Abstract
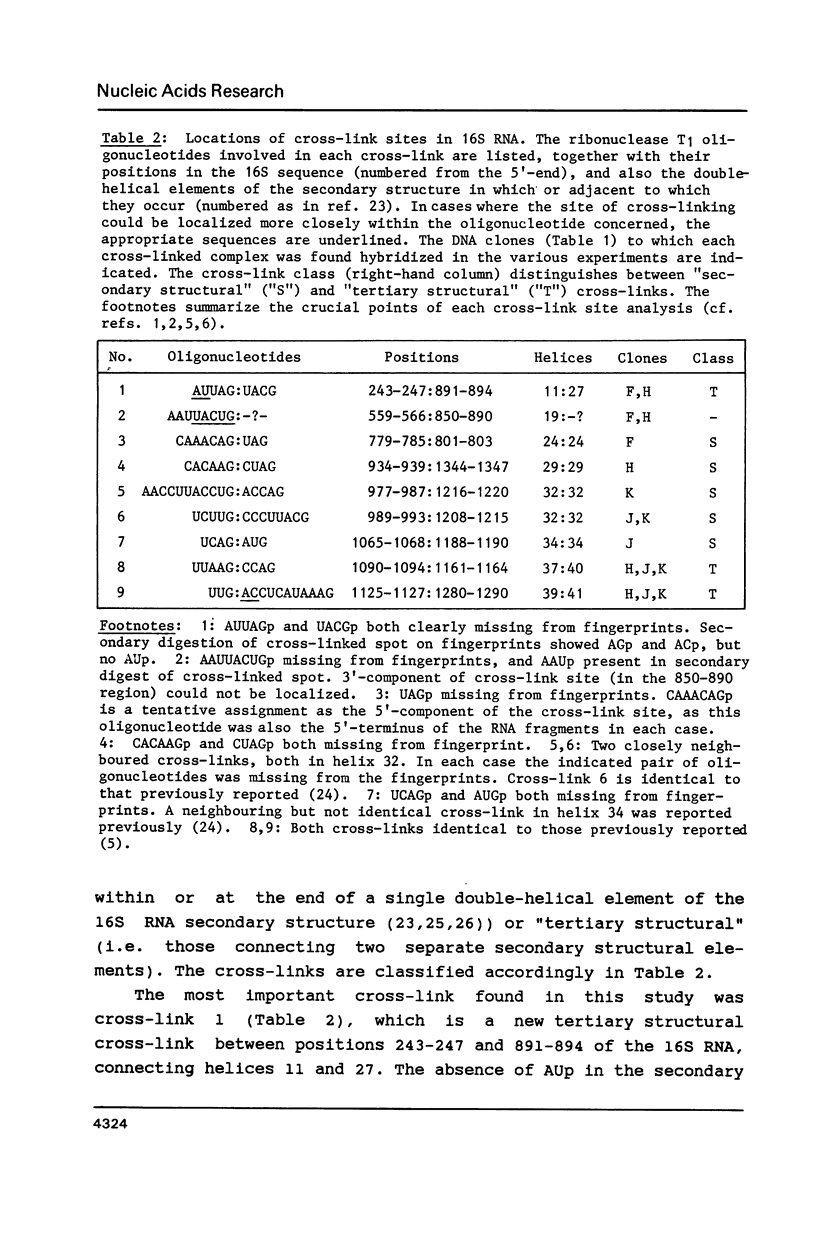
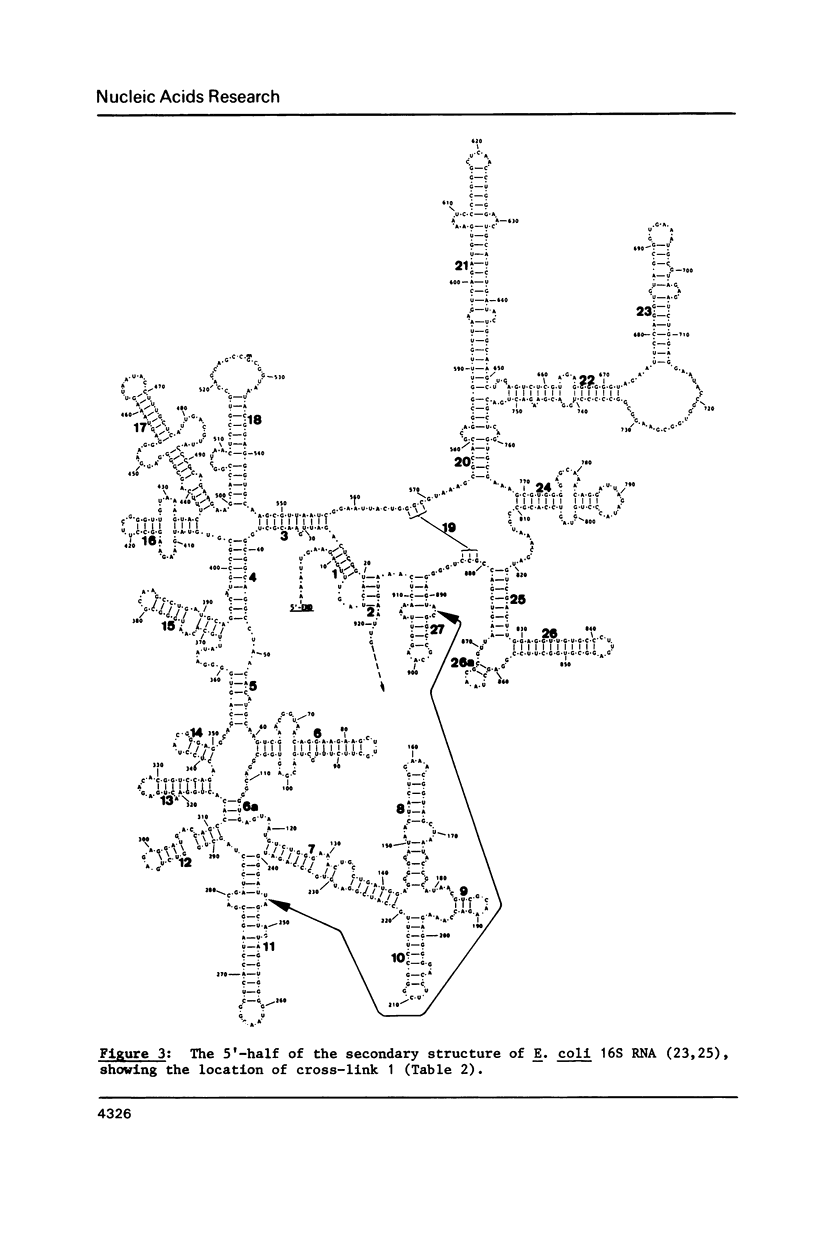
M13 clones were constructed with cDNA inserts corresponding to specific regions of E. coli ribosomal RNA. The DNA from the clones was immobilized by coupling to diazobenzyloxymethyl cellulose, and was used for the selective isolation by hybridization of cross-linked RNA complexes containing the complementary sequences. Immobilized DNA samples with inserts complementary to four different regions covering bases 735-1384 of the 16S RNA were hybridized with a mixture of 16S RNA fragments generated by partial digestion of 30S subunits that had been cross-linked by ultraviolet irradiation in vivo. After dehybridization, the individual RNA fragments and cross-linked complexes were separated by gel electrophoresis and analysed by our usual procedures. Nine cross-links are described; four of these are hitherto unobserved "secondary structural" cross-links, and one is a new "tertiary structural" cross-link between positions 243-247 and 891-894 of the 16S RNA.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Jovin T. M., Bähr W., Frischauf A. M., Marquardt M. Covalent attachment of DNA to agarose. Improved synthesis and use in affinity chromatography. Eur J Biochem. 1975 Jun;54(2):411–418. doi: 10.1111/j.1432-1033.1975.tb04151.x. [DOI] [PubMed] [Google Scholar]
- Atmadja J., Brimacombe R., Blöcker H., Frank R. Investigation of the tertiary folding of Escherichia coli 16S RNA by in situ intra-RNA cross-linking within 30S ribosomal subunits. Nucleic Acids Res. 1985 Oct 11;13(19):6919–6936. doi: 10.1093/nar/13.19.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmadja J., Stiege W., Zobawa M., Greuer B., Osswald M., Brimacombe R. The tertiary folding of Escherichia coli 16S RNA, as studied by in situ intra-RNA cross-linking of 30S ribosomal subunits with bis-(2-chloroethyl)-methylamine. Nucleic Acids Res. 1986 Jan 24;14(2):659–673. doi: 10.1093/nar/14.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
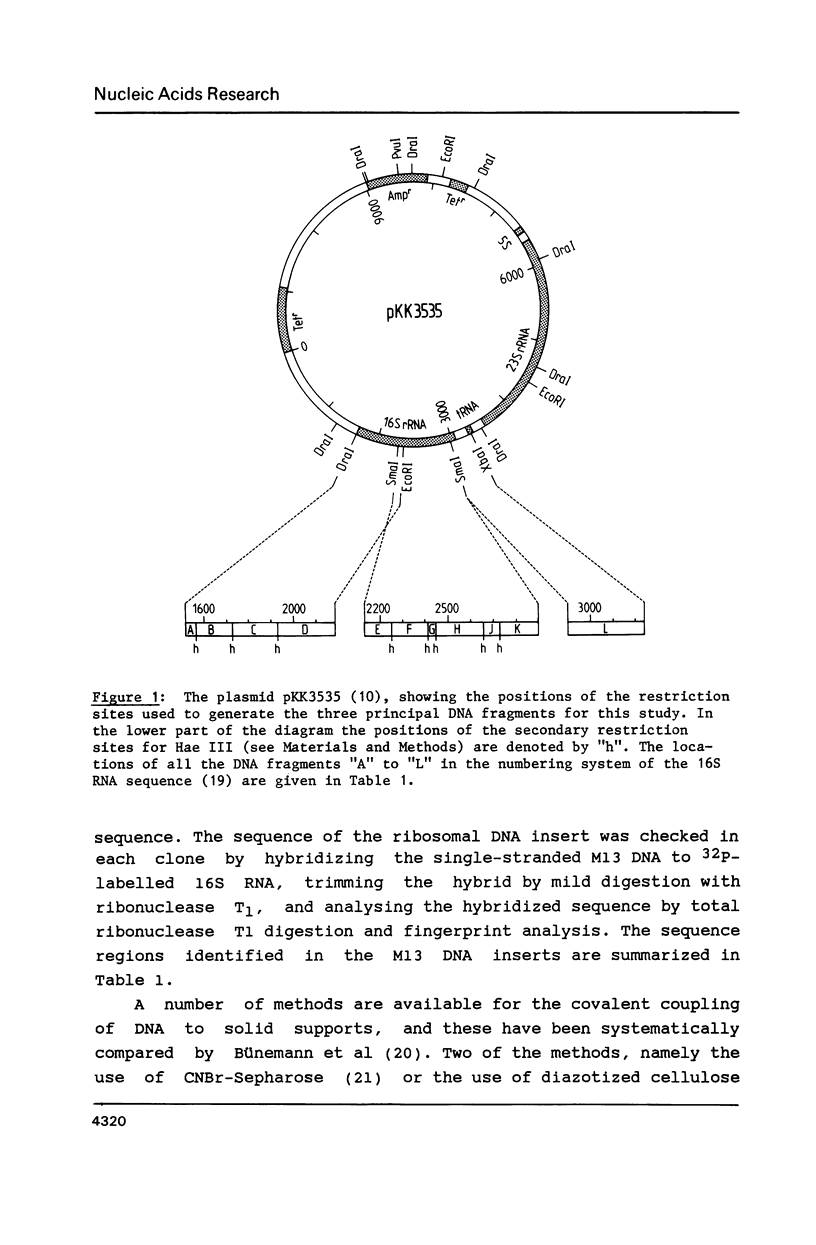
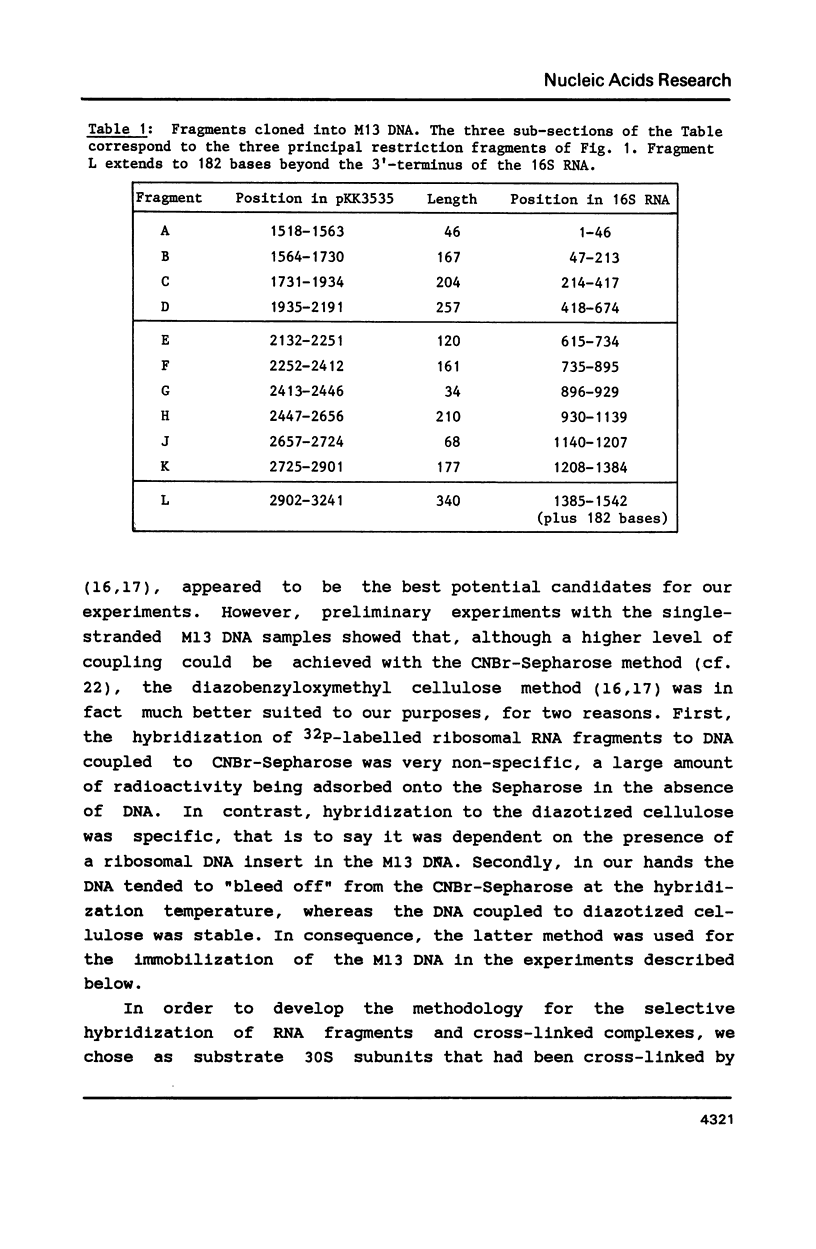
- Brimacombe R., Atmadja J., Stiege W., Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16 S ribosomal RNA in situ in the 30 S subunit. J Mol Biol. 1988 Jan 5;199(1):115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Ullrich A., Raker M. A., Gray A., Dull T. J., Gutell R. R., Noller H. F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981 Jul;6(1):112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Bünemann H. Immobilization of denatured DNA to macroporous supports: II. Steric and kinetic parameters of heterogeneous hybridization reactions. Nucleic Acids Res. 1982 Nov 25;10(22):7181–7196. doi: 10.1093/nar/10.22.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann H., Westhoff P., Herrmann R. G. Immobilization of denatured DNA to macroporous supports: I. Efficiency of different coupling procedures. Nucleic Acids Res. 1982 Nov 25;10(22):7163–7180. doi: 10.1093/nar/10.22.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaruttini C., Milet M., Hayes D. H., Expert-Bezancon A. Multiple crosslinks of proteins S7 and S9 to domains 3 and 4 of 16S ribosomal RNA in the Escherichia coli 30S particle. Eur J Biochem. 1986 Oct 15;160(2):363–370. doi: 10.1111/j.1432-1033.1986.tb09980.x. [DOI] [PubMed] [Google Scholar]
- Goldberg M. L., Lifton R. P., Stark G. R., Williams J. G. Isolation of specific RNA's using DNA covalently linked to diazobenzyloxymethyl cellulose or paper. Methods Enzymol. 1979;68:206–220. doi: 10.1016/0076-6879(79)68016-3. [DOI] [PubMed] [Google Scholar]
- Gronenborn B., Messing J. Methylation of single-stranded DNA in vitro introduces new restriction endonuclease cleavage sites. Nature. 1978 Mar 23;272(5651):375–377. doi: 10.1038/272375a0. [DOI] [PubMed] [Google Scholar]
- Jinks-Robertson S., Gourse R. L., Nomura M. Expression of rRNA and tRNA genes in Escherichia coli: evidence for feedback regulation by products of rRNA operons. Cell. 1983 Jul;33(3):865–876. doi: 10.1016/0092-8674(83)90029-6. [DOI] [PubMed] [Google Scholar]
- Maly P., Brimacombe R. Refined secondary structure models for the 16S and 23S ribosomal RNA of Escherichia coli. Nucleic Acids Res. 1983 Nov 11;11(21):7263–7286. doi: 10.1093/nar/11.21.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Noyes B. E., Stark G. R. Nucleic acid hybridization using DNA covalently coupled to cellulose. Cell. 1975 Jul;5(3):301–310. doi: 10.1016/0092-8674(75)90105-1. [DOI] [PubMed] [Google Scholar]
- Stiege W., Atmadja J., Zobawa M., Brimacombe R. Investigation of the tertiary folding of Escherichia coli ribosomal RNA by intra-RNA cross-linking in vivo. J Mol Biol. 1986 Sep 5;191(1):135–138. doi: 10.1016/0022-2836(86)90429-8. [DOI] [PubMed] [Google Scholar]
- Stiege W., Glotz C., Brimacombe R. Localisation of a series of intra-RNA cross-links in the secondary and tertiary structure of 23S RNA, induced by ultraviolet irradiation of Escherichia coli 50S ribosomal subunits. Nucleic Acids Res. 1983 Mar 25;11(6):1687–1706. doi: 10.1093/nar/11.6.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiege W., Zwieb C., Brimacombe R. Precise localisation of three intra-RNA cross-links in 23S RNA and one in 5S RNA, induced by treatment of Escherichia coli 50S ribosomal subunits with bis-(2-chloroethyl)-methylamine. Nucleic Acids Res. 1982 Nov 25;10(22):7211–7229. doi: 10.1093/nar/10.22.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. F., Hearst J. E. Structure of E. coli 16S RNA elucidated by psoralen crosslinking. Cell. 1983 Apr;32(4):1355–1365. doi: 10.1016/0092-8674(83)90316-1. [DOI] [PubMed] [Google Scholar]
- Vasilenko S. K., Ryte V. C. [Isolation of highly purified ribonuclease from cobra (Naja oxiana) venom]. Biokhimiia. 1975 May-Jun;40(3):578–583. [PubMed] [Google Scholar]
- Volckaert G., Fiers W. Micro thin-layer techniques for rapid sequence analysis of 32P-labeled RNA: double digestion and pancreatic ribonuclease analyses. Anal Biochem. 1977 Nov;83(1):228–239. doi: 10.1016/0003-2697(77)90531-0. [DOI] [PubMed] [Google Scholar]
- Wollenzien P. L., Murphy R. F., Cantor C. R., Expert-Bezançon A., Hayes D. H. Structure of the Escherichia coli 16 S ribosomal RNA. Psoralen crosslinks and N-acetyl-N'-(p-glyoxylylbenzoyl)cystamine crosslinks detected by electron microscopy. J Mol Biol. 1985 Jul 5;184(1):67–80. doi: 10.1016/0022-2836(85)90044-0. [DOI] [PubMed] [Google Scholar]
- Zwieb C., Brimacombe R. Localisation of a series of intra-RNA cross-links in 16S RNA, induced by ultraviolet irradiation of Escherichia coli 30S ribosomal subunits. Nucleic Acids Res. 1980 Jun 11;8(11):2397–2411. doi: 10.1093/nar/8.11.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]