Cytokinesis in animal cells is achieved by a circumferential ring of differentiated cortex, enriched in actin and myosin, that contracts to divide the cytoplasm (reviewed in ref. 1). The placement of the contractile ring is specified by the mitotic apparatus such that contraction bisects the mitotic spindle, ensuring that division occurs between the separating chromosomes (reviewed in ref. 2). Experiments in which the influence of the mitotic apparatus (MA) on the cortex can be controlled, usually by ablation of the MA at various times, indicate that the signal from the MA to the cortex occurs between anaphase and telophase. Despite many years of experimentation, the nature of this signal remains elusive (2).
The paper by Rieder et al. in this issue of the Proceedings (3) follows mitosis in living PtK1 cells that contain two independent spindles in a common cytoplasm. The main focus of their work, which is well addressed in their paper, is to understand how unattached kinetochores inhibit the metaphase-to-anaphase transition. Their data also provide information on the mechanism of positioning the cleavage furrow. We will focus on this aspect of the work.
Much of what we know about cleavage furrow induction is derived from studies performed in marine invertebrate eggs. To address which part of the MA provides the cleavage stimulus, a now classic experiment was performed by Raymond Rappaport in sand dollar eggs (4). Rappaport generated donut shaped sand dollar eggs by pressing a glass sphere through them. The result, at second mitosis, was a horseshoe-shaped cell containing two spindles. As the cleavages bisecting the two spindles are completed, the asters in the resulting binucleate cell, which had never been joined by a spindle, move closer together, and another furrow appears between them (illustrated on p. 935 of ref. 5). This extra “Rappaport furrow” starts late but proceeds to completion, demonstrating that cleavage can occur between appropriately positioned asters not connected by a spindle or chromosomes.
Rappaport furrows have also been observed in eggs under other experimental conditions. Hiramoto observed Rappaport furrows in sea urchin eggs after selective removal of the central spindle with a micropipet, leaving the asters behind (6). Salmon and Wolniak also observed Rappaport furrows in sea urchin embryos by using hydrostatic pressure to suppress the initiation of furrowing during the first division (7). The result is an egg containing two spindles that share a common cytoplasm. At cytokinesis, furrows initially form in the plane of the spindle equators, but a short time later furrows also form between adjacent spindle asters not connected by spindles, resulting in division of the egg directly into four cells. In the paper by Rieder et al. (3), Rappaport cleavage is observed in somatic tissue culture cells. Eight of 15 PtK1 cells containing two independent spindles in a common cytoplasm initiated cleavage between spindle poles derived from adjacent spindles at positions that did not have intervening chromosomes. The spindle poles between which cleavage was initiated could be separated by up to 60 μm. The fact that Rappaport cleavages can be observed in both large invertebrate eggs and mammalian tissue culture cells suggests that induction of cleavage may be mechanistically similar in these disparate systems.
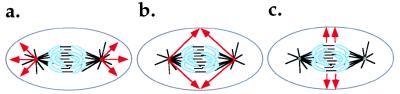
Three models have been proposed to explain how the MA signals the cortex to position the cleavage furrow (Fig. 1).
Figure 1.
Models to explain how the MA signals the cortex to determine the position of the cleavage furrow. (a) Astral relaxation. Signals from the astral centers induce relaxation of the cortex near the poles. (b) Equatorial stimulation by signals from the astral centers. Signals from the astral centers induce contraction of the equatorial cortex. (c) Equatorial stimulation by signals from the spindle midzone. Signals from the spindle midzone induce contraction of the equatorial cortex.
The first model, astral relaxation, is an extension by White and Borisy (8) of a model originally proposed by Wolpert (9) in which signals emanating from the asters of the MA induce relaxation of the cortex. If the signals from the asters are maximal at the poles and minimal at the equator (Fig. 1a), cortical relaxation would produce a differential in tension between the equator and the poles, resulting in equatorial contraction. One interesting component of White and Borisy’s model is the idea that the contractile elements that compose the cleavage furrow are laterally mobile in the plane of the cortex, an idea for which there is some experimental support (10–12). This assumption allows the initially broad tension gradient generated by astral relaxation to refine itself as contraction promotes the further alignment of tension-producing elements (8, 13). This concept of self-focusing gradients of tension and of the proteins that generate tension (myosin and actin) is useful beyond the confines of the polar relaxation model, and we will return to it later.
The second model, equatorial stimulation, proposes stimulation of the equatorial cortex by signals from the astral centers. In this model, championed largely by Rappaport (14, 15), the position of the cleavage furrow is determined by signals inducing contraction of the cortex that emanate from the asters. In this case, the signals from the asters are predicted to be maximal at the equator where the contractile ring will form (Fig. 1b).
Computer modeling has demonstrated that, depending on the precise relationship between the magnitude of the signal and the distance from the asters, cleavage could result from either signals that promote cortical contraction or cortical relaxation (16). In the absence of additional information on the nature or distribution of the signal, it has been impossible to rule out either of these models. However, the ability of a very small block placed between the spindle midzone and the cortex to inhibit furrowing in flattened echinoderm eggs seems inconsistent with the astral relaxation mechanism (17, 18). This result would also not necessarily be predicted if furrow induction is the result of stimulatory signals emanating from the astral centers but is, instead, most consistent with a model in which the origin of the signal is the spindle midzone.
The third model for cleavage furrow positioning is equatorial stimulation by signals from the spindle midzone. This model posits that the signal that stimulates the equatorial cortex emerges from the spindle midzone rather than the asters (Fig. 1c). This view, which we favor, was suggested initially by studies in grasshopper spermatocytes (19, 20), echinoderm eggs (21), and newt kidney epithelial cells (21). Recently, this model has gained support from studies in a number of systems, including mammalian tissue culture cells, amphibian eggs, and insect spermatocytes.
By analyzing where furrows form in perforated cells and in cells undergoing multipolar mitosis, Wang and coworkers (22, 23) concluded that a signal triggering cortical contraction is released from the spindle midzone coincident with anaphase onset in NRK (normal rat kidney) epithelial cells. Cleavage activity was correlated with the distribution of midzone microtubule bundles (23). Wang and coworkers’ data also argue against a signaling mechanism mediated by freely diffusible molecules, suggesting that the signal may migrate along an equatorially localized structure (22).
One interesting possibility for such a structure is that the spindle midzone elaborates a “telophase disc” that extends beyond the confines of the spindle until it comes in contact with the cortex, where it functions to determine the position of the cleavage furrow (24). There is good evidence for the existence of such a structure in several systems. In mammalian cells, bundles of antiparallel microtubules form in the spindle midzone during anaphase (25). A number of proteins are known to localize to an amorphous deposit of electron dense material that is associated with these bundles. These proteins include the kinesin CHO-1/MKLP-1 as well as a number of proteins (including TD-60, CENP-E, CENP-F, and INCENP) that have been termed “chromosomal passengers” because they appear to be carried to the metaphase plate by association with the chromosomes (26). The localization of one of these proteins, TD-60, extends beyond the region of the spindle to the cell cortex during late anaphase (24, 27). Where it has been examined, the presence of the telophase disc (as assayed by TD-60 staining) has been shown to correlate with the ability to initiate cleavage (23, 27). Earnshaw and coworkers have put forward a model (reviewed in ref. 26) in which alignment of the chromosomes at the metaphase plate brings the chromosomal passenger proteins to the spindle midzone, facilitating the formation of the telophase disc when these proteins dissociate from the chromosomes during anaphase. However, chromosomes are not needed in all cells because, during insect meiosis, apparently normal positioning of the cleavage furrow can be signaled by spindles from which all the chromosomes have been removed (28). There is also strong evidence in this system for a nondiffusible signal emanating from the spindle midzone (D. Zhang and B. Nicklas, personal communication) so it will be interesting to ask if components of the telophase disc that are normally passengers on chromosomes can also be positioned by other mechanisms.
A signal originating from the spindle midzone has also been invoked to explain cleavage in amphibian eggs. In these eggs, coincident with deposition of the cleavage signal at the cortex, a large telophase disc (the diastema) appears as a differentially stained zone of cytoplasm that bisects the MA (29). In this system, a narrow band of equatorial cytoplasm underlying the future site of the cleavage furrow has been found to contain a factor that can induce ectopic furrow formation when transplanted to other parts of the surface not in the equatorial plane (30).
Is it possible to reconcile the formation of a Rappaport furrow (between two asters not connected by a spindle) with a model in which the source of the signal is the spindle midzone/telophase disc? Until now, proponents of the idea that the cleavage stimulus arises from the spindle midzone have tended to argue that the mechanism for signaling furrow position may simply differ between large eggs and somatic cells. However, the data of Rieder et al. (3) clearly show that classic Rappaport furrows can be generated in tissue culture cells in the appropriate experimental context. We can see two ways of resolving this issue that should be amenable to experimental testing using the Rieder et al. assay (3).
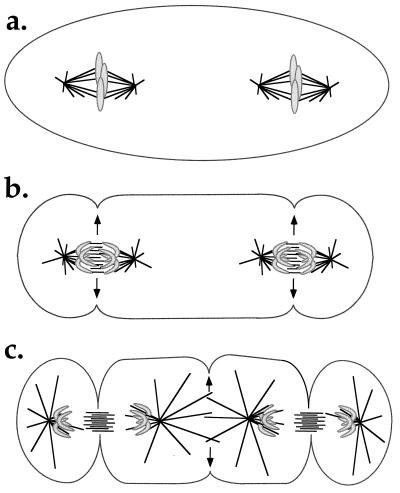
The first possibility is that signals for furrow positioning are always generated by spindle midzone-like structures, for example, overlapping antiparallel microtubule arrays. If this idea is correct, then such arrays must also be transiently generated between the two apposed asters during anaphase in the Rieder et al. (3) experimental situation (Fig. 2), and also in the original Rappaport experiments in manipulated marine eggs. Rieder et al. (3) showed that no microtubule overlap between the two spindles exists in metaphase, but they did not look in anaphase. Because astral microtubules are known to rapidly elongate during late anaphase, presumably in response to declining levels of mitotic kinases (31, 32), formation of overlap microtubule structures between the spindles at anaphase/telophase is a real, and experimentally testable, possibility.
Figure 2.
Model for induction of a Rappaport furrow directly by spindle midzone components. (a) At metaphase the two spindles are separate. (b) At anaphase the spindle midzones signal to the cortex (arrows), initiating formation of the two primary furrows. (c) As the spindles elongate and the astral microtubules grow out, a new zone of microtubule overlap is created in the center of the cell. This new midzone signals to the cortex (arrows) initiating the Rappaport furrow. This model predicts the formation of overlap microtubule structures in the cell center in the Rieder et al. experiment (3) that could be detected by tubulin staining of cells fixed in late anaphase/telophase.
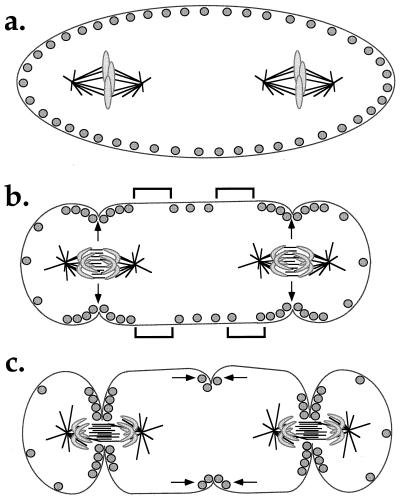
Alternatively, multiple overlapping mechanisms, which collectively insure the correct positioning of the cleavage furrow, may exist within a single cell type. We find the cumulative evidence supporting signaling from the spindle midzone highly persuasive, although the true nature of the telophase disc and the extent to which the signal depends on components normally brought to the midzone on chromosomes, are less clear. If Rappaport furrows form in the absence of any midzone components, or overlapping antiparallel microtubules, then we would suspect that an alternative mechanism is coming into play. The White and Borisy idea (8) that signals induce mobile contractile elements to form self-sharpening gradients of both molecules (myosin and actin) and tension is an attractive addition to any model for furrow positioning. It is mechanistically plausible, and it helps turn an initially biased signal into a single, defined furrow. If we apply this idea to the Rieder et al. (and Rappaport) experimental situations (3, 4) and assume primary signaling by the spindle midzone, an interesting consequence arises. The two spindles sending local, positive signals induce the formation of furrows that bisect them (Fig. 3a). White/Borisy focusing leads to the depletion of contractile elements distal to these furrows. If the self-focusing mechanism has a distance dependence, these depletions may, in turn, lead to a locally high concentration of contractile elements in the middle of the cell (Fig. 3b). Self-focusing of this region by the same mechanism would lead to a Rappaport furrow between the two spindles (Fig. 3c). This model predicts that formation of the Rappaport furrow would be delayed relative to formation of the two furrows that bisect the spindles and that it might be somewhat weaker or more transient. This is indeed what is observed in both the Rieder et al. (3) and original Rappaport experiments (4).
Figure 3.
Model for induction of a Rappaport furrow indirectly as a result of local depletion of contractile elements near the primary furrows, followed by White/Borisy focusing. (a) In metaphase the contractile elements (circles) are uniformly distributed in the cortex. These elements presumably include myosin-II filaments and actin filaments. (b) At anaphase, the spindle midzones signal as in Fig. 1 (arrows), leading to local enrichment of contractile elements and primary furrow assembly. As a consequence, regions of cortex depleted in contractile elements are generated next to the furrows (denoted by brackets). (c) The gradient of contractile elements in the mid-region of the cell self-focuses by the White/Borisy mechanism (arrows), leading to assembly of a Rappaport furrow. This model predicts the absence of overlap microtubules, and of proteins normally associated with midzone signaling, in the cell center in the Rieder et al. experiment (3). Myosin and actin are predicted to be enriched in the Rappaport furrow, along with other proteins that have a role in furrow function.
If the Rappaport furrow is in fact produced by White/Borisy focusing secondary to normal furrow assembly, what might we expect to see by cytology in the Rieder et al. experiment (3)? We would expect overlap microtubules to be absent and myosin and actin to be enriched in the Rappaport furrow. More interesting would be the distribution of other components. Those proteins hypothesized to be involved strictly in spindle midzone signaling, such as TD-60 and INCENP, should be absent. The localization of other furrow-enriched proteins such as anillin (33) and the septins (reviewed in ref. 34) is more difficult to predict because we do not know if they are involved primarily in signaling, or more directly in assembly and function of the contractile apparatus. Asking whether these components localize to Rappaport furrows might tell us more about the function of these proteins and the mechanism of furrow assembly.
Unfortunately, none of these cytological observations would conclusively distinguish whether the Rappaport furrow was generated by White/Borisy focusing secondary to normal furrow assembly elsewhere versus the older models in which signals emanating from the asters directly affect the cortex. The best way of distinguishing these models might be to artificially generate tension gradients in anaphase cells with no asters and ask if they self-focus into bona fide furrows. Local application of myosin inhibitors to the cortex of enucleated, activated Xenopus eggs, at the appropriate cell cycle stage, might provide such an experimental approach.
Note Added in Proof
Eckley et al. (35) have recently observed a low frequency of furrowing between poles not linked by chromosomes in human cells containing tripolar spindles. The low incidence of furrowing they observe between poles not connected by chromosomes supports the work of Wheatley and Wang (23) in rodent cells, in which furrowing in cells containing tripolar spindles was observed primarily between poles connected by chromosomes. In both cases, the authors conclude that the lack or low incidence of Rappaport furrows suggests that the mechanisms for furrow positioning in tissue culture cells and in echinoderm eggs must be distinct. However, another possibility is that the geometry of the Rieder et al. (3) and original Rappaport (4) experiments, in which two independent spindles are present in a common cytoplasm, is more conducive to the formation of Rappaport furrows than the geometry in the Eckley et al. (35) and Wheatley and Wang (23) experiments in cells containing tripolar spindles.
References
- 1.Satterwhite L L, Pollard T D. Curr Opin Cell Biol. 1992;4:43–52. doi: 10.1016/0955-0674(92)90057-j. [DOI] [PubMed] [Google Scholar]
- 2.Rappaport R. Cytokinesis in Animal Cells. Cambridge, U.K.: Cambridge Univ. Press; 1996. [Google Scholar]
- 3.Rieder C L, Khodjakov A, Paliulis L V, Fortier T M, Cole R W, Sluder G. Proc Natl Acad Sci USA. 1997;94:5107–5112. doi: 10.1073/pnas.94.10.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rappaport R. J Exp Zool. 1961;148:81–89. doi: 10.1002/jez.1401480107. [DOI] [PubMed] [Google Scholar]
- 5.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J D. Molecular Biology of the Cell. New York: Garland; 1994. [Google Scholar]
- 6.Hiramoto Y. Exp Cell Res. 1971;68:291–298. doi: 10.1016/0014-4827(71)90153-4. [DOI] [PubMed] [Google Scholar]
- 7.Salmon E D, Wolniak S M. Ann NY Acad Sci. 1990;582:88–98. doi: 10.1111/j.1749-6632.1990.tb21670.x. [DOI] [PubMed] [Google Scholar]
- 8.White J G, Borisy G G. J Theor Biol. 1983;101:289–316. doi: 10.1016/0022-5193(83)90342-9. [DOI] [PubMed] [Google Scholar]
- 9.Wolpert L. Int Rev Cytol. 1960;10:163–216. [Google Scholar]
- 10.Rappaport R. J Exp Zool. 1985;234:167–171. doi: 10.1002/jez.1402340120. [DOI] [PubMed] [Google Scholar]
- 11.Cao L, Wang Y. J Cell Biol. 1990;111:1905–1911. doi: 10.1083/jcb.111.5.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y L, Silverman J D, Cao L G. J Cell Biol. 1994;127:963–971. doi: 10.1083/jcb.127.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White J G. BioEssays. 1985;2:267–272. [Google Scholar]
- 14.Rappaport R. Int Rev Cytol. 1986;105:245–281. doi: 10.1016/s0074-7696(08)61065-7. [DOI] [PubMed] [Google Scholar]
- 15.Devore J J, Conrad G W, Rappaport R. J Cell Biol. 1989;109:2225–2232. doi: 10.1083/jcb.109.5.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris A K, Gewalt S L. J Cell Biol. 1989;109:2215–2223. doi: 10.1083/jcb.109.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rappaport R. Embryologia. 1968;10:115–130. doi: 10.1111/j.1440-169x.1968.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 18.Rappaport R, Rappaport B. J Exp Zool. 1983;227:213–227. [Google Scholar]
- 19.Ris H. Biol Bull (Woods Hole, Mass) 1949;96:90–106. [PubMed] [Google Scholar]
- 20.Kawamura K. Exp Cell Res. 1977;106:127–137. doi: 10.1016/0014-4827(77)90249-x. [DOI] [PubMed] [Google Scholar]
- 21.Rappaport R, Rappaport B N. J Exp Zool. 1974;189:189–196. doi: 10.1002/jez.1401890206. [DOI] [PubMed] [Google Scholar]
- 22.Cao L G, Wang Y L. Mol Biol Cell. 1996;7:225–232. doi: 10.1091/mbc.7.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheatley S P, Wang Y. J Cell Biol. 1996;135:981–989. doi: 10.1083/jcb.135.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreassen P R, Palmer D K, Wener M H, Margolis R L. J Cell Sci. 1991;99:523–534. doi: 10.1242/jcs.99.3.523. [DOI] [PubMed] [Google Scholar]
- 25.Mastronarde D N, McDonald K L, Ding R, McIntosh J R. J Cell Biol. 1993;123:1475–1489. doi: 10.1083/jcb.123.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Earnshaw W C, Mackay A M. FASEB J. 1994;8:947–956. doi: 10.1096/fasebj.8.12.8088460. [DOI] [PubMed] [Google Scholar]
- 27.Martineau S N, Andreassen P R, Margolis R L. J Cell Biol. 1995;131:191–205. doi: 10.1083/jcb.131.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D, Nicklas R B. Nature (London) 1996;382:466–468. doi: 10.1038/382466a0. [DOI] [PubMed] [Google Scholar]
- 29.Sawai T, Yomota A. Ann NY Acad Sci. 1990;582:40–49. doi: 10.1111/j.1749-6632.1990.tb21666.x. [DOI] [PubMed] [Google Scholar]
- 30.Sawai T. J Cell Sci. 1972;11:543–556. doi: 10.1242/jcs.11.2.543. [DOI] [PubMed] [Google Scholar]
- 31.Verde F, Dogterom M, Stelzer E, Karsenti E, Leibler S. J Cell Biol. 1992;118:1097–1108. doi: 10.1083/jcb.118.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kellogg D R, Mitchison T J, Alberts B M. Development (Cambridge, UK) 1988;103:675–686. doi: 10.1242/dev.103.4.675. [DOI] [PubMed] [Google Scholar]
- 33.Field C M, Alberts B M. J Cell Biol. 1995;131:165–178. doi: 10.1083/jcb.131.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longtine M S, DeMarini D J, Valencik M L, Al-Awar O S, Fares H, De Virgilio C, Pringle J R. Curr Opin Cell Biol. 1996;8:106–119. doi: 10.1016/s0955-0674(96)80054-8. [DOI] [PubMed] [Google Scholar]
- 35.Eckley M D, Ainsztein A M, Mackay A M, Goldberg I G, Earnshaw W C. J Cell Biol. 1997;136:1169–1183. doi: 10.1083/jcb.136.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]