroviruses and Opportunistic Infections (Boston, Massachusetts, 2008).
All protocols and materials were approved by the GWU and DCDOH Institutional Review Boards prior to study implementation.
REFERENCES
- 1.Centers for Disease Control and Prevention (US) HIV/AIDS surveillance report, 2006. Vol. 18. Atlanta: CDC; 2008. [Google Scholar]
- 2.District of Columbia Department of Health. District of Columbia HIV/AIDS, Hepatitis, STD, and TB Epidemiology annual report: 2009 update. 2010. [cited 2010 Jul 7]. Available from: URL: http://dchealth.dc.gov/doh/frames.asp?doc=/doh/lib/doh/services/administration_offices/hiv_aids/pdf/annual_report_hahsta_march_2010.pdf.
- 3.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 4.District of Columbia Department of Health Administration for HIV Policy and Programs. Washington: District of Columbia Department of Health Administration for HIV Policy and Programs; 2007. Jul, OraQuick Advance rapid HIV test distribution program: district-wide HIV testing protocol. [Google Scholar]
- 5.Centers for Disease Control and Prevention (US) Atlanta: CDC; 2007. [cited 2011 Feb 4]. Quality assurance guidelines for testing using rapid HIV antibody tests waived under the Clinical Laboratory Improvement Amendments of 1988. Also available from: URL: http://www.cdc.gov/hiv/topics/testing/resources/guidelines/pdf/qa_guidlines.pdf. [Google Scholar]
- 6.Delaney KP, Branson BM, Uniyal A, Kerndt PR, Keenan PA, Jafa K, et al. Performance of an oral fluid rapid HIV-1/2 test: experience from four CDC studies. AIDS. 2006;20:1655–60. doi: 10.1097/01.aids.0000238412.75324.82. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds SJ, Muwonga J. OraQuick ADVANCE Rapid HIV-1/2 antibody test. Expert Rev Mol Diagn. 2004;4:587–91. doi: 10.1586/14737159.4.5.587. [DOI] [PubMed] [Google Scholar]
- 8.Wesolowski LG, MacKellar DA, Facente SN, Dowling T, Ethridge SF, Zhu JH, et al. Post-marketing surveillance of OraQuick whole blood and oral fluid rapid HIV testing. AIDS. 2006;20:1661–6. doi: 10.1097/01.aids.0000238413.13442.ed. [DOI] [PubMed] [Google Scholar]
- 9.SAS Institute, Inc. SASr: Version 8.0. Cary (NC): SAS Institute, Inc; 2000. [Google Scholar]
- 10.Scientific Software Development. ATLAS.ti: Version 4.2. Berlin: Scientific Software Development; 2000. [Google Scholar]
- 11.Bowles KE, Clark HA, Tai E, Sulllivan PS, Song B, Tsang J, et al. Implementing rapid HIV testing in outreach and community settings: results from an advancing HIV prevention demonstration project conducted in seven U.S. cities. Public Health Rep. 2008;123(Suppl 3):78–85. doi: 10.1177/00333549081230S310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown J, Shesser R, Simon G, Bahn M, Czarnogorski M, Kuo I, et al. Routine HIV screening in the emergency department using the new US Centers for Disease Control and Prevention guidelines: results from a high-prevalence area. J Acquir Immune Defic Syndr. 2007;46:395–401. doi: 10.1097/qai.0b013e3181582d82. [DOI] [PubMed] [Google Scholar]
- 13.Voluntary HIV testing as part of routine medical care—Massachusetts, 2002. MMWR Morb Mortal Wkly Rep. 2004;53(24):523–6. [PubMed] [Google Scholar]
- 14.Heffelfinger JD, Sullivan PS, Branson BM, Mastro TD, Purcell DW, Griffiths SD, et al. Advancing HIV prevention demonstration projects: new strategies for a changing epidemic. Public Health Rep. 2008;123(Suppl 3):5–15. doi: 10.1177/00333549081230S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark HA, Bowles KE, Song B, Heffelfinger JD. Implementation of rapid HIV testing programs in community and outreach settings: perspectives from staff at eight community-based organizations in seven U.S. cities. Public Health Rep. 2008;123(Suppl 3):86–93. doi: 10.1177/00333549081230S311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rapid HIV testing in outreach and other community settings—United States, 2004-2006. MMWR Morb Mortal Wkly Rep. 2007;56(47):1233–7. [PubMed] [Google Scholar]
- 17.Hutchinson AB, Branson BM, Kim A, Farnham PG. A meta-analysis of the effectiveness of alternative HIV counseling and testing methods to increase knowledge of HIV status. AIDS. 2006;20:1597–604. doi: 10.1097/01.aids.0000238405.93249.16. [DOI] [PubMed] [Google Scholar]
- 18.Jain CL, Jue JS, MacKay R, Wallach F, Factor SH, Wyatt CM. Acceptance of rapid HIV testing among medical inpatients in New York City. AIDS Patient Care STDS. 2008;22:657–62. doi: 10.1089/apc.2007.0189. [DOI] [PubMed] [Google Scholar]
- 19.Hutchinson AB, Corbie-Smith G, Thomas SB, Mohanan S, del Rio C. Understanding the patient's perspective on rapid and routine HIV testing in an inner-city urgent care center. AIDS Educ Prev. 2004;16:101–14. doi: 10.1521/aeap.16.2.101.29394. [DOI] [PubMed] [Google Scholar]
- 20.Liang TS, Erbelding E, Jacob CA, Wicker H, Christmyer C, Brunson S, et al. Rapid HIV testing of clients of a mobile STD/HIV clinic. AIDS Patient Care STDS. 2005;19:253–7. doi: 10.1089/apc.2005.19.253. [DOI] [PubMed] [Google Scholar]
- 21.Samet JH, Freedberg KA, Stein MD, Lewis R, Savetsky J, Sullivan L, et al. Trillion virion delay: time from testing positive for HIV to presentation for primary care. Arch Intern Med. 1998;158:734–40. doi: 10.1001/archinte.158.7.734. [DOI] [PubMed] [Google Scholar]
- 22.Jafa K, Patel P, MacKellar DA, Sullivan PS, Delaney KP, Sides TL, et al. Investigation of false positive results with an oral fluid rapid HIV-1/2 antibody test. PLoS One. 2007;2:e185. doi: 10.1371/journal.pone.0000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.False-positive oral fluid rapid HIV tests—New York City, 2005–2008. MMWR Morb Mortal Wkly Rep. 2008;57(24):660–5. [PubMed] [Google Scholar]
- 24.Get Screened Oakland. HIV prevention campaign. [cited 2011 Feb 28]. Available from: URL: http://www.getscreenedoakland.org/hivprevention.html.
- 25.Miami-Dade County Health Department. HIV/AIDS services: Test Miami. [cited 2011 May 9]. Available from: URL: http://www.dadehealth.org/hiv/HIVservices.asp.
- 26.New York City Department of Health and Mental Hygiene. The Bronx Knows. [cited 2011 May 9]. Available from: URL: http://www.nyc.gov/html/doh/html/ah/bronx_test.shtml.
- 27.HIV Prevention Trials Network. HPTN 065, TLC-Plus: a study to evaluate the feasibility of an enhanced test, link to care, plus treat approach for HIV prevention in the United States . [cited 2011 May 9]. Available from: URL: http://www.hptn.org/research_studies/hptn065.asp#StudySummary. [DOI] [PMC free article] [PubMed]