Abstract
Diagnosis and management of coronary artery disease represents major challenges to our health care system, affecting millions of patients each year. Until recently, the diagnosis of coronary artery disease was possible only through cardiac catheterization and invasive coronary angiography. To avoid the risks of an invasive procedure, stress testing is often employed for an initial assessment of patients with suspected coronary artery disease, serving as a gatekeeper for cardiac catheterization. With the emergence of non-invasive coronary angiography, the question arises if such a strategy is still sensible, particularly, in view of only a modest agreement between stress testing results and the presence of coronary artery disease established by cardiac catheterization. Much data in support of the diagnostic accuracy and prognostic value of non-invasive coronary angiography by computed tomography have emerged within the last few years. These data challenge the role of stress testing as the initial imaging modality in patients with suspected coronary artery disease. This article reviews the clinical utility, limitations, as well as the hazards of stress testing compared with non-invasive coronary artery imaging by computed tomography. Finally, the implications of this review are discussed in relation to clinical practice.
Key words: CT angiography, stress testing, cardiac CT, coronary artery disease.
Introduction
In 2008, more than 5 million Americans required emergency unit care for the evaluation of chest pain and more than 16 million Americans required a visit to the surgery for chest pain evaluation.1 As a result, more than 10 million stress tests are being performed in the US every year, as well as at least one million diagnostic catheterizations.1,2 The assessment of patients with suspected coronary artery disease (CAD) places an enormous and increasing burden on our health care system.3 Because effective treatment is available to reduce the risk of CAD associated adverse events, i.e., myocardial infarction and acute cardiac death, accurate and efficient diagnosis of CAD is critical. In this review, the benefits and risks of current and evolving strategies of evaluating patients with suspected CAD will be discussed.
Diagnosis of coronary artery disease
The diagnosis of CAD begins with a dilemma: while any definition of CAD includes a description of coronary atherosclerotic disease, there is no consensus on how much atherosclerosis is required to fulfill criteria for CAD. Most seemingly healthy adult Americans have evidence of coronary atherosclerosis, with approximately 10% having obstructive disease.4,5 Thus, unlike other diseases, such as cancer or infections, the diagnosis of CAD is typically not categorical, i.e. present or absent. Rather, its significance is defined by its quantity and extent. CAD is commonly used to describe the condition of stenosed coronary arteries that are associated with symptoms, often termed obstructive or significant CAD. In the light of ample evidence that many myocardial infarctions arise from coronary atherosclerotic lesions that are only mildly stenotic, the rationale for this terminology should be questioned.6,7 The diagnosis of CAD is further complicated when patients present with acute symptoms. In patients with acute chest pain, the diagnosis of CAD is made if an acute coronary syndrome is confirmed by biomarkers and electrocardiogram (ECG), even in the presence of only mild CAD on cardiac catheterization. Conversely, CAD is diagnosed in the absence of an acute coronary syndrome if angiography shows obstructive CAD. These ambiguities represent a major challenge to any diagnostic tool which aims at reliably establishing a diagnosis. The introduction of catheter-based coronary angiography in 1958 greatly helped the understanding and the treatment of CAD, but its associated risks restricted its application to selected patients with high pre-test probability of CAD. In most cases, the initial approach to a patient with suspected coronary artery disease has been to perform myocardial stress testing which has served as a gatekeeper for invasive coronary angiography.
Stress testing for diagnosis and prognosis of coronary artery disease
Stress testing has been used since the late 1920s as a convenient, non-invasive way to assess for exercise induced myocardial ischemia.8 Exercise increases myocardial oxygen demand that may not be met in the presence of a coronary artery stenosis, leading to myocardial ischemia with associated ST segment changes on the ECG, as well as symptoms. For patients unable to exercise, dobutamine has been used to elicit an increase of cardiac output, and thus myocardial oxygen demand, similar to that resulting from exercise. Most pharmacological stress testing is being performed today using vasodilators, which do not apply cardiac stress per se, but rather expose the inability of stenotic coronary arteries to match the coronary blood flow of unobstructed vessels, leading to relative differences in myocardial perfusion. The vast majority of stress testing is being performed using adjunctive imaging, which increases the sensitivity and specificity over that of the ECG itself.9 Most commonly, this is nuclear perfusion imaging, which uses the single-photon-emission-computed-tomography (SPECT) as the imaging modality of choice.
Accuracy of stress testing to diagnose coronary artery disease
Numerous studies and meta-analyses reported the diagnostic accuracy of stress testing to identify patients with obstructive CAD, defined as 50% diameter or more stenosis by quantitative coronary angiography (QCA).10–13 Table 1 summarizes data for different types of stress tests. When combined with imaging, pooled sensitivity for stress testing ranges from 80–90% and specificity ranges from 70–80%; however, several caveats need to be considered. These results are almost exclusively based on single-center experiences that are typically more accurate than centers in the community. A recent European multicenter study assessing the diagnostic accuracy of stress MRI versus SPECT found an area under the receiver operating characteristic (ROC) curve of only 0.67–0.75 for SPECT; this is likely more representative of widespread practice.14 Such modest diagnostic accuracy barely yields 70% sensitivity for equal specificity. Furthermore, diagnostic accuracy studies using cardiac catheterization as the gold standard are subject to referral bias: only patients with sufficient suspicion for obstructive CAD, typically based on a positive non-invasive test, are being referred for invasive angiography and meet inclusion criterion for study enrollment. Accordingly, sensitivity is inflated while specificity is decreased.13 Considering these limitations, it is unreasonable to assume 80% or greater sensitivity and specificity for stress testing to diagnose CAD in clinical practice, even under the best of circumstances. Thus, at least 20% of stress tests with imaging are either false positive or false negative for the diagnosis of obstructive CAD in patients. Not surprisingly, the rate of obstructive disease found on cardiac catheterization in a large registry was found to be only 38% in patients without prior history of CAD, despite the fact that non-invasive testing was performed in 89% of these patients prior to invasive coronary angiography.15 Of note, a positive non-invasive test only marginally increased the rate of obstructive disease in these patients from 36 to 41%.
Table 1. Accuracy of stress testing for detecting obstructive coronary artery disease as defined by quantitative coronary angiography.
| Test | N | Sensitivity | Specificity |
|---|---|---|---|
| Exercise treadmill test | 24,074 | 68 | 77 |
| Exercise nuclear MPI | 2,360 | 88 | 70 |
| Vasodilator nuclear MPI | 4,582 | 89 | 77 |
| Dobutamine nuclear MPI | 1,359 | 84 | 79 |
| Exercise echocardiography | 2,637 | 85 | 77 |
| Dobutamine echocardiography | 6,881 | 81 | 82 |
MPI, myocardial perfusion imaging.
Prognostic value of stress testing
Similar to the diagnostic data, there have been numerous studies, notably almost exclusively single-center studies, reporting patient outcome after stress testing.16–19 In general, two important variables must be considered when reviewing the outcome after any test: the type of outcome and the morbidity in the study population. Total mortality is the easiest and most reliable outcome measure to obtain, but it is substantially influenced by the morbidity in the study population. Furthermore, it does not adequately inform on the outcome that is associated with the diagnosis to be tested, i.e. coronary artery disease. Depending on the co-morbidity in the patient population, there may be substantial mortality attributed to non-cardiac (or more specifically, to non-coronary artery disease) related deaths, which is still to be attributed to events after testing. Therefore, myocardial infarction and cardiac death, though more vulnerable to attribution bias, are the preferred outcome measurements after testing for coronary artery disease.
Unfortunately, outcome studies after stress testing vary widely in their end points, hindering pooling of data. Table 2 summarizes available data for myocardial infarction and cardiac death occurring after stress testing. The most robust data are available for nuclear stress perfusion studies, whereas exercise treadmill testing data are difficult to extract due to end point variability. With a 0.65% annualized event rate, myocardial infarction and cardiac death is fairly low for normal exercise SPECT; on the other hand, 1.78% is rather substantial for a normal pharmacological SPECT.16 When extrapolating the latter event rate to ten years, almost 18% suffer myocardial infarction or cardiac death after a normal pharmacological nuclear stress test, which is a risk level bordering on that of established CAD. Because at least 40% of nuclear stress tests are being performed with pharmacological stress, such a scenario applies to millions of patients each year.16 Bangalore et al. risk stratified patients in the same patient cohort and found annualized event rates that more than quadrupled (0.3% vs 1.4%) in moderate/ high versus low risk patients with normal stress echocardiograms.19 These very different event rates for normal exercise versus normal pharmacological stress tests are generally attributed to greater co-morbidity and the associated atherosclerotic disease burden within the pharmacological stress group. Thus, when assessing prognosis for patients based on stress testing results, it is important to consider the patient's co-morbidity and risk categorization. A normal stress test in a patient with significant co-morbidity by no means indicates a benign prognosis. An important limitation of stress testing is its inability to detect non-obstructive coronary atherosclerotic plaque, which is capable of triggering events. An overall (exercise and pharmacological stress testing combined) event rate of approximately 1% for myocardial infarction and cardiac death after a normal stress test, while generally considered a low event rate, has substantial implications because of the large numbers of patients affected. Given that at least 10 million patients undergo stress testing in the US annualy, with approximately 70% reported normal, up to 70,000 patients suffer myocardial infarction or cardiac death each year after a normal stress test.
Table 2. Annualized rates of myocardial infarction and cardiac death at follow up according to stress testing results.
| Test | N | Median follow up (months) |
MI/Cardiac death with normal test |
MI/Cardiac death with abnormal test |
|---|---|---|---|---|
| Exercise treadmill test | 1,647 | 30 | 0.80* | 2.00° |
| Exercise Nuclear MPI | 9,930 | 20 | 0.65 | 4.30 |
| Pharmacologic Nuclear MPI | 4,988 | 22 | 1.78 | 9.98 |
| Exercise Echocardiography | 4,347 | 36 | 0.50 | 2.06 |
| Dobutamine Echocardiography | 1,930 | 32 | 1.13 | 4.33 |
MI, myocardial infarction; MPI, myocardial perfusion imaging.
indicates low risk Duke treadmill score;
indicates high risk Duke treadmill score.
Non-invasive coronary artery imaging for diagnosis and prognosis of coronary artery disease
Non-invasive imaging of the coronary arteries has been extremely challenging because of the high demands on temporal and spatial resolution for imaging, which allows detailed depiction of the rather small coronary arteries. Both whole heart magnetic resonance imaging (MRI) and multidetector computed tomography angiography (CTA) have emerged as non-invasive imaging tools that have significantly advanced the field of non-invasive coronary imaging. Current generation MRI, 1.5 Tesla or greater, yields good temporal resolution (approximately 20–40 ms) compared to invasive coronary angiography (10–20 ms) but its spatial resolution is far inferior (1.5 vs 0.15 mm).20 CT angiography, on the other hand, has fairly good spatial resolution (0.35–0.60 mm), but its temporal resolution varies widely, typically between 80–180 ms. This requires beta blockers to lengthen diastasis for motion free image reconstruction. Because of its better spatial resolution and speed of image acquisition, as well as its wide spread availability, CT angiography has come to the forefront as the more accurate and robust method for non-invasive coronary imaging, despite the disadvantage of requiring X-ray radiation.21 Furthermore, CT technology has evolved very rapidly over the past few years with major improvements in temporal resolution, with radiation doses much lower than conventional CT imaging.22 Despite these advancements, however, beta blocker application prior to scanning is still required in the vast majority of cases. Because of the more prominent role of CT for non-invasive coronary artery imaging, compared to other modalities, this review will focus on CT coronary angiography (CTA) for this purpose.
Computed tomography angiography diagnostic accuracy for detecting obstructive coronary artery disease
Similar to stress testing, there have been numerous single-center studies documenting the diagnostic accuracy of CTA compared to QCA for stable patients with suspected CAD.23–25 Meta-analyses reveal exceedingly high diagnostic accuracy to identify CAD in patients with pooled AUC ranging between 0.97–0.99, depending on whether patients with known CAD were included or not. Pooled sensitivity ranges between 98–99%; specificity ranges between 82–89% in meta-analyses (Figure 1).23,24 Similar to the data on stress testing, however, many of the studies on CTA are subject to referral bias, suggesting that true sensitivity is likely lower, while specificity is higher in less selected populations. There have also been several multicenter studies that yielded AUCs between 0.93 and 0.96 for 64-slice detector technology among patients with a wide range of disease prevalence, coronary calcification, and image quality.26–28 Predictive values, while frequently referenced in the context of diagnostic accuracy, are highly dependent on the disease prevalence in the study population and should, therefore, be viewed accordingly.26 Positive predictive values are generally lower and negative predictive values are higher in low-risk populations and vice versa. Despite some criticism on the positive predictive values by CTA, single-center studies with very different study populations yielded median positive and negative predictive values of 93% and 100% in patients, respectively.23 When pooling the data from the three major multicenter studies (n=961) with a higher disease prevalence (53%) than typically encountered in symptomatic patients without known CAD, positive and negative predictive values are 85% and 92%, respectively.
Figure 1.
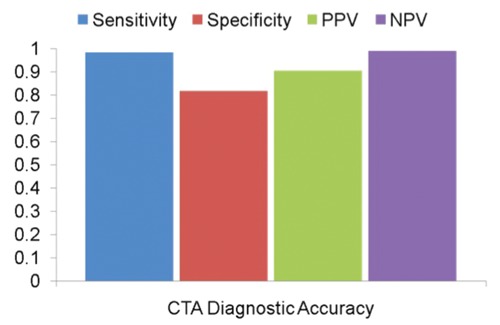
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) of computed tomography angiography for detecting 50% stenosis or over by quantitative coronary angiography based on a meta-analysis of 3,674 symptomatic patients without history of coronary artery disease enrolled in 28 studies.24
It is important to note that the diagnostic data available for CTA are at least equivalent to those for stress testing with regards to number of subjects, length of follow up, characteristics of patient populations, gold standards used, and end points. In fact, diagnostic accuracy data are more robust for CTA than for stress testing, as the former includes several multicenter studies. Thus, data sets for CTA and stress testing are sufficiently congruent to allow comparison. Furthermore, data from seven clinical studies, comparing CTA directly to stress testing for the diagnosis of obstructive CAD are currently available (Table 3).29–35 Pooled sensitivity and specificity for 483 patients were 96% and 88% for CTA, but only 66% and 69% for nuclear stress testing, respectively. These disappointing results for nuclear stress testing are consistent with data from an independent core laboratory for 225 patients from 18 centers evaluating SPECT in comparison with stress MRI.14 On the other hand, results for CTA are in agreement with those obtained in single and multi-center studies. Hence, data from studies directly comparing CTA with stress testing support the results from diagnostic accuracy studies performed separately for these modalities, confirming substantially superior diagnostic performance for CTA. The remarkable consistency of these study results and the magnitude of differences in diagnostic performance between CTA and stress testing allow us to have great confidence in this conclusion.
Table 3. Direct comparison of computed tomography angiography vs single-photon-emission-computed-tomography for detecting ≥50% coronary artery stenoses as defined by quantitative coronary angiography.
| Study | N | CTA sensitivity |
CTA specificity |
SPECT sensitivity |
SPECT specificity |
|---|---|---|---|---|---|
| Budoff et al.30 | 30 | 95 | 89 | 81 | 78 |
| Schuijf et al.32 | 58 | 100 | 81 | 70 | 58 |
| Gaemperli et al.31 | 78 | 94 | 100 | 53 | 83 |
| Gallagher et al.29 | 85 | 86 | 92 | 71 | 90 |
| Budoff et al.35 | 48 | 92 | 78 | 76 | 57 |
| Arbab-Zadeh et al.34 | 62 | 95 | 100 | 86 | 45 |
| Hamirani et al.33 | 122 | 99 | 74 | 56 | 39 |
| Pooled results | 483 | 96 | 88 | 66 | 69 |
CTA, computed tomography angiography; SPECT, single-photon-emission-computed-tomography.
Prognostic value of computed tomography angiography
Three recent meta-analyses and one large registry document patient outcomes for patients with suspected CAD who underwent CTA.36–39 The number of patients included in these investigations, ranging from 5,000–23,000, is comparable to data available from the nuclear medicine literature. In addition, the patient populations (patients referred to evaluate chest pain) and the median follow up (1–2 years) are similar to the nuclear medicine literature. These data, therefore, are quite applicable for comparison among these modalities. On the other hand, most of the prognostic data available for CTA only report total mortality as outcome, while few provide breakdowns of cardiac mortality and myocardial infarction. Since total mortality is substantially influenced by the co-morbidity and characteristics of the study population, comparisons of total mortality between different populations have to be interpreted with caution. Unfortunately, several meta-analyses included studies with both cardiac and total mortality without adequately differentiating the results. Furthermore, results for CTA were categorized into normal, non-obstructive, and obstructive CAD, as opposed to SPECT data which were reported as normal versus abnormal.
Overall, a CTA with obstructive CAD has similar event rates to an abnormal SPECT: approximately 4% annualized rate of death or myocardial infarction (Figure 2). Conversely, a normal CTA has lower rates of death and myocardial infarction than a normal SPEC, which is a key finding. Specifically, in patients with normal CTA, total mortality is similar to a reference population (0.15% annualized total mortality) in a recent meta-analysis.36 For a subset of 1,681 patients with a normal CTA, information on cardiac mortality is also available.36,40 Among these, notably, there was no case of CAD related mortality (one patient died of a myosarcoma which was counted as cardiac mortality).40 Equally important, there was no case of myocardial infarction in those followed after a normal CTA.36 Rates for myocardial infarction and death were approximately 1% per year for non-obstructive CAD equivalent to event rates with a normal SPECT, which is unable to detect non-obstructive CAD.
Figure 2.
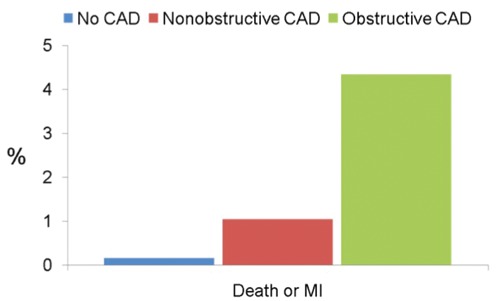
Annualized rates of death or myocardial infarction after computed tomography angiography according to study results based on a meta-analysis of 9,592 patients enrolled in 18 studies.36 CAD, coronary artery disease.
A recently published large international registry, consisting mainly of low-intermediate risk patients, confirmed very low total mortality in patients with normal CTA, but information for cardiac mortality or myocardial infarction is not available from these data.38 Only one study compared patient outcomes from CTA to SPECT in the same patient cohort41 and after a median follow up of 672 days, CTA analysis resulted in incremental prognostic value for total mortality over SPECT in 517 patients. Importantly, the annualized mortality rate was more than three times greater in patients with normal SPECT compared to those with normal CTA (1.1 vs 0.3%), and because no breakdown of events was provided, it remained unclear if any CAD related events occurred in patients after a normal CTA. Another study compared outcome data after CTA (n=693) with SPECT (n=3,067) using a matched cohort comparison study design, which concluded similar prognostic performance.42 No separate analysis was performed for patients with a normal CTA, i.e. with no evidence of CAD, limiting the results.
For patients presenting with acute symptoms, few follow-up data are available. The ROMICAT study, similar to studies in stable patients, revealed no major adverse cardiovascular events in 183 patients two years after presenting with acute chest pain and normal ECG/cardiac makers.43 Conversely, 185 patients with non-obstructive or obstructive CAD had annualized event rates of 2.3 and 15.2%, respectively. A multicenter study randomized low-risk patients with acute chest pain to a CTA vs SPECT guided management, with time to diagnosis as the primary end point.44 As a result, CTA led to a more rapid diagnosis while other outcome was similar within the groups. Of note, myocardial infarction occurred in 0.3% among patients found to have normal or mild CAD by CTA, versus 1.5% in patients with low-risk SPECT, after six months (P=0.11); no analysis was provided for patients with normal CTA, i.e. the absence of any CAD.
Overall, CTA, using simple categorization of no CAD, non-obstructive CAD, and obstructive CAD, conveys risk stratification that appears superior to that obtained by stress testing because it identifies a sizable subgroup (20–30%) of patients who are at exceedingly low risk of adverse events. In fact, available data suggest that there is a total absence of CAD related events in patients with normal CTA for at least two years after testing.36,40,45 It is conceivable that other CTA characteristics, such as individual plaque burden and composition, total atherosclerotic plaque burden etc., further improve predictive power. For example, Ostrom et al. followed 2,538 individuals for a mean of 6.5 years after CTA and found an area under the curve of 0.89, predicting total (not cardiac!) mortality, based on simple cardiac CT categories of normal arteries, non-obstructive, and obstructive coronary artery disease, combined with a calcium score and traditional risk factors.46
Anatomy versus physiology: what matters most for patients with suspected coronary artery disease?
The key objective in the evaluation and management of patients with suspected CAD is the prevention/risk reduction of acute coronary events, i.e. myocardial infarction and cardiac death. The pathophysiology of such acute coronary events has been extensively studied, and central to this concept is the alteration of coronary atherosclerotic plaque, e.g. erosion and rupture, in the presence of a thrombosis conducive state.47 Numerous studies established a strong, consistent relationship between the presence and extent of coronary atherosclerotic disease and the occurrence of acute coronary events. Most impressively, an analysis of more than 25,000 patients showed an increase in mortality even for a small increment in coronary calcium score, a surrogate of coronary atherosclerotic burden.48 A larger coronary atherosclerotic plaque burden is associated with more frequent plaque ruptures or erosions increasing the probability that one of them coincides with a thrombosis conducive state triggering an event.47
While the relationship between the presence and extent of CAD and coronary events is well established, the role of inducible myocardial ischemia for predicting acute events is less certain. Although many studies suggest a greater number of acute coronary events in patients with inducible perfusion abnormalities by stress testing, compared to those without, it remains unclear if stress induced ischemia provides independent prognostic value, or if inducible ischemia simply represents a surrogate for the severity of underlying CAD. Several observations suggest the latter. As outlined above, meta-analyses of thousands of patients revealed a relatively low but consistent number of acute coronary events in patients despite normal stress test findings, documenting that the absence of myocardial ischemia does not preclude acute events soon after testing. Furthermore, the COURAGE and several other randomized trials have failed to demonstrate a reduction in myocardial infarction and death in patients with chronic angina who underwent revascularization despite reduced ischemic burden on stress testing.49 A sub-analysis of the COURAGE trial suggested a worse outcome in patients with large residual ischemic burden compared to those without; however, these results lost statistical significance after risk adjustments, even before considering the severity of the underlying CAD.
In studies employing both calcium scanning and stress testing in the same patients, the presence of calcified coronary atherosclerotic plaque conveyed increased risk for subsequent cardiac events in patients, even with a normal stress perfusion study.50 Conversely, long-term follow up of patients with chest pain and normal coronary arteries by invasive angiography revealed a total annualized mortality equivalent to a normal reference population despite the presence of ischemia on stress testing in some.51 Importantly, follow up of patients with chest pain and abnormal stress test results were found to have repeated hospitalizations for chest pain evaluation, but there were no acute coronary events.52 Hence, while coronary atherosclerosis clearly is integral to the pathophysiology of acute coronary events, inducible ischemia has not been shown to be of prognostic importance for myocardial infarction and death that is independent from the presence and extent of coronary artery disease. It remains to be seen if the presence and severity of inducible myocardial ischemia can provide information that is incremental to a comprehensive assessment of coronary anatomy.
Risk - benefit considerations of stress testing versus computed tomography angiography
Potential benefits derived from imaging
Any imaging to procedure should be of sufficient benefit to a patient, helping to outweigh potential risks and side effects. In the case of cardiac imaging for CAD, the benefit is assumed because it establishes the diagnosis of CAD, which may then prompt treatment and reduce the risk of subsequent events. In contrast, ruling out CAD may alleviate the need for medications and further testing, lowering the risk from drug interactions and adverse effects. Since there are no prospective, controlled studies documenting outcome benefit for patients undergoing imaging, it remains unclear if all or most patients benefit, or if the risk-benefit ratio is unfavorable for many. A key problem for testing these hypotheses is that there is no adequate control. Not testing appears to be impractical and not a valid alternative in most cases. Demonstrating superiority of a specific testing strategy over another, on the other hand, requires a very large number of participants and/or extensive follow up, which may be cost prohibitive and impractical.
To come to some reasonable estimates with regards to the benefits of diagnosing CAD, the following assumptions are made: approximately 20% of relative risk reduction for myocardial infarction and death are secondary to preventative measures (including risk factor interdiction, pharmacotherapy, and revascularization); approximately 30% of stress tests are abnormal; sensitivity and specificity are (optimistically) 80% for stress testing; there is a 4% annualized event risk for untreated patients. Accordingly, 24% of all patients will have a correct diagnosis of obstructive CAD, resulting in an absolute annualized reduction of 0.192% (0.960 minus 0.768%) for myocardial infarction and death, applied to all patients undergoing stress testing. Thus, of 521 patients undergoing stress testing for suspected CAD, there would be one myocardial infarction or death prevented per year as a result of testing.
Because of the superior diagnostic accuracy for CTA, its benefit is somewhat greater. Assuming a conservative 90% sensitivity and specificity, 27% of patients will benefit from the correct diagnosis of obstructive CAD. The associated annualized 20% relative reduction in myocardial infarction and death will result in a 0.216% absolute risk reduction each year for myocardial infarction and death for all patients undergoing CTA testing. In other words, 463 patients would need to undergo testing for one myocardial infarction or death per year to be prevented. In addition to diagnosing obstructive disease, CTA is capable of diagnosing non-obstructive disease. Assuming similar risk reductions when treated and a similar normal/abnormal study ratio for stress testing with 50% of normal studies having non-obstructive CAD,36 an annular event rate of approximately 1%, with an additional absolute reduction in annular event rate of 0.07%, would be applied to all patients undergoing CTA, for a total of a 0.286% absolute reduction in annular myocardial infarction and death (for every 350 patients undergoing CTA for evaluation for CAD one hard event is prevented per year). Furthermore, CTA has another very important advantage over stress testing in its capability to exclude any disease as opposed to excluding only obstructive CAD. Since a normal CTA is associated with the absence of myocardial infarction and CAD related deaths for at least two years after testing,36,40,45 patients are very unlikely to benefit from further short-term testing and CAD targeted pharmacotherapy, which will reduce risk of adverse events associated with these measures. Drawing from long-term follow-up data after a normal CTA which, however, provided only information on total mortality, survival is 100% after five years with possible longer event free survival if cardiac mortality is considered.46 Thus, the data currently available suggest that the warranty period for a normal CTA in properly selected patients is at least 2–5 years, considering the absence of any hard events or mortality, but could possibly be much longer. The extent of this potentially enormous impact on the testing and treatment strategies for millions of patients is difficult to estimate at the present time, but it must be viewed as extremely positive. Other important benefits from testing, diagnosis, and treatment, e.g. symptom relief, quality of life, etc., must also be considered, even though these benefits are currently more difficult to estimate.
Direct and indirect risks of stress testing
Risks from stress testing vary substantially, and these risks depend on the form of the stress and the imaging combined. In its most basic form, the risk from exercise treadmill testing is confined to strenuous exercise that is associated with approximately 0.04% risk of myocardial infarction or death.53 Fewer data are available for dobutamine instead of exercise stress, with reported rates of myocardial infarction or death of 0.025%.54,55 Myocardial infarction or death associated with vasodilator stress appears to be similarly rare, with an approximate rate of 0.028%.56 However, if nuclear perfusion imaging is performed, the radiation dose must be added to the risk profile. A typical radiation dose for a sestamibi rest-stress protocol encompasses 11 mSv, whereas a dual isotope protocol involving thallium yields a dose of 24 mSv.57 Adopting the guidelines set by BEIR VII at the National Academy of Sciences, a 0.1% risk of cancer is assumed for any 10 mSv dose.58 Accordingly, a reasonable assumption of risk for myocardial infarction, cancer, or death results from stress testing that ranges from 0.025% with dobutamine echocardiography, to 0.28% for an exercise nuclear perfusion study using thallium.
In addition to the direct risks from stress testing, there are indirect risks, i.e. adverse effects from false positive or false negative test results. Since there is a 70% normal testing result and an optimistic 80% sensitivity and specificity, approximately 1.4 million patients in the US will have a false positive or negative stress test result every year. Assuming that one fourth will undergo cardiac catheterization for a false positive result with a major complication rate of 1.7%, including 0.23% for myocardial infarction, stroke, or death,59 5,950 patients every year (0.06% of all patients) will suffer major complications, and 805 patients (0.008%) will suffer life threatening complications from an invasive procedure that was prompted by a false positive stress test. Even more important, however, is the effect of false negative testing results. These results can be divided into two categories: a) false negative test result for obstructive CAD; and b) the categorical inability of stress testing to detect non-obstructive CAD. At least 20% of patients will have false negative results for obstructive CAD, and an annualized 0.8% excess risk of myocardial infarction and death must be considered using the same assumptions as made above. This adds a 0.048% annualized or a 0.48% 10-year risk of myocardial infarction and death when applied to the entire stress testing population. As 70% of patients with normal stress testing results have on average a 1% event rate because of undetected non-obstructive disease, an additional annualized excess risk of 0.14% (1.4% 10-year risk) for the entire group applies.
It becomes apparent, therefore, that the adverse effects from false positive or false negative test results for CAD have a larger impact than the risks associated with the tests themselves. By accruing all excessive risks from stress testing for a 10-year follow-up period (assuming it fulfills requirements for cancer development from radiation), an excessive risk of 2% for myocardial infarction, stroke, cancer, or death from stress testing is realistic. Conversely, considering a 1.9% absolute risk reduction for myocardial infarction or death over ten years, for the correct diagnosis of obstructive CAD, the risk-benefit ratio for stress testing is not convincing.
Direct and indirect risks of computed tomography coronary angiography
Direct risks from CTA are confined to contrast application, beta blocker application, and radiation dose. Risk from myocardial infarction or death associated with contrast application is exceedingly low, and was 0.003% in a series of over 29,000 patients undergoing CT angiography.60 Risk from beta blocker application in preparation for a CTA has been studied only in a few case series, which suggests an exceedingly low risk for severe adverse events with no reported cases of myocardial infarction or death.61 Radiation doses from a coronary CTA vary drastically with the image acquisition protocol and technology used. More recently, a dramatic decrease in radiation doses, from approximately 20 to less than 1 mSv in some studies, has become noticeable.22 The Protection I study surveyed the radiation doses in clinical practice at 50 worldwide centers and found a median dose of 12 mSv with a wide range of median doses among the participating centers.62 However, this study reflects the practice pattern from 2007, and likely represents an overestimation of doses from CTA compared to current practice today. Growing use of prospective scan triggering, low tube voltage settings, and novel image reconstruction techniques have resulted in an average estimated dose of 2.7 mSv (95% CI 2.2–3.2) in 960 patients assessed in a recent meta-analysis of 16 studies.25 Even when considering differences in image acquisition in the community, it is reasonable to assume that the majority of laboratories yield effective doses from CTA that on average do not currently exceed 5 mSv, and have a strong trend for further reductions. Considering BEIR VII guidelines, such doses are associated with an approximately 0.05% excess risk of cancer later in life.58
As with stress testing, indirect risks are more relevant than direct risks. When applying the same assumptions for stress testing, but then adjusting for the superior diagnostic accuracy by CT, approximately 10% of patients will have false positive results for obstructive CAD. Again, assuming that one fourth will undergo cardiac catheterization with the associated event rate of 0.23% for myocardial infarction, stroke, or death, an estimated 0.0058% of patients undergoing CTA will fall into this category, or 0.058% over ten years. Furthermore, approximately 10% of patients will have false negative results for obstructive CAD (with an annualized 0.02% or 0.2% 10-year excess risk of myocardial infarction and death) when applied to the entire testing population. CTA has the ability to detect non-obstructive disease, but its impact is unclear because of an inadequate reference standard. Assuming a 10% false negative rate and an event rate of 1% in this group, an excess risk of 0.014% likely applies. The cumulative 10-year excess risk of myocardial infarction, stroke, cancer, or death of 0.451% is outweighed by a 2.9% event reduction for correct diagnoses, which is a favorable risk-benefit ratio.
One factor that was not discussed in this context is the need for follow up imaging for incidental non-cardiac findings seen on CT imaging. Depending on the study population, significant non-cardiac findings that require follow up or a change in management occur in 1–30% of cases.63,64 For a rather low risk population encountered in patients without known CAD, this rate is approximately 5%.64 It is difficult to ascertain if incidental findings on CT imaging have to be regarded as an advantage or a limitation. Although there are limited data available about radiation exposure and toxicity from follow-up imaging, recent data suggest that screening patients for lung cancer, using low dose CT, reduces mortality.65 It remains unclear if this benefit also applies to patients with a much lower risk for lung cancer. Likewise, it is unclear if detecting other pathologies, such as pulmonary embolism, pneumonia, or aortic aneurysm/dissection, outweighs the risk from radiation and contrast. Given the low dose and contrast requirements for contemporary cardiac CT and the seriousness of detected conditions, a favorable risk-benefit ratio must be regarded as likely.
Cost considerations
Direct costs for SPECT and CTA are similar. Medicare reimbursement for exercise SPECT averages $500 for technical and $200 for professional services versus $550 and $180 for CTA, respectively.66 Several studies addressed cost effectiveness for CTA versus SPECT. Min et al. examined the use of CTA as a first-line test for patients with suspected CAD in comparison with SPECT and found CTA was more cost effective even when not considering a detailed risk-benefit analysis as outlined above.66 In a randomized controlled trial, CTA was more cost effective also in patients with acute chest pain.44 It is conceivable that the benefit of CTA is even more apparent if costs from adverse effects are fully considered. Ironically, relatively low reimbursement for CTA has been instrumental in limiting its wide spread application. Equipment and overhead costs are higher for CTA than for SPECT, and practices struggle to recover their expenses for CTA, particularly since study interpretation is substantially more time consuming compared to SPECT. Thus, economic considerations hinder the application of imaging tools with superior diagnostic accuracy for many patients.
Conclusions and implications for clinical management
There is remarkably little prospective, randomized data that steer image guided clinical management toward patient outcomes.67 Some studies addressed the question of medical treatment versus revascularization, based on SPECT imaging findings in patients with CAD. But despite widespread acceptance in the medical community, these investigations were limited by observational and/or retrospective designs.68,69 Stress testing has been used for decades to obtain a reasonable estimate for the likelihood of obstructive coronary artery disease and its associated cardiac event risk. Because of a lack of valid alternatives, such an approach was never prospectively investigated. Since the emergence of non-invasive imaging, there have been several meta-analyses documenting both greater diagnostic accuracy and prognostic information for CTA, compared to stress testing in patients with suspected coronary artery disease. Despite equal or lesser toxicity and cost for CT coronary angiography, when comparing CTA to stress testing, there has been a reluctance on the part of the medical community to embrace CT as the primary diagnostic tool for the assessment of patients with suspected coronary artery disease. Given the abundance of clinical data, it is time to accept CTA as an established imaging modality for the assessment of patients with suspected CAD.70
While there is conclusive evidence that the presence and extent of CAD strongly relates to cardiac event risk, it is still unclear if the risk from myocardial ischemia, as detected by stress testing, is merely related to the underlying CAD, or if there is an independent risk associated with ischemia. Nonetheless, health care experts demand large scale studies to prove better outcome for patients assessed by CTA compared to alternative management, even though no such evidence exists for stress testing or other cardiac imaging.67 The outlined estimates for outcome benefits from stress testing and CTA reveal that much larger patient numbers than commonly enrolled are necessary to show outcome benefit, given the low number of events and relatively small reduction in risk. However, in view of the many millions of patients undergoing testing for CAD, these differences are indeed relevant for the outcome of thousands of patients each year.
When given the choice between stress testing and CTA for an initial assessment of patients with suspected coronary artery disease, there is evidence to support the superiority of an assessment using CTA, and it appears prudent to implement CTA as the preferred tool pending evidence to the contrary. Based on the data available, it is plausible that thousands of patients suffer myocardial infarction and cardiac death each year after normal stress testing results that could have been identified and treated if CTA was employed instead of stress testing. Therefore, the burden of proof must be on stress testing and not on CTA to deliver evidence of this well based hypothesis. Such evidence may not be generated any time soon because of the inflated cost involved. Even large scale studies, such as the PROMISE study, are unlikely to demonstrate differences in hard end points unless longer follow up is implemented. Furthermore, the diagnostic and prognostic advantage of CTA over stress testing is so clearly evident that it appears prudent to invest research dollars into clinical studies that inform clinicians of the best management strategies based on test results, rather than on comparison studies among imaging modalities. Until such data are available, we are left with our best judgment to apply imaging results in clinical practice. A suggested algorithm for initial testing in patients with suspected CAD is provided in Figure 3. Naturally, these suggested strategies will change with the emergence of new data, and it is conceivable that fewer patients will have to undergo cardiac catheterization if CTA can identify patients who will not benefit from revascularization.
Figure 3.
Schema for a proposed algorithm of triaging patients with suspected but without known coronary artery disease. CAD, coronary artery disease; CTA, computed tomography angiography; tx, therapy.
The coming years will likely see stress testing evolving into a secondary role for the assessment of patients with suspected CAD, providing information on hemodynamic significance and ischemic burden in patients with CAD in the moderate-severe range, or for patients not eligible for CTA. Clinically, it is uncertain if ischemia testing provides information that is of incremental value for patient management over anatomical assessment alone. For this purpose, clinical studies, such as the ISCHEMIA study, need to disentangle the information provided by stress testing that is not directed towards the severity and location of CAD, which is currently available from coronary angiography. Looking forward, it is feasible that the integration of emerging CAD char-acteristics, e.g. plaque configuration and consistency, shear stress analysis, and blood flow dynamics, will evolve into a comprehensive coronary artery assessment, even though it will likely require a substantial effort to clarify the use of this information for patient management and outcome. However, a comprehensive coronary artery assessment would finally enable us to move beyond simplistic categorization of CAD for a more differentiated approach to patients with coronary artery disease.71
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics - 2011 update: a report from the American Heart Association. Circulation. 2011;123:933–44. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berrington de Gonzalez A, Kim KP, Smith-Bindman R, McAreavey D. Myocardial perfusion scans: projected population cancer risks from current levels of use in the United States. Circulation. 2010;122:2403–10. doi: 10.1161/CIRCULATIONAHA.110.941625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–44. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.Nemetz PN, Roger VL, Ransom JE, et al. Recent trends in the prevalence of coronary disease: a population-based autopsy study of nonnatural deaths. Arch Intern Med. 2008;168:264–70. doi: 10.1001/archinternmed.2007.79. [DOI] [PubMed] [Google Scholar]
- 5.Tuzcu EM, Kapadia SR, Tutar E, et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103:2705–10. doi: 10.1161/01.cir.103.22.2705. [DOI] [PubMed] [Google Scholar]
- 6.Hackett D, Davies G, Maseri A. Pre-existing coronary stenoses in patients with first myocardial infarction are not necessarily severe. Eur Heart J. 1988;9:1317–23. doi: 10.1093/oxfordjournals.eurheartj.a062449. [DOI] [PubMed] [Google Scholar]
- 7.Little WC, Constantinescu M, Applegate RJ, et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78:1157–66. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 8.Master A, Oppenheimer E. A simple exercise tolerance test for circulatory efficiency with standard tables for normal individuals. Am J Med Sci. 1929;177:223–43. [Google Scholar]
- 9.Gibbons RJ, Balady GJ, Beasley JW, et al. ACC/AHA guidelines for exercise testing. A report of the American College of Cardiology/American heart association task force on practice guidelines (committee on exercise testing) J Am Coll Cardiol. 1997;30:260–311. doi: 10.1016/s0735-1097(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 10.Underwood SR, Anagnostopoulos C, Cerqueira M, et al. Myocardial perfusion scintigraphy: the evidence. Eur J Nucl Med Mol Imaging. 2004;31:261–91. doi: 10.1007/s00259-003-1344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianrossi R, Detrano R, Mulvihill D, et al. Exercise-induced ST depression in the diagnosis of coronary artery disease. A meta-analysis. Circulation. 1989;80:87–98. doi: 10.1161/01.cir.80.1.87. [DOI] [PubMed] [Google Scholar]
- 12.Fleischmann KE, Hunink MG, Kuntz KM, Douglas PS. Exercise echocardiography or exercise SPECT imaging? A meta-analysis of diagnostic test performance. JAMA. 1998;280:913–20. doi: 10.1001/jama.280.10.913. [DOI] [PubMed] [Google Scholar]
- 13.Geleijnse ML, Krenning BJ, van Dalen BM, et al. Factors affecting sensitivity and specificity of diagnostic testing: dobutamine stress echocardiography. J Am Soc Echocardiogr. 2009;22:1199–208. doi: 10.1016/j.echo.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: Comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480–9. doi: 10.1093/eurheartj/ehm617. [DOI] [PubMed] [Google Scholar]
- 15.Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navare SM, Mather JF, Shaw LJ, et al. Comparison of risk stratification with pharmacologic and exercise stress myocardial perfusion imaging: a meta-analysis. J Nucl Cardiol. 2004;11:551–61. doi: 10.1016/j.nuclcard.2004.06.128. [DOI] [PubMed] [Google Scholar]
- 17.Metz LD, Beattie M, Hom R, et al. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: a meta-analysis. J Am Coll Cardiol. 2007;49:227–37. doi: 10.1016/j.jacc.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 18.Peteiro J, Monserrrat L, Pineiro M, et al. Comparison of exercise echocardiography and the duke treadmill score for risk stratification in patients with known or suspected coronary artery disease and normal resting electrocardiogram. Am Heart J. 2006;151:1324–1324. doi: 10.1016/j.ahj.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Bangalore S, Gopinath D, Yao SS, Chaudhry FA. Risk stratification using stress echocardiography: incremental prognostic value over historic, clinical, and stress electrocardiographic variables across a wide spectrum of bayesian pretest probabilities for coronary artery disease. J Am Soc Echocardiogr. 2007;20:244–52. doi: 10.1016/j.echo.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Gerber BL. MRI versus CT for the detection of coronary artery disease: current state and future promises. Curr Cardiol Rep. 2007;9:72–8. doi: 10.1007/s11886-007-0013-x. [DOI] [PubMed] [Google Scholar]
- 21.Schuetz GM, Zacharopoulou NM, Schlattmann P, Dewey M. Meta-analysis: Noninvasive coronary angiography using computed tomography versus magnetic resonance imaging. Ann Intern Med. 2010;152:167–77. doi: 10.7326/0003-4819-152-3-201002020-00008. [DOI] [PubMed] [Google Scholar]
- 22.Achenbach S, Marwan M, Ropers D, et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J. 2010;31:340–6. doi: 10.1093/eurheartj/ehp470. [DOI] [PubMed] [Google Scholar]
- 23.Mowatt G, Cook JA, Hillis GS, et al. 64-slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart. 2008;94:1386–93. doi: 10.1136/hrt.2008.145292. [DOI] [PubMed] [Google Scholar]
- 24.Paech DC, Weston AR. A systematic review of the clinical effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of suspected coronary artery disease. BMC Cardiovasc Disord. 2011;11:32–32. doi: 10.1186/1471-2261-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Ballmoos MW, Haring B, Juillerat P, Alkadhi H. Meta-analysis: diagnostic performance of low-radiation-dose coronary computed tomography angiography. Ann Intern Med. 2011;154:413–20. doi: 10.7326/0003-4819-154-6-201103150-00007. [DOI] [PubMed] [Google Scholar]
- 26.Arbab-Zadeh A, Miller J, Rochitte C, et al. Diagnostic accuracy of CT coronary angiography according to pretest probability of coronary artery disease and severity of coronary arterial calcification: the CorE-64 international, multicenter study. J Am Coll Cardiol. 2012;59:379–87. doi: 10.1016/j.jacc.2011.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–44. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 28.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (assessment by coronary computed tomographic angiography of individuals undergoing invasive coronary angiography) trial. J Am Coll Cardiol. 2008;52:1724–32. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher MJ, Ross MA, Raff GL, et al. The diagnostic accuracy of 64-slice computed tomography coronary angiography compared with stress nuclear imaging in emergency department low-risk chest pain patients. Ann Emerg Med. 2007;49:125–36. doi: 10.1016/j.annemergmed.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 30.Budoff MJ, Rasouli ML, Shavelle DM, et al. Cardiac CT angiography (CTA) and nuclear myocardial perfusion imaging (MPI)-a comparison in detecting significant coronary artery disease. Acad Radiol. 2007;14:252–7. doi: 10.1016/j.acra.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Gaemperli O, Husmann L, Schepis T, et al. Coronary CT angiography and myocardial perfusion imaging to detect flow-limiting stenoses: a potential gatekeeper for coronary revascularization? Eur Heart J. 2009;30:2921–9. doi: 10.1093/eurheartj/ehp304. [DOI] [PubMed] [Google Scholar]
- 32.Schuijf JD, Wijns W, Jukema JW, et al. Relationship between noninvasive coronary angiography with multi-slice computed tomography and myocardial perfusion imaging. J Am Coll Cardiol. 2006;19(48):2508–14. doi: 10.1016/j.jacc.2006.05.080. [DOI] [PubMed] [Google Scholar]
- 33.Hamirani YS, Isma'eel H, Larijani V, et al. The diagnostic accuracy of 64-detector cardiac computed tomography compared with stress nuclear imaging in patients undergoing invasive cardiac catheterization. J Comput Assist Tomogr. 2010;34:645–51. doi: 10.1097/RCT.0b013e3181e3d0b1. [DOI] [PubMed] [Google Scholar]
- 34.Arbab-Zadeh A, Lima JAC, Lemos PA, et al. Association of myocardial ischemia as assessed by stress testing and the presence of coronary stenoses by computed tomography and conventional angiography: a sub-analysis from the CORE-64 multicenter trial. J Am Coll Cardiol. 2008;51:278–906. [Google Scholar]
- 35.Budoff MJ, Kazerooni EA, Thomas GS, et al. Diagnostic accuracy of noninvasive 64-row computed tomographic coronary angiography (CCTA) compared with myocardial perfusion imaging (MPI): the PICTURE study, a prospective multicenter trial. Circulation. 2008;118:S935–S935. doi: 10.1016/j.acra.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Hulten EA, Carbonaro S, Petrillo SP, et al. Prognostic value of cardiac computed tomography angiography. A systematic review and meta-analysis. J Am Coll Cardiol. 2011;57:1237–47. doi: 10.1016/j.jacc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 37.Bamberg F, Sommer WH, Hoffmann V, et al. Meta-analysis and systematic review of the long-term predictive value of assessment of coronary atherosclerosis by contrast-enhanced coronary computed tomography angiography. J Am Coll Cardiol. 2011;57:2426–36. doi: 10.1016/j.jacc.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 38.Min JK, Dunning A, Lin FY, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the international multicenter CONFIRM (coronary CT angiography evaluation for clinical outcomes: An international multicenter registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–60. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 39.Abdulla J, Asferg C, Kofoed KF. Prognostic value of absence or presence of coronary artery disease determined by 64-slice computed tomography coronary angiography A systematic review and meta-analysis. Int J Cardiovasc Imaging. 2011;27:413–20. doi: 10.1007/s10554-010-9652-x. [DOI] [PubMed] [Google Scholar]
- 40.Chow BJ, Wells GA, Chen L, et al. Prognostic value of 64-slice cardiac computed tomography severity of coronary artery disease, coronary atherosclerosis, and left ventricular ejection fraction. J Am Coll Cardiol. 2010;55:1017–28. doi: 10.1016/j.jacc.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 41.van Werkhoven JM, Schuijf JD, Gaemperli O, et al. Prognostic value of multislice computed tomography and gated single-photon emission computed tomography in patients with suspected coronary artery disease. J Am Coll Cardiol. 2009;53:623–32. doi: 10.1016/j.jacc.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 42.Shaw LJ, Berman DS, Hendel RC, et al. Prognosis by coronary computed tomographic angiography: Matched comparison with myocardial perfusion single-photon emission computed tomography. J Cardiovasc Comput Tomogr. 2008;2:93–101. doi: 10.1016/j.jcct.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Schlett CL, Banerji D, Siegel E, et al. Prognostic value of CT angiography for major adverse cardiac events in patients with acute chest pain from the emergency department: 2-year outcomes of the ROMI-CAT trial. JACC Cardiovasc Imaging. 2011;4:481–91. doi: 10.1016/j.jcmg.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldstein JA, Chinnaiyan KM, Abidov A, et al. The CT-STAT (coronary computed tomographic angiography for systematic triage of acute chest pain patients to treatment) trial. J Am Coll Cardiol. 2011;58:1414–22. doi: 10.1016/j.jacc.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 45.La Grutta L, Runza G, Gentile G, et al. Prognostic outcome of routine clinical noninvasive multidetector-row computed tomography coronary angiography in patients with suspected coronary artery disease: a 2-year follow-up study. Radiol Med. 2011;116:521–31. doi: 10.1007/s11547-011-0655-z. [DOI] [PubMed] [Google Scholar]
- 46.Ostrom MP, Gopal A, Ahmadi N, et al. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol. 2008;52:1335–43. doi: 10.1016/j.jacc.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 47.Finn AV, Nakano M, Narula J, et al. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–92. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 48.Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–70. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 49.Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 50.Chang SM, Nabi F, Xu J, et al. The coronary artery calcium score and stress myocardial perfusion imaging provide independent and complementary prediction of cardiac risk. J Am Coll Cardiol. 2009;54:1872–82. doi: 10.1016/j.jacc.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 51.Papanicolaou MN, Califf RM, Hlatky MA, et al. Prognostic implications of angiographically normal and insignificantly narrowed coronary arteries. Am J Cardiol. 1986;58:1181–7. doi: 10.1016/0002-9149(86)90378-4. [DOI] [PubMed] [Google Scholar]
- 52.Johnson BD, Shaw LJ, Buchthal SD, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the national institutes of health-national heart, lung, and blood institute-sponsored women's ischemia syndrome evaluation (WISE) Circulation. 2004;109:2993–9. doi: 10.1161/01.CIR.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- 53.Stuart RJ, Jr, Ellestad MH. National survey of exercise stress testing facilities. Chest. 1980;77:94–7. doi: 10.1378/chest.77.1.94. [DOI] [PubMed] [Google Scholar]
- 54.Mathias W, Jr, Arruda A, Santos FC, et al. Safety of dobutamine-atropine stress echocardiography: a prospective experience of 4,033 consecutive studies. J Am Soc Echocardiogr. 1999;12:785–91. doi: 10.1016/s0894-7317(99)70182-3. [DOI] [PubMed] [Google Scholar]
- 55.Geleijnse ML, Fioretti PM, Roelandt JR. Methodology, feasibility, safety and diagnostic accuracy of dobutamine stress echocardiography. J Am Coll Cardiol. 1997;30:595–606. doi: 10.1016/s0735-1097(97)00206-4. [DOI] [PubMed] [Google Scholar]
- 56.Lette J, Tatum JL, Fraser S, et al. Safety of dipyridamole testing in 73,806 patients: The multicenter dipyridamole safety study. J Nucl Cardiol. 1995;2:3–17. doi: 10.1016/s1071-3581(05)80003-0. [DOI] [PubMed] [Google Scholar]
- 57.Einstein AJ, Moser KW, Thompson RC, et al. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116:1290–305. doi: 10.1161/CIRCULATIONAHA.107.688101. [DOI] [PubMed] [Google Scholar]
- 58.Health risks from exposure to low levels of ionizing radiation: BEIR VII – phase 2. National Academy of Sciences; Washington DC, USA: 2006. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, National Research Council. [PubMed] [Google Scholar]
- 59.Noto TJ, Jr, Johnson LW, Krone R, et al. Cardiac catheterization 1990: A report of the registry of the society for cardiac angiography and interventions (SCA&I) Cathet Cardiovasc Diagn. 199;24:75–83. doi: 10.1002/ccd.1810240202. [DOI] [PubMed] [Google Scholar]
- 60.Mortele KJ, Oliva MR, Ondategui S, et al. Universal use of nonionic iodinated contrast medium for CT: Evaluation of safety in a large urban teaching hospital. AJR Am J Roentgenol. 2005;184:31–4. doi: 10.2214/ajr.184.1.01840031. [DOI] [PubMed] [Google Scholar]
- 61.Mahabadi AA, Achenbach S, Burgstahler C, et al. Safety, efficacy, and indications of beta-adrenergic receptor blockade to reduce heart rate prior to coronary CT angiography. Radiology. 2010;257:614–23. doi: 10.1148/radiol.10100140. [DOI] [PubMed] [Google Scholar]
- 62.Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301:500–7. doi: 10.1001/jama.2009.54. [DOI] [PubMed] [Google Scholar]
- 63.Killeen RP, Cury RC, McErlean A, Dodd JD. Noncardiac findings on cardiac CT. part II: Spectrum of imaging findings. J Cardiovasc Comput Tomogr. 2009;3:361–71. doi: 10.1016/j.jcct.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 64.Hoffmann U, Bamberg F, Chae CU, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: The ROMICAT (rule out myocardial infarction using computer assisted tomography) trial. J Am Coll Cardiol. 2009;53:1642–50. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aberle DR, Adams AM, et al. National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Min JK, Gilmore A, Budoff MJ, et al. Cost-effectiveness of coronary CT angiography versus myocardial perfusion SPECT for evaluation of patients with chest pain and no known coronary artery disease. Radiology. 2010;254:801–8. doi: 10.1148/radiol.09090349. [DOI] [PubMed] [Google Scholar]
- 67.Douglas PS, Taylor A, Bild D, et al. Outcomes research in cardiovascular imaging: Report of a workshop sponsored by the national heart, lung, and blood institute. Circ Cardiovasc Imaging. 2009;2:339–48. doi: 10.1161/CIRCIMAGING.108.123999. [DOI] [PubMed] [Google Scholar]
- 68.Hachamovitch R, Hayes SW, Friedman JD, et al. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–7. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 69.Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the clinical outcomes utilizing revascularization and aggressive drug evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–91. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 70.Shaw LJ, Narula J. Coronary CT angiography: An established, not emerging, basis of diagnosis and risk stratification. JACC Cardiovasc Imaging. 2011;4:565–6. doi: 10.1016/j.jcmg.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 71.Arbab-Zadeh A, Hoe J. Quantification of coronary arterial stenoses by multidetector CT angiography in comparison with conventional angiography methods, caveats, and implications. JACC Cardiovasc Imaging. 2011;4:191–202. doi: 10.1016/j.jcmg.2010.10.011. [DOI] [PubMed] [Google Scholar]



