Abstract
The development of a serological marker for early diagnosis of isocyanate-induced occupational asthma (isocyanate-OA) may improve clinical outcome. Our previous proteomic study found that expression of vitamin D-binding protein (VDBP) was upregulated in the patients with isocyanate-OA. In the present study, we evaluated the clinical relevance of VDBP as a serological marker in screening for isocyanate-OA among exposed workers and its role in the pathogenesis of isocyanate-OA. Three study groups including 61 patients with isocyanate-OA (group I), 180 asymptomatic exposed controls (AECs, group II), 58 unexposed healthy controls (NCs, group III) were enrolled in this study. The baseline serum VDBP level was significantly higher in group I compared with groups II and III. The sensitivity and specificity for predicting the phenotype of isocyanate-OA with VDBP were 69% and 81%, respectively. The group I subjects with high VDBP (≥ 311 µg/ml) had significantly lower PC20 methacholine levels than did subjects with low VDBP. The in vitro studies showed that TDI suppressed the uptake of VDBP into RLE-6TN cells, which was mediated by the downregulation of megalin, an endocytic receptor of the 25-hydroxycholecalciferol-VDBP complex. Furthermore, toluene diisocyanate (TDI) increased VEGF production and secretion from this epithelial cells by suppression of 1,25-dihydroxycholecalciferol [1,25(OH)2D3] production. The findings of this study suggest that the serum VDBP level may be used as a serological marker for the detection of isocyanate-OA among workers exposed to isocyanate. The TDI-induced VEGF production/secretion was reversed by 1,25(OH)2D3 treatment, which may provide a potential therapeutic strategy for patients with isocyanate-OA.
Keywords: asthma, occupational; biological markers; isocyanates; low density lipoprotein receptor-related protein-2; vascular endothelial growth factorA; vitamin D-binding protein
Introduction
Three major isocyanates (toluene diisocyanate, TDI; hexamethylene diisocyanate, HDI; and diphenylmethane diisocyanate, MDI) are highly reactive chemicals used in a variety of chemical manufacturing processes, such as production of polyurethane forms and paints. Isocyanate-induced occupational asthma (OA) is the most frequent cause of OA worldwide (Meredith and Nordman, 1996; Mannino, 2000), with a prevalence rate of around 10% of exposed workers (Park and Nahm, 1997). Although the specific inhalation challenge test with isocyanate is the gold standard for the confirmative diagnosis of isocyanate-OA, this procedure requires special equipment and is both time and labor intensive. Furthermore, study of the long-term prognosis has shown that more than 50% of patients with isocyanate-OA have persistent asthma symptoms even after complete avoidance of exposure and treatment with asthma medications (Park and Nahm, 1997). Therefore, early identification of patients may improve the long-term outcome. Previous studies focused on the detection of serum-specific antibodies, which were not sufficiently specific to be used for diagnostic purposes (Cartier et al., 1989; Park et al., 2002; Ye et al., 2006; Wisnewski, 2007; Palikhe et al., 2011).
More recently, proteomic analysis, a process that includes purifying and identifying individual proteins from body fluids, has been used to develop serological markers for diagnosis (Colantonio and Chan, 2005). The results of our previous study using proteomic analysis of patients with diphenyl-methane diisocyanate occupational asthma (MDI-OA) showed upregulation of vitamin D-binding protein (VDBP) expression (Hur et al., 2008a). There has been much interest in the role of the vitamin D axis in lung disease such as asthma, chronic obstructive pulmonary disease and tuberculosis (Devereux et al., 2009; Janssens et al., 2009; Wilkinson and Lange, 2009; Chishimba et al., 2010), which includes vitamin D and VDBP. Various cytokines, cellular elements, oxidative stress and protease/antiprotease levels affect lung fibroproliferation, remodeling and function, which may be influenced by vitamin D level (Gilbert et al., 2009). Moreover, several previous studies have suggested the active involvement of VEGF in the pathogenesis of isocyanate-OA, which may be mediated by 1,25-dihydroxycholecalciferol [1,25(OH)2D3], the active form of vitamin D (Nakagawa et al., 2005; Gruber et al., 2008). VEGF (Nakagawa et al., 2005; Gruber et al., 2008), a potent proangiogenic cytokine, plays a central role in angiogenesis, chronic airway inflammation, and airway remodeling in asthma (Voelkel et al., 2006; Walters et al., 2008).
In the present study, the clinical relevance of the serum VDBP level was investigated as a potential candidate marker for screening susceptible subjects exposed to isocyanate. In addition, the mechanism associated with serum VDBP levels and isocyanate exposure was studied.
Results
Clinical characteristics of the study subjects
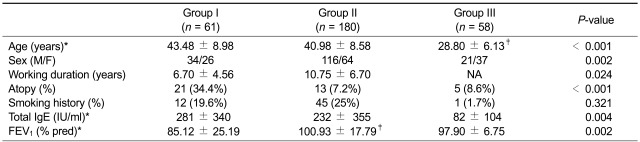
Sixty-one patients with isocyanate-OA (group I), confirmed by positive responses to isocyanate bronchial challenge test, and 180 asymptomatic exposed workers (AECs, group II) from same working environment, including spray-painting and polishing departments of the furniture and musical instrument industries or working in a car upholstery factory and a control group, 58 unexposed healthy controls (NCs, group III) were enrolled in this study. The clinical features of the three study groups are summarized in Table 1. Atopy was determined by a positive skin test to at least one common inhalant allergen, including house dust mites, Alternaria, cat fur, dog fur, a tree pollen mixture, a grass pollen mixture, and mugwort and ragweed pollens (Bencard, Bradford, UK). Serum samples from all study subjects were collected at initial examination; all subjects stopped using inhaled or oral steroids for 4 weeks before the study and underwent an interview, chest radiography, and skin prick test. In addition, five patients with isocyanate-OA and seven with AECs had additional blood sampling; the changes in the serum VDBP were observed before and after the isocyanate bronchial challenge tests. This study was reviewed and approved by the Institutional Review Board of Ajou Medical Center, Suwon, Korea, and all subjects gave written informed consent prior to enrollment in the study.
Table 1.
Clinical characteristics of the subjects in the three study groups
Group I, isocyanate-induced occupational asthma; group II, asymptomatic exposed control; group III, unexposed healthy controls; NA, not available.
*Values are expressed as means ± SD, †P < 0.05, ‡P < 0.05 (group I vs. group II, or group III). Statistical significance of differences was determined by ANOVA with Bonferroni's correction.
Comparison of serum VDBP levels in the three study groups
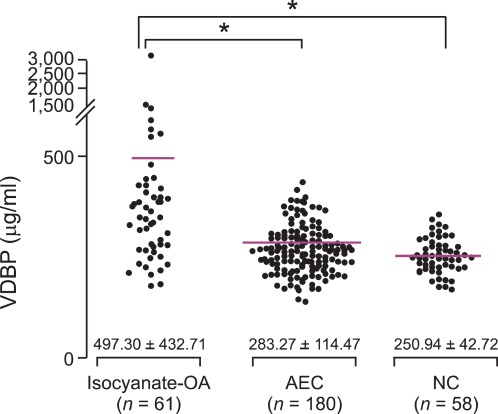
To validate the proteins identified by previous proteomic analysis, we compared baseline VDBP levels in the three study groups. Baseline serum levels of VDBP were compared among the three study groups, and the level was significantly higher in group I compared with groups II and III (497.30 ± 432.71 µg/ml, 283.27 ± 114.47 µg/ml, 250.94 ± 42.72 µg/ml respectively, P < 0.001); this remained significant after adjusting for age and gender (P < 0.001), as shown in Figure 1.
Figure 1.
Comparison of serum vitamin D-binding protein (VDBP) levels in the three study groups. group I, isocyanate induced occupational asthma (isocyanate-IA); group II, asymptomatic exposed controls (AEC); group III, unexposed healthy controls (NC). The line indicates the mean value for each group. *P < 0.001.
Determination of the optimal cutoff levels for serum VDBP
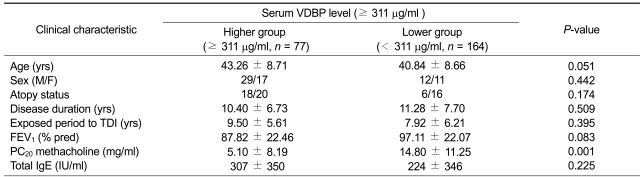
To evaluate the diagnostic value of the serum VDBP level for discriminating between group I and II, a receiver operating characteristic (ROC) curve analysis was performed. An optimal cutoff value was selected from the ROC curve to obtain the highest sensitivity and specificity. When the cutoff for VDBP was selected as ≥ 311 µg/ml, the sensitivity and specificity were 69% and 81%, respectively, with 0.765 [95% confidence interval (CI), 0.688-0.843, P < 0.001)] as the AUC, with a 55% positive predictive value and an 88% negative predictive value.
When the clinical parameters were compared according to the serum VDBP level in group I and II subjects, the subjects with a high serum VDBP level ≥ 311 µg/ml had significantly lower PC20 methacholine levels (P = 0.001). The baseline FEV1 level (percent predicted value) tended to be lower in subjects with high serum VDBP levels, although statistical significance was not reached. No significant associations were noted between the serum VDBP level and other demographic and clinical parameters, such as age, gender, atopy status, total IgE level, and duration of working (Table 2).
Table 2.
Comparison of the clinical characteristics between positive and negative groups among the isocyanate-exposed subjects
Isocyanate-exposed subjects were divided into two groups according to the cutoff value of serum VDBP level: positive (≥ 11 µg/ml) and negative (<311 µg/ml) groups. Statistical significance was evaluated by Student's t-test.
Suppression of FITC-VDBP uptake into RLE-6TN cells by toluene diisocyanate (TDI)
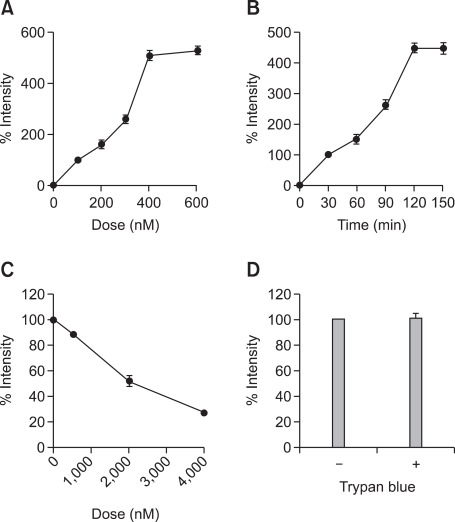
To examine whether TDI exposure regulates VDBP uptake into RLE-6TN cells, in a rat alveolar epithelial cell line, the characteristics of FITC-VDBP uptake were determined using immunofluorescence analysis as described in the Materials and Methods section. The RLE-6TN cells were incubated in the absence or presence of FITC-VDBP at the indicated doses for 2 h. The results showed a dose-dependent increase in fluorescence intensity, which approached saturation at about 400 nM (Figure 2A). The RLE-6TN cells were incubated with 400 nM FITC-VDBP for the indicated times, and the fluorescence intensity increased in a time-dependent manner, reaching saturation at 120 min (Figure 2B). Therefore, these conditions were used for further studies. To evaluate the effects of native VDBP on FITC-VDBP uptake into RLE-6TN cells, the cells were preincubated with 0.5, 2, or 4 µM native VDBP for 30 min and then incubated with 400 nM of FITC-VDBP for 2 h. The uptake of FITC-VDBP was suppressed up to 88%, 51%, and 27%, respectively, compared with the untreated controls (Figure 2C). In addition, we performed a trypan blue quenching assay to determine whether the measured fluorescence intensity was due to a nonspecific membrane-bound protein. As shown in Figure 2D, when the RLE-6TN cells were treated with or without trypan blue, no significant changes were observed in the fluorescence intensities, suggesting that this FITC-VDBP uptake assay was highly specific.
Figure 2.
Characteristics of FITC-VDBP uptake. RLE-6TN cells were incubated in serum-free medium at 37℃ for 2 h with FITC-VDBP over a concentration range of 100-600 nM (A), and for a range of times from 30 to 150 min with 400 nM FITC-VDBP (B). Serum-starved RLE-6TN cells were preincubated with a range of doses of 0.5-4 µM native VDBP for 30 min and then incubated with 400 nM FITC-VDBP at 37℃ for 2 h (C). RLE-6TN cells were incubated in serum-free medium at 37℃ for 2 h with 400 nM FITC-VDBP. The cells were then washed with PBS and treated with 1.2 mg/ml trypan blue. Then, FITC intensity was quantified by immunofluorescence assay as described in the Materials and Methods section. Fluorescence intensities of the cells were counted using an image browser, and data are presented as the percent intensity (D). All data are representative of three independent experiments. Values represent the means ± SEM.
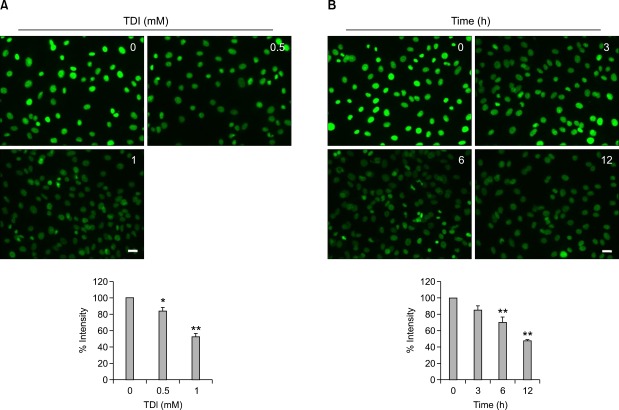
When the RLE-6TN cells were incubated with 0.5 and 1 mM TDI for 12 h, the FITC-VDBP uptake was decreased by 84.1% and 52.2%, respectively, compared with dimethyl sulfoxide (DMSO)-treated controls (Figure 3A). Similarly, when the RLE-6TN cells were incubated with 1 mM TDI for 3, 6, or 12 h, its uptake was decreased by 84.9%, 74.3%, or 47.6%, respectively, compared with the DMSO-treated controls (Figure 3B), indicating that TDI exposure inhibited VDBP uptake into the RLE-6TN cells in a time- and dose-dependent manner. There was no cytotoxicity associated with TDI under the present experimental conditions (data not shown).
Figure 3.
Time- and dose-dependent effects of toluene diisocyanate (TDI) on FITC-VDBP uptake in RLE-6TN cells. RLE-6TN cells were preincubated with 1 mM TDI for the indicated times and then incubated in serum-free medium with 400 nM FITC-VDBP at 37℃ for 2 h (A). RLE-6TN cells were preincubated with the indicated doses of TDI for 6 h and then incubated in serum-free medium with 400 nM FITC-VDBP at 37℃ for 2 h. Fluorescence was examined using a fluorescence microscope. Scale bar, 20 µm. In the lower panel, fluorescent intensities of the cells were counted using an image browser, and data are presented as percent intensity (B). All data are representative of three independent experiments. Values represent the means ± SEM. *P < 0.05, **P < 0.001 vs. control.
Downregulation of megalin mRNA and protein by TDI treatment in RLE-6TN cells
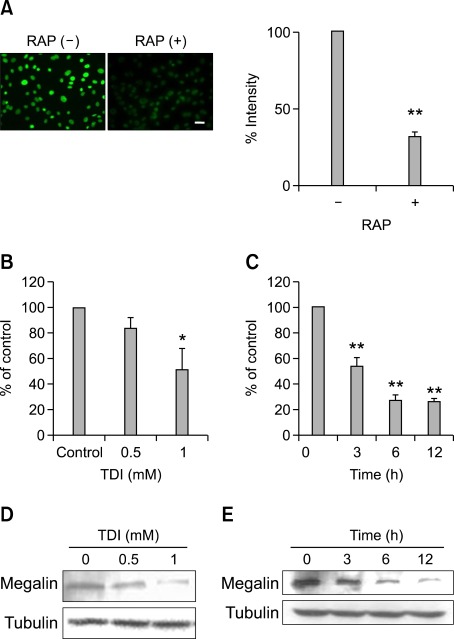
VDBP as a form of the 25-hydroxycholecalciferol [25(OH)D3]-VDBP complex is taken up into target cells by megalin (Nykjaer et al., 1999). To confirm the importance of megalin in VDBP uptake into the RLE-6TN cells, the RLE-6TN cells were preincubated with receptor-associated protein, a known inhibitor of megalin-mediated endocytosis. The uptake of FITC-VDBP was markedly blunted by up to 60% compared with the untreated controls (Figure 4A), suggesting that megalin mediated endocytic uptake of FITC-VDBP into RLE-6TN cells. Real-time PCR analysis was performed to examine the regulatory effect of TDI on the level of megalin expression. When RLE-6TN cells were incubated with 0.5 and 1 mM TDI for 3 h, the level of megalin mRNA was decreased to 83.8% and 53.3% of the control, respectively (Figure 4B). When RLE-6TN cells were incubated with 1 mM TDI for 3, 6, or 9 h, megalin mRNA level was decreased to 53%, 27.3%, or 26% of the control, respectively (Figure 4C). In addition, Western blotting analysis indicated that megalin expression was downregulated by TDI treatment (Figures 4D and 4E).
Figure 4.
Effects of TDI on megalin expression. RLE-6TN cells were preincubated in serum-free medium with or without 1 µM receptor-associated protein (RAP) for 30 min and then incubated with 400 nM FITC-VDBP at 37℃ for 2 h. The fluorescence was observed using a fluorescence microscope. Scale bar, 20 µm. Fluorescence intensities of the cells were counted using an image browser, and data are presented as percent intensity (A). RLE-6TN cells were incubated with the indicated doses of TDI for 3 h, and then real-time PCR was performed (B). RLE-6TN cells were incubated with 1 mM TDI for the indicated times, and then real-time PCR was performed. The values are normalized relative to the GAPDH standard (C). RLE-6TN cells were incubated with the indicated doses of TDI for 12 h (D). RLE-6TN cells were incubated with 1 mM TDI for the indicated times, and then Western blotting analysis for megalin was performed. Tubulin was used as a loading control (E). All data are representative of three independent experiments. Values represent the means ± SEM. *P < 0.05, **P < 0.001 vs. control.
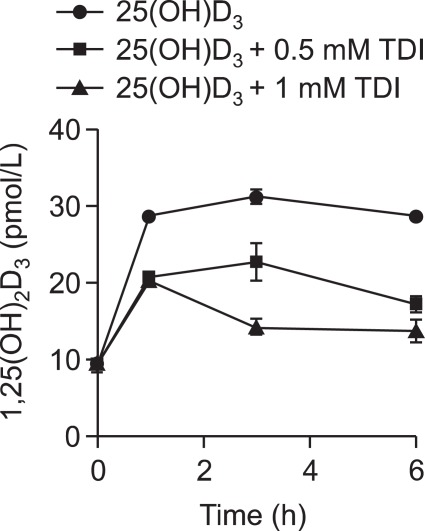
To evaluate whether TDI inhibits 25(OH)D3 uptake by downregulating megalin expression, RLE-6TN cells were incubated in the absence or presence of TDI (0.5-1 mM) for 6 h before incubation with 1 µM 25(OH)D3 for 6 h. The level of 1,25-dihydroxycholecalciferol [1,25(OH)2D3] production was decreased in a dose-dependent manner, as shown in Figure 5. These observations suggested that 1,25(OH)2D3 mediated its inhibitory action on VEGF secretion via an autocrine loop and that TDI inhibited the intracellular production of 1,25(OH)2D3 by inhibiting 25(OH)D3 uptake by downregulating megalin expression.
Figure 5.
Effects of toluene diisocyanate (TDI) on 1,25(OH)2D3 production. RLE-6TN cells were preincubated with the indicated doses of TDI for 6 h and then incubated with 1 µM 25(OH)D3 for 1, 3, or 6 h. 1,25(OH)2D3 in the supernatant was quantified using an enzyme immunoassay kit. Data are representative of two independent experiments. Values represent the means ± SEM.
Increased VEGF production and secretion through suppression of 1,25(OH)2D3 production
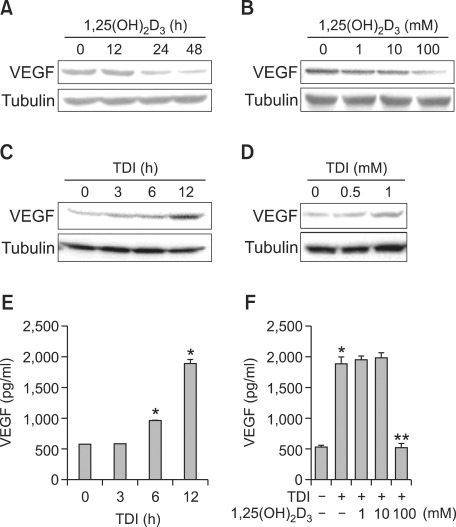
The production of 1,25(OH)2D3 has been associated with VEGF production and secretion (Nakagawa et al., 2005; Gruber et al., 2008). To examine the possibility that TDI regulates VEGF production and secretion by regulating 1,25(OH)2D3 production in RLE-6TN cells, the effects of 1,25(OH)2D3 on VEGF production were investigated by Western blotting analysis. The cells were incubated with 100 nM 1,25(OH)2D3 for 12, 24, or 48 h, and the resultant expression levels of VEGF were 100%, 52%, and 45%, respectively, compared with the controls (Figure 6A). In addition, when RLE-6TN cells were incubated with 1, 10, or 100 nM 1,25(OH)2D3 for 24 h, the resultant VEGF expression levels were 98%, 89%, and 58%, respectively, compared with the controls (Figure 6B). These results suggest that 1,25(OH)2D3 inhibited VEGF production in the RLE-6TN cells. Next, when RLE-6TN cells were incubated with 1 mM TDI for 3, 6, or 12 h, the expression level of VEGF was increased by 40%, 66%, and 180%, respectively, compared with the control (Figure 6C). When the RLE-6TN cells were incubated with 0.5 and 1 mM TDI for 12 h, the level of VEGF expression was increased by 54% and 210%, respectively, compared with the control (Figure 6D). Similar effects of TDI on VEGF secretion were observed on ELISA (Figures 6E and 6F). Furthermore, when the cells were preincubated with 1,25(OH)2D3 for 24 h before treatment with 1 mM TDI for 12 h, VEGF secretion was completely rescued by 100 nM 1,25(OH)2D3 (Figure 6F). These findings suggested that TDI exposure increased VEGF production and secretion in RLE-6TN cells by suppression of 1,25(OH)2D3 production.
Figure 6.
Effects of toluene diisocyanate (TDI) and 1,25(OH)2D3 on VEGF production and secretion. RLE-6TN cells were incubated with 100 nM 1,25(OH)2D3 alone for the indicated times (A). RLE-6TN cells were incubated with the indicated doses of 1,25(OH)2D3 alone for 24 h, and then Western blotting analysis for VEGF was performed. Tubulin was used as a loading control (B). RLE-6TN cells were incubated with 1 mM TDI for the indicated times (C). RLE-6TN cells were incubated with the indicated doses of TDI for 12 h, and then Western blotting analysis for VEGF was performed. Tubulin was used as a loading control (D). RLE-6TN cells were incubated with 1 mM TDI for 3, 6, or 12 h, and then the VEGF concentration in each supernatant was quantified using a rat ELISA kit (E). RLE-6TN cells were preincubated with the indicated doses of 1,25(OH)2D3 for 24 h and then incubated with or without 1 mM TDI for 12 h (F). All data are representative of three independent experiments. Values represent the means ± SEM. *P < 0.001 vs. TDI(-) and 1,25(OH)2D3(-); **P < 0.001 vs. TDI(+).
Discussion
VDBP, also known as Gc globulin or group-specific component, is a multifunctional serum protein. VDBP has been reported to have a variety of immunological functions, such as in the actin cleavage system, macrophage activation, and chemotaxis of neutrophils, monocytes, and fibroblasts (White and Cooke, 2000; Meier et al., 2006). However, its major function is as a carrier of 25(OH)D3, a major form of circulating vitamin D that is present in the form of the 25(OH)D3-VDBP complex. In our previous study, a proteomic approach with bronchoalveolar lavage fluid (BALF) was used as a screening tool to identify inflammatory mediators involved in the development of isocyanate-OA, where VDBP expression was upregulated in BALF of isocyanate-OA patients compared with AECs (Hur et al., 2008a). These findings were consistent with those of previous studies where VDBP was identified in the BALF of asthma patients by proteomic analysis (Noel-Georis et al., 2002; Wu et al., 2005). In the present study, the serum VDBP level was found to be significantly higher in patients with isocyanate-OA compared with controls. Moreover, more severe airway hyperresponsiveness to methacholine was observed in subjects with elevated serum VDBP levels than in those without elevated serum VDBP levels. In addition, the diagnostic value of the serum VDBP level showed significantly increased sensitivity for identifying isocyanate-OA; the sensitivity and specificity for discrimination of isocyanate-OA in exposed workers were 69% and 81%, respectively. These values were comparable to those of previous studies in which serum-specific antibodies including serum-specific IgE and IgG antibodies to TDI-human serum albumin (HAS) conjugate, specific IgG to cytokeratin 19, and specific IgE and IgG antibodies to MDI-HSA (Cartier et al., 1989; Choi et al., 2004a; Ye et al., 2006; Wisnewski, 2007; Hur et al., 2008b) were evaluated as potential serological markers for TDI- or MDI-OA. When we measured serum VDBP level in the sera of 45 patients with allergic asthma recruited from Ajou University Hospital, it showed a similar VDBP level to that of group II, but showed a significantly lower level than that of group I (data not shown), indicating that increased serum level of VDBP may be a specific finding for isocyanate-OA, not found in allergic asthma. In the previous study, we reported that the combined levels of serum ferritin and transferrin may be useful as markers for differentiating subjects with MDI-OA among MDI-exposed workers with 71.43% sensitivity and 85.71% specificity (Hur et al., 2008a). In this study, we found that measurement of serum VDBP level is a simple and reproducible method that can be used in larger cohorts of exposed workers with a commercial ELISA kit; it is less expensive to measure one protein than two proteins, i.e., ferritin and transferrin. Based on these findings, the serum VDBP level may be a useful serological marker for differentiating subjects with isocyanate-OA from AEC and for predicting the severity of airway hyperresponsiveness among patients with isocyanate-OA.
This is the first study to suggest the potential involvement of VDBP in the pathogenesis of isocyanate-OA. First, we focused on the function of VDBP as a major carrier protein of vitamin D, which is known to be associated with immune modulation of T helper 2 inflammation and an individual's susceptibility to asthma (Cantorna et al., 2004; Hughes and Norton, 2009). Previous studies demonstrated that the 25(OH)D3-VDBP complex moved compounds to target tissues that were taken up into cells; this process was mediated by an endocytic mechanism via megalin (Nykjaer et al., 1999, 2001; Rowling et al., 2006). Mice lacking megalin were found to develop vitamin D deficiency or rickets due to loss of VDBP and vitamin D metabolites (Dusso et al., 2005), indicating that megalin is a major endocytic receptor for the uptake of vitamin D. Once taken up into the target cells, 25(OH)D3 was dissociated from VDBP and metabolized to 1,25(OH)2D3. Megalin is expressed not only in the kidney cells (Nykjaer et al., 1999; Hosojima et al., 2009) but also in the alveolar epithelial cells (Lundgren et al., 1997). The results of the present study showed that TDI exposure suppresses VDBP and 25(OH)D3 uptake along with downregulating megalin expression in a dose- and time-dependent manner in RLE-6TN cells. These results suggest that TDI exposure may lead to higher VDBP levels in exposed tissue, such as BALF, in susceptible workers by suppressing megalin expression in lung alveolar cells.
It is postulated that VEGF could act as an angiogenic factor that is capable of altering subepithelial vascularity, which would increase pulmonary microvascular permeability, thus enhancing airway inflammation and remodeling in patients with TDI- and MDI-OA (Weyel and Schaffer, 1985; Mapp et al., 1988; Nabe et al., 2005). In addition, the VEGF level increased in the BALF after TDI challenge in a murine model of TDI-OA (Lee et al., 2002) and in the induced sputum of TDI-induced asthma patients. (Choi et al., 2004b) VEGF appears to play an important role, at least in part, in the pathogenesis of isocyanate-OA (Weyel and Schaffer, 1985; Mapp et al., 1988; Nabe et al., 2005). Recently, 1,25(OH)2D3 has been reported to have a negative regulatory effect on VEGF production and secretion (Nakagawa et al., 2005; Gruber et al., 2008). The mechanism by which 1,25(OH)2D3 regulates VEGF expression and secretion is currently unclear. However, a few mechanisms have been suggested. First, it may be related to the rapid induction of non-transcriptional responses, which may occur via activation of transmembrane signal-transduction pathways, such as protein kinase C, phosphatidylinositol 3-kinase/Akt, and p42/p44 MAP kinase (Nemere et al., 1998; Ma et al., 2006; Gruber et al., 2008), where all of the kinases were closely associated with VEGF expression (Berra et al., 2000; Aida et al., 2005). 1,25(OH)2D3 induced rapid and sustained activation of phosphatidylinositol 3-kinase/Akt, this effect was nongenomic (Ma et al., 2006). Swain et al. reported 1,25(OH)2D3 may regulate phopholipase C production by the cells, which, in turn, may modulate signal transduction by receptors with tyrosine kinase activity, including VEGF. (Swain et al., 1992). Second, 1,25(OH)2D3 may modulate the expression of growth factor receptors (Koga et al., 1988). Finally, growth factors could modulate the expression of the nuclear vitamin D receptor (Haussler et al., 1998). The central role of vitamin D receptor in the biology of vitamin D action has been known at the molecular level. In the present study, TDI exposure increased VEGF production and secretion from RLE-6TN cells by suppression of 1,25(OH)2D3 production, which was reversed by 1,25(OH)2D3 treatment. This is the first study to demonstrate the regulatory effects of 1,25(OH)2D3 on TDI-induced VEGF production and secretion. The administration of 1,25(OH)2D3 may reduce VEGF-induced airway inflammation in patients with isocyanate-OA. Although further studies are needed to elucidate the mechanisms by which TDI decreases megalin expression and 1,25(OH)2D3 decreases VEGF production and secretion, this information may provide insight to facilitate the development of new therapeutic strategies for the treatment of isocyanate-OA.
In conclusion, the results of this study demonstrated that the measurement of serum VDBP levels may be a useful serological marker for the early detection of isocyanate-OA among exposed workers. TDI-induced VEGF production/secretion may be reversed by 1,25(OH)2D3 treatment, which may provide a potential therapeutic strategy for isocyanate-OA.
Methods
Bronchial challenge testing with methacholine and isocyanate
All subjects with asthma underwent lung function measurement and inhalation challenge tests with methacholine and isocyanate. Airway responsiveness to methacholine was tested using the five-breath dosimeter protocol, as described previously (Park et al., 1999; Ye et al., 2006). The methacholine PC20 level was determined by interpolation from the dose-response curve. The isocyanate (TDI or MDI) bronchial challenge tests were performed according to the method described previously (Park et al., 1999; Ye et al., 2006).
Measurement of VDBP levels in the sera
The level of VDBP in sera was measured using a commercially available ELISA kit (Immunodiagnostik AG, Bensheim, Germany) according to the manufacturer's instructions. The serum samples were diluted 1:10000 just before the assay to ensure that the measured values were within the optimal range of the standard curve. The inter- and intra-assay variations of this ELISA kit were under 19.3% and 5.0%, respectively.
Cell culture and treatment with TDI
The rat alveolar epithelial cell line RLE-6TN, which was characterized and found to be similar to alveolar type 2 cells, including the expression of cytokeratin 19 (Paine et al., 1988; Driscoll et al., 1995), was purchased from the American Type Culture Collection (Manassas, VA). The cells were cultured in D-MEM/F-12 containing 10% FBS, 100 IU/ml penicillin, and 100 µg/ml streptomycin, in an atmosphere of 5% CO2 and 95% air at 37℃. TDI (Sigma, St. Louis, MO) was thawed at 37℃, then diluted in DMSO (1:20, v/v). When the cells were treated with TDI, controls were treated with an equivalent amount of DMSO; the final DMSO concentration was less than 0.3% (v/v).
Real-time reverse transcriptase-PCR
Total RNA was isolated from RLE-6TN cells using an Easy-BLUE Total RNA Extraction Kit (iNtRON Biotechnologies, Seoul, Korea) after exposure to 0.5-1 mM TDI for 3-12 h. Total RNA (2 µg) was reverse transcribed using the oligo (dT) primer and MMLV reverse transcriptase (Promega, Madison, WI) at 42℃ for 90 min. Real-time PCR was performed using an ABI Prism 7500 instrument according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). The following primer pairs were used: megalin, forward 5'-TGGAATCTCCCTTGATCCTG-3' and reverse, 5'-TGTTGCTGCCATCAGTCTTC-3'; and GAPDH, forward 5'-GGCCAAAAGGGTCATCATC-3' and reverse, 5'-GTGATGGCATGGACTGTGG-3'. After an initial hot start for 10 min, amplification was performed for 40 cycles consisting of denaturation for 10 s at 94℃, annealing for 30 s at 56℃, and extension for 40 s at 72℃. The amplification kinetics was recorded as sigmoid progress curves for which fluorescence was plotted against the number of amplification cycles. The threshold cycle number (CT) was used to define the initial amount of each template. The CT was the first cycle for which a detectable fluorescent signal was observed. The mRNA expression levels were determined and compared with the GAPDH standard.
Preparation of FITC-VDBP conjugate and immunofluorescence staining
VDBP was purchased from Calbiochem (La Jolla, CA). FITC-VDBP conjugate was prepared using a Pierce FITC antibody labeling kit according to the manufacturer's instructions (Pierce, Rockford, IL). For the in vitro VDBP-uptake studies, aliquots of 2 × 104 of the RLE-6TN cells were seeded on cover slips in 24-well plates. The next day, the cells were preincubated with or without 0.5-1 mM TDI for 3-12 h, washed twice with PBS, and incubated with 400 nM FITC-VDBP conjugate in serum-free medium for 2 h. For competition assay, the cells were incubated in the absence or presence of the indicated doses of native VDBP for 30 min and then incubated with 400 nM FITC-VDBP for 2 h. For the trypan blue quenching assay, the cells were incubated with FITC-VDBP conjugate and then incubated with or without 1.2 mg/ml trypan blue (Sigma, St. Louis, MO) for 15 min to quench the extracellular fluorescence signal. After washing, the cells were fixed with 4% paraformaldehyde and observed under a fluorescence microscope (Carl Zeiss, Jena, Germany). More than 30 cells per field were randomly selected from each of five fields in three separate experiments, and the fluorescence intensity was determined by processing FITC images at the cellular level; the percent intensity was calculated as the mean fluorescence intensity of the sample divided by that of the control.
Western blotting analysis
Cell lysates were separated by 5% (for megalin) or 10% SDS-PAGE. The membranes were blocked in blocking solution (5% nonfat dried milk in PBS) for 1 h at room temperature and then probed with anti-megalin, anti-VEGF (Santa Cruz Technology, Santa Cruz, CA), and anti-tubulin (Sigma) antibodies for 1 h at room temperature. After washing three times with PBS containing 0.1% Tween-20 (PBS-T), the membranes were incubated with secondary antibodies (Cell Signaling Technology, Beverly, MA) for 1 h at room temperature. After washing an additional three times in PBS-T, the membranes were developed using an ECL solution (Pierce, Rockford, IL) and exposed to Chemidoc XRS Gel Documentation System (Bio-Rad, Hercules, CA).
Measurement of 1,25(OH)2D3 production and VEGF secretion
RLE-6TN cells were incubated with 1 mM TDI for 6 h and then further incubated with 1 µM 25(OH)2D3 (Sigma) for 1, 3, or 6 h. 1,25(OH)2D3 in the supernatant was quantified using an enzyme immunoassay kit for 1,25(OH)2D3 (Immunodiagnostic Systems, Boldon, UK) according to the manufacturer's instructions. For detecting VEGF, the RLE-6TN cells were incubated with 1 mM TDI for 3, 6, or 12 h or incubated with 1, 10, or 100 nM 1,25(OH)2D3 for 24 h before treatment with 1 mM TDI for 12 h. The VEGF level in the supernatant was quantified using an ELISA kit for rat VEGF (Invitrogen, Camarillo, CA) according to the manufacturer's instructions.
Statistical Analyses
All values are expressed as the means ± SEM, unless otherwise stated. The significance of differences among groups was evaluated by the Student's t-test and χ2 test. Comparison of the serum VDBP in each group was performed by ANOVA with Bonferroni's correction. Wilcoxon's test was used to compare VDBP levels in sera obtained before and after isocyanate bronchial challenge tests within the groups. A ROC curve was constructed to evaluate the diagnostic value of the serum VDBP for discrimination between isocyanate-OA and AECs, and the AUC with 95% CI was computed. Sensitivity and specificity were calculated according to the identified optimal cutoffs. All analyses were carried out using SPSS 13.0 software (SPSS Inc., Chicago, IL). In all analyses, P < 0.05 was taken to indicate statistical significance.
Acknowledgements
This work was supported in part by a Korean Science and Engineering Foundation (KOSEF) grant (MEST, 2009-00786746 to H. S. P.) and a Korea Science and Engineering Foundation through Chronic Inflammatory Disease Research Center Ajou University Grant (R13-2003-019 to S. M. P.) funded by the Korean government.
Abbreviations
- AEC
asymptomatic exposed controls
- BALF
bronchoalveolar lavage fluid
- HSA
human serum albumin
- MDI
diphenyl-methane diisocyanate
- NC
unexposed healthy controls
- OA
occupational asthma
- TDI
toluene diisocyanate
- VDBP
vitamin D-binding protein
- 1,25(OH)2D3
1,25-dihydroxycholecalciferol
- 25(OH)D3
25-hydroxycholecalciferol
References
- 1.Aida M, Irie T, Aida T, Tachikawa T. Expression of protein kinases C betaI, betaII, and VEGF during the differentiation of enamel epithelium in tooth development. J Dent Res. 2005;84:234–239. doi: 10.1177/154405910508400305. [DOI] [PubMed] [Google Scholar]
- 2.Berra E, Pages G, Pouyssegur J. MAP kinases and hypoxia in the control of VEGF expression. Cancer Metastasis Rev. 2000;19:139–145. doi: 10.1023/a:1026506011458. [DOI] [PubMed] [Google Scholar]
- 3.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80:1717S–1720S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 4.Cartier A, Grammer L, Malo JL, Lagier F, Ghezzo H, Harris K, et al. Specific serum antibodies against isocyanates: association with occupational asthma. J Allergy Clin Immunol. 1989;84:507–514. doi: 10.1016/0091-6749(89)90364-3. [DOI] [PubMed] [Google Scholar]
- 5.Chishimba L, Thickett D, Stockley R, Wood A. The vitamin D axis in the lung: a key role for vitamin D-binding protein. Thorax. 2010;65:456–462. doi: 10.1136/thx.2009.128793. [DOI] [PubMed] [Google Scholar]
- 6.Choi JH, Nahm DH, Kim SH, Kim YS, Suh CH, Park HS, Ahn SW. Increased levels of IgG to cytokeratin 19 in sera of patients with toluene diisocyanate-induced asthma. Ann Allergy Asthma Immunol. 2004a;93:293–298. doi: 10.1016/S1081-1206(10)61504-9. [DOI] [PubMed] [Google Scholar]
- 7.Choi JH, Suh YJ, Lee SK, Suh CH, Nahm DH, Park HS. Acute and chronic changes of vascular endothelial growth factor (VEGF) in induced sputum of toluene diisocyanate (TDI)-induced asthma patients. J Korean Med Sci. 2004b;19:359–363. doi: 10.3346/jkms.2004.19.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colantonio DA, Chan DW. The clinical application of proteomics. Clin Chim Acta. 2005;357:151–158. doi: 10.1016/j.cccn.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Devereux G, Macdonald H, Hawrylowicz C. Vitamin D and Asthma: Time for Intervention? Am J Respir Crit Care Med. 2009;179:739–740. doi: 10.1164/rccm.200901-0145ED. [DOI] [PubMed] [Google Scholar]
- 10.Driscoll KE, Carter JM, Iype PT, Kumari HL, Crosby LL, Aardema MJ, Isfort RJ, Cody D, Chestnut MH, Burns JL, Leboeuf RA. Establishment of immortalized alveolar type II epithelial cell lines from adult rats. In Vitro Cell Dev Biol Anim. 1995;31:516–527. doi: 10.1007/BF02634029. [DOI] [PubMed] [Google Scholar]
- 11.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert CR, Arum SM, Smith CM. Vitamin D deficiency and chronic lung disease. Can Respir J. 2009;16:75–80. doi: 10.1155/2009/829130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruber HE, Hoelscher G, Ingram JA, Chow Y, Loeffler B, Hanley EN., Jr 1,25(OH)2-vitamin D3 inhibits proliferation and decreases production of monocyte chemoattractant protein-1, thrombopoietin, VEGF, and angiogenin by human annulus cells in vitro. Spine (Phila Pa 1976) 2008;33:755–756. doi: 10.1097/BRS.0b013e3181695d59. [DOI] [PubMed] [Google Scholar]
- 14.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 15.Hosojima M, Sato H, Yamamoto K, Kaseda R, Soma T, Kobayashi A, Suzuki A, Kabasawa H, Takeyama A, Ikuyam K, Iino N, Nishiyama A, Thekkumkara TJ, Takeda T, Suzuki Y, Gejyo F, Saito A. Regulation of megalin expression in cultured proximal tubule cells by angiotensin II type 1A receptor- and insulin-mediated signaling cross talk. Endocrinology. 2009;150:871–878. doi: 10.1210/en.2008-0886. [DOI] [PubMed] [Google Scholar]
- 16.Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol. 2009;158:20–25. doi: 10.1111/j.1365-2249.2009.04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hur GY, Choi GS, Sheen SS, Lee HY, Park HJ, Choi SJ, Ye YM, Park HS. Serum ferritin and transferrin levels as serologic markers of methylene diphenyl diisocyanate-induced occupational asthma. J Allergy Clin Immunol. 2008;122:774–780. doi: 10.1016/j.jaci.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Hur GY, Koh DH, Choi GS, Park HJ, Choi SJ, Ye YM, Kim KS, Park HS. Clinical and immunologic findings of methylene diphenyl diisocyanate-induced occupational asthma in a car upholstery factory. Clin Exp Allergy. 2008;38:586–593. doi: 10.1111/j.1365-2222.2008.02935.x. [DOI] [PubMed] [Google Scholar]
- 19.Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179:630–636. doi: 10.1164/rccm.200810-1576PP. [DOI] [PubMed] [Google Scholar]
- 20.Koga M, Eisman JA, Sutherland RL. Regulation of epidermal growth factor receptor levels by 1, 25-dihydroxyvitamin D3 in human breast cancer cells. Cancer Res. 1988;48:2734–2739. [PubMed] [Google Scholar]
- 21.Lee YC, Kwak YG, Song CH. Contribution of vascular endothelial growth factor to airway hyperresponsiveness and inflammation in a murine model of toluene diisocyanate-induced asthma. J Immunol. 2002;168:3595–3600. doi: 10.4049/jimmunol.168.7.3595. [DOI] [PubMed] [Google Scholar]
- 22.Lundgren S, Carling T, Hjalm G, Juhlin C, Rastad J, Pihlgren U, Rask L, Akerstrom G, Hellman P. Tissue distribution of human gp330/megalin, a putative Ca2+-sensing protein. J Histochem Cytochem. 1997;45:383–392. doi: 10.1177/002215549704500306. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y, Yu WD, Kong RX, Trump DL, Johnson CS. Role of nongenomic activation of phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase 1/2 pathways in 1,25D3-mediated apoptosis in squamous cell carcinoma cells. Cancer Res. 2006;66:8131–8138. doi: 10.1158/0008-5472.CAN-06-1333. [DOI] [PubMed] [Google Scholar]
- 24.Mannino DM. How much asthma is occupationally related? Occup Med. 2000;15:359–368. [PubMed] [Google Scholar]
- 25.Mapp CE, Corona PC, De Marzo N, Fabbri L. Persistent asthma due to isocyanates. A follow-up study of subjects with occupational asthma due to toluene diisocyanate (TDI) Am Rev Respir Dis. 1988;137:1326–1329. doi: 10.1164/ajrccm/137.6.1326. [DOI] [PubMed] [Google Scholar]
- 26.Meier U, Gressner O, Lammert F, Gressner AM. Gc-globulin: roles in response to injury. Clin Chem. 2006;52:1247–1253. doi: 10.1373/clinchem.2005.065680. [DOI] [PubMed] [Google Scholar]
- 27.Meredith S, Nordman H. Occupational asthma: measures of frequency from four countries. Thorax. 1996;51:435–440. doi: 10.1136/thx.51.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nabe T, Yamauchi K, Shinjo Y, Niwa T, Imoto K, Koda A, Kohno S. Delayed-type asthmatic response induced by repeated intratracheal exposure to toluene-2,4-diisocyanate in guinea pigs. Int Arch Allergy Immunol. 2005;137:115–124. doi: 10.1159/000085466. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa K, Kawaura A, Kato S, Takeda E, Okano T. 1 alpha,25-Dihydroxyvitamin D(3) is a preventive factor in the metastasis of lung cancer. Carcinogenesis. 2005;26:429–440. doi: 10.1093/carcin/bgh332. [DOI] [PubMed] [Google Scholar]
- 30.Nemere I, Schwartz Z, Pedrozo H, Sylvia VL, Dean DD, Boyan BD. Identification of a membrane receptor for 1,25-dihydroxyvitamin D3 which mediates rapid activation of protein kinase C. J Bone Miner Res. 1998;13:1353–1359. doi: 10.1359/jbmr.1998.13.9.1353. [DOI] [PubMed] [Google Scholar]
- 31.Noel-Georis I, Bernard A, Falmagne P, Wattiez R. Database of bronchoalveolar lavage fluid proteins. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;771:221–236. doi: 10.1016/s1570-0232(02)00114-9. [DOI] [PubMed] [Google Scholar]
- 32.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 33.Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, Ray R, Gliemann J, Willnow TE, Christensen EI. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3) Proc Natl Acad Sci USA. 2001;98:13895–13900. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paine R, Ben-Ze'ev A, Farmer SR, Brody JS. The pattern of cytokeratin synthesis is a marker of type 2 cell differentiation in adult and maturing fetal lung alveolar cells. Dev Biol. 1988;129:505–515. doi: 10.1016/0012-1606(88)90396-x. [DOI] [PubMed] [Google Scholar]
- 35.Palikhe NS, Kim JH, Park HS. Biomarkers predicting isocyanate-induced asthma. Allergy Asthma Immunol Res. 2011;3:21–26. doi: 10.4168/aair.2011.3.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park HS, Nahm DH. Prognostic factors for toluene diisocyanate-induced occupational asthma after removal from exposure. Clin Exp Allergy. 1997;27:1145–1150. [PubMed] [Google Scholar]
- 37.Park HS, Kim HY, Nahm DH, Son JW, Kim YY. Specific IgG, but not specific IgE, antibodies to toluene diisocyanate-human serum albumin conjugate are associated with toluene diisocyanate bronchoprovocation test results. J Allergy Clin Immunol. 1999;104:847–851. doi: 10.1016/s0091-6749(99)70297-6. [DOI] [PubMed] [Google Scholar]
- 38.Park HS, Lee SK, Kim HY, Nahm DH, Kim SS. Specific immunoglobulin E and immunoglobulin G antibodies to toluene diisocyanate-human serum albumin conjugate: useful markers for predicting long-term prognosis in toluene diisocyanate-induced asthma. Clin Exp Allergy. 2002;32:551–555. doi: 10.1046/j.0954-7894.2002.01349.x. [DOI] [PubMed] [Google Scholar]
- 39.Rowling MJ, Kemmis CM, Taffany DA, Welsh J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J Nutr. 2006;136:2754–2759. doi: 10.1093/jn/136.11.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swain LD, Schwartz Z, Boyan BD. 1, 25-(OH) 2D3 and 24, 25-(OH) 2D3 regulation of arachidonic acid turnover in chondrocyte cultures is cell maturation-specific and may involve direct effects on phospholipase A2. Biochim Biophys Acta. 1992;1136:45–51. doi: 10.1016/0167-4889(92)90083-n. [DOI] [PubMed] [Google Scholar]
- 41.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006;290:L209–L221. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- 42.Walters EH, Soltani A, Reid DW, Ward C. Vascular remodelling in asthma. Curr Opin Allergy Clin Immunol. 2008;8:39–43. doi: 10.1097/ACI.0b013e3282f42696. [DOI] [PubMed] [Google Scholar]
- 43.Weyel DA, Schaffer RB. Pulmonary and sensory irritation of diphenylmethane-4,4'- and dicyclohexylmethane-4,4'-diisocyanate. Toxicol Appl Pharmacol. 1985;77:427–433. doi: 10.1016/0041-008x(85)90182-6. [DOI] [PubMed] [Google Scholar]
- 44.White P, Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol Metab. 2000;11:320–327. doi: 10.1016/s1043-2760(00)00317-9. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson RJ, Lange C. Vitamin D and tuberculosis: new light on a potent biologic therapy? Am J Respir Crit Care Med. 2009;179:740–742. doi: 10.1164/rccm.200902-0186ED. [DOI] [PubMed] [Google Scholar]
- 46.Wisnewski AV. Developments in laboratory diagnostics for isocyanate asthma. Curr Opin Allergy Clin Immunol. 2007;7:138–145. doi: 10.1097/ACI.0b013e3280895d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu J, Kobayashi M, Sousa EA, Liu W, Cai J, Goldman SJ, Dorner AJ, Projan SJ, Kavuru MS, Qiu Y, Thomassen MJ. Differential proteomic analysis of bronchoalveolar lavage fluid in asthmatics following segmental antigen challenge. Mol Cell Proteomics. 2005;4:1251–1264. doi: 10.1074/mcp.M500041-MCP200. [DOI] [PubMed] [Google Scholar]
- 48.Ye YM, Kim CW, Kim HR, Kim HM, Suh CH, Nahm DH, Park HS, Redlich CA, Wisnewski AV. Biophysical determinants of toluene diisocyanate antigenicity associated with exposure and asthma. J Allergy Clin Immunol. 2006;118:885–891. doi: 10.1016/j.jaci.2006.06.026. [DOI] [PubMed] [Google Scholar]