Abstract
Methamphetamine use has been associated with HIV transmission among men who have sex with men (MSM). However, providers have been hesitant to utilize post-exposure prophylaxis (PEP) in populations of stimulant users. This single-arm, open label pilot study sought to demonstrate the safety, feasibility, and acceptability of PEP combined with the drug abstinence intervention of contingency management (CM) in methamphetamine-using MSM. HIV-uninfected MSM reporting recent methamphetamine use were recruited to a CM intervention. Those who reported a recent high-risk sexual or injection drug exposure to an HIV-infected or serostatus unknown source were initiated on tenofovir/emtricitabine (Truvada)-based PEP. Participants were followed over 3 months for infectious/biologic, behavioral, and drug use outcomes. Fifty-three participants enrolled in the study; 35 participants (66%) initiated PEP after a high-risk exposure. The median time from exposure to medication administration was 37.8 h (range 12.5–68.0 h). Twenty-five (71.4%) PEP initiators successfully completed the treatment course. Median medication adherence was 96% (IQR 57–100%), and medication was generally well tolerated. Methamphetamine abstinence during CM treatment increased PEP adherence (2% [95% CI +1–+3%]) per clean urine toxicology sample provided), and increased the odds of PEP course completion (OR 1.17, 95% CI 1.04–1.31). One incident of HIV seroconversion was observed in a participant who did not complete PEP treatment, and reported multiple subsequent exposures. Findings demonstrate that PEP, when combined with CM, is safe, feasible, and acceptable as an HIV prevention strategy in methamphetamine-using MSM.
Introduction
In the United States, men who have sex with men (MSM) are at disproportionate risk for HIV infection. In 2008, MSM accounted for 68% of newly diagnosed infections, with an overall HIV prevalence rate of 19%.1,2 In Los Angeles County (LAC), MSM represent 84% of all prevalent AIDS cases.
Research suggests that high rates of HIV infection among MSM are associated with substance use, in particular methamphetamine use.3,4 Thirteen percent of MSM in LAC reported methamphetamine use in the past year, when assessed as part of behavioral surveillance (T.A. Bingham, unpublished observation). Among urban MSM, methamphetamine use is strongly associated with HIV infection,3 presumably mediated through increases in risky sexual behavior, such as an increased number of sexual partners;5 decreased condom use;5,6 sex with casual, anonymous,7 and multiple partners;8 and unprotected receptive and insertive anal sex with casual partners;6 all of which increase the likelihood of HIV and sexually transmitted infection (STI) acquisition.3,5,8–13 There is an ecological association between intensity of methamphetamine use and HIV infection among MSM; as drug use increases, the greater the likelihood of prevalent HIV infection, with prevalence rates increasing from 23% among MSM reporting occasional use of the drug (approximating domestic and LAC prevalence rates among MSM of 19%2,14), 42% among MSM reporting regular monthly use, 61% among those seeking outpatient treatment for methamphetamine use, and 86% among those seeking residential treatment for methamphetamine dependence.4 Therefore, there is a pressing need for novel and effective interventions to prevent HIV acquisition by methamphetamine-using MSM.15
There is increasing interest in biomedical approaches to HIV prevention – particularly using HIV antiretroviral therapy (ART) in a preventive capacity among HIV-uninfected high-risk populations. PEP, the strategy of taking 28 days of ART, initiated rapidly after an exposure to HIV, has been estimated to reduce the odds of HIV acquisition by 81% after occupational needle-stick injuries,16 and is recommended for the prevention of HIV transmission in high-risk cases of sexual exposure and needle-sharing, despite a lack of efficacy data for this indication.17 The available data suggest that important parameters for PEP efficacy are (1) the time from exposure to initiation of medication,18,19 (2) medication adherence,20 and (3) medication course completion.21 These parameters may be adversely influenced by active substance use,22–25 explaining some the reluctance of providers to use PEP in actively substance-using populations.26 Additional concerns include the potential for risk compensation,27 a reactionary increase in high-risk behavior induced by a perception of protection against HIV afforded by prophylactic therapy. In mathematical models, such behavior changes have the potential to mitigate or abrogate the efficacy of ART-based prevention strategies,28 although the available data suggest that PEP is not associated with risk compensation when accompanied by directed risk-reduction counseling.29–31
Long-standing reluctance to endorse PEP use among substance-using populations is reinforced by observations of poor medical compliance among methamphetamine-using HIV-infected persons.24,25 This concern was supported in a Los Angeles-based pilot program of PEP administration, in which findings demonstrated that the odds for PEP treatment course completion were 4.6-fold lower for participants reporting substance use at the time of their high-risk exposure episode than among the non-substance users.32
This project sought to assess whether incorporating a contingency management (CM) intervention to reduce methamphetamine use could facilitate PEP initiation, adherence, and completion for HIV prevention among methamphetamine-using MSM.
CM is a behavioral intervention that utilizes positive reinforcement in the form of vouchers, gift certificates, goods and services to shape targeted operant behaviors. CM has been successfully implemented in research, community, and public health settings, and has targeted a variety of behaviors such as medication adherence, weight loss, and smoking cessation.33–36 Randomized controlled trials have shown CM to be an effective intervention for reducing stimulant use with effects sustained to at least 1 year, as well as reducing HIV-transmission risk behaviors.37–41 Voucher-based CM for substance users involves providing participants with “vouchers” in exchange for verified urine samples that are negative for metabolites of the targeted drug. Vouchers are then exchanged for gift certificates, goods and services, or other items that promote pro-social and healthy behaviors.42–44
Local success with CM, particularly in groups of methamphetamine-using MSM, and the observation that the HIV epidemic in Los Angeles is notable for high rates of methamphetamine use among MSM, suggested a potential synergy of combination prevention interventions in this population. We hypothesized that the application of a CM intervention for out-of-treatment methamphetamine-using MSM would optimize the use of PEP with regard to initiation, adherence, and completion. This single-arm, open-label pilot study evaluated the safety and feasibility of the combination prevention approach of coupling CM with PEP as a method for helping HIV-uninfected methamphetamine-using MSM to remain HIV-negative. Results are contextualized with other reports from cohorts of PEP recipients that were not specifically targeted as substance-using MSM.
Methods
The Institutional Review Boards for the University of California, Los Angeles and Friends Research Institute provided oversight for all study activities, and approved all study-related documents, materials, and procedures.
Participants
Participants were recruited through targeted advertisements posted in local gay magazines; the distribution of flyers and club cards in the settings where methamphetamine-using MSM congregate such as cruising areas of parks, bars, dance clubs, bathhouses, bookstores, video stores, sex clubs, and at gay-specific events; and at in-services at local agencies. Potential participants were eligible if they self-identified as MSM, were at least 18 years of age, were HIV uninfected on rapid HIV ELISA testing, self-reported methamphetamine use within the previous 30 days, and reported unprotected anal intercourse (UAI) with an HIV-positive or HIV-serostatus-unknown partner in the previous 90 days. The protocol was amended early in the study to remove an inclusion criterion obligating a positive urine toxicology screen for methamphetamine metabolites at study entry. This was done after observed frequent non-correlation of urine test results with self-report of recent methamphetamine use.
Study procedures
All study procedures were conducted at a community research site in Los Angeles, California. At the baseline visit, all eligible participants underwent informed consent; completed baseline assessments (discussed subsequently); received rapid HIV testing (OraQuick Advance, OraSure technologies, Bethlehem, PA); provided specimens for syphilis, gonorrhea and chlamydia testing; and received a medical examination. Participants also received an orientation to the CM intervention. Those who reported a high-risk sexual or drug exposure episode with an HIV-positive or serostatus-unknown source within the preceding 72 h immediately initiated tenofovir disoproxil fumarate+emtricitabine (Truvada, Gilead Sciences), one tablet daily, for 28 days (Immediate Initiators). All other participants received a 4-day “starter pack” of Truvada to be initiated only in the case of a future high-risk exposure to HIV. Not all participants initiated PEP during the study period (Non-Initiators). Participants who reported a post-entry high-risk exposure during the study period initiated PEP via the provided starter pack (Delayed Initiators).
Behavioral intervention (CM)
All participants began the voucher-based CM intervention upon study entry. For the initial 8 weeks of study conduct, participants presented to the study site three times weekly for a urine drug screen for methamphetamine metabolites. Participants who provided urine samples that were negative for methamphetamine metabolites earned vouchers, which escalated in value for successive negative urine samples. A participant with a missing sample or a sample positive for methamphetamine metabolites did not earn vouchers. The participant's initial urine sample that was negative for methamphetamine metabolites earned $2.50. Vouchers increased in value by $1.25 for each consecutive methamphetamine-free sample to a maximum additive value of $20. Participants earned a $10 bonus voucher for every three consecutive methamphetamine-free samples. If a participant provided methamphetamine-free urine samples for the entire 8 week intervention, maximum earnings of $430 in vouchers were possible. Accrued vouchers were never forfeited and could be redeemed at any time during the study for gift certificates or goods or services that promote healthy, pro-social behaviors; vouchers could not be redeemed for cash.
Assessments
Baseline assessments included demographics, diagnosis of methamphetamine abuse or dependence (Diagnostic and Statistical Manual of Mental Disorders, 4th ed.[DSM-IV]), sexual risk behaviors (Behavioral Questionnaire-Amphetamine [BQA - II]), depression (Beck Depression Inventory [BDI]), HIV serostatus, and STIs, urine sample, and self-performed rectal swab for nucleic acid amplification [NAAT] for Neisseria gonorrhoeae and Chlamydia trachomatis, pharyngeal swab for N. gonorrheae, and syphilis testing via serum rapid plasma reagin [RPR] assay).
A urine test strip for methamphetamine metabolites was used for drug screening. Sample validity was confirmed by urine temperature, creatinine content, and pH. BDI was administered weekly and the BQA-II was additionally performed at the 3-month follow-up visit. HIV and STI testing was performed at the 3-month follow-up; HIV RNA testing was performed only in the event of clinical suspicion of acute HIV seroconversion.
For all PEP initiators (immediate or delayed), additional HIV re-testing was performed at 4–6 weeks post-exposure and at 3 months post-exposure. Delayed initiators were also retested at the time of PEP initiation. Serum electrolytes, creatinine, liver function tests (alanine transaminase [ALT], aspartate transaminase [AST], alkaline phosphatase, and total bilirubin), complete blood count (CBC) with differential, and hepatitis B surface antigen (HBsAg) were collected at baseline and upon PEP initiation (for delayed initiators), and directed laboratory evaluations were performed to evaluate potential medication toxicity.
PEP initiators found to be HBsAg positive at baseline were followed with serial liver function test monitoring on a monthly basis for 6 months after PEP completion, or until evaluation by a hepatologist.
Medication adherence was assessed by 4-day medication recall, self-report, and pill counts at day 14 and 28 of PEP treatment; if CM and PEP were concurrent, self-report and pill counts were additionally assessed at all CM visits. Adverse events were assessed at all visits and graded according to the 2004 DAIDS Toxicity Tables (2009 update). All participants initiating PEP were followed for 3 months post-exposure, regardless of the time interval from study enrollment.
Statistical analysis
T-tests were used to test for differences between outcome variables that were measured at both baseline and follow-up. Multivariate ordinary least squares (OLS) regression was used for analyses of PEP adherence and hours to PEP initiation, and multivariate logistic regression was used for analysis of course completion. Because of the small sample size, and the potentially collinear effects of the two included covariates (i.e., years of heavy methamphetamine use and CM-induced abstinence), significance is reported for any coefficient with a p<0.1. All analyses were performed using Stata version 10SE (StataCorp, College Station, TX).
Missing data
Missing data were not imputed. A small number of participants refused to answer questions about methamphetamine usage at baseline (n=3), about their number of sexual partners at follow-up (n=7), or had missing or indeterminate STI testing at baseline (n=2). Two participants who initiated PEP withdrew consent shortly after beginning treatment, and all data after withdrawal of consent were considered missing. Analysis of missing urine drug screen samples revealed no significant differences between participants who did or did not initiate PEP (50% vs. 41.2%, p=0.536), implying that the data are “missing at random” and appropriate for statistical analysis.
Results
Study recruitment and retention
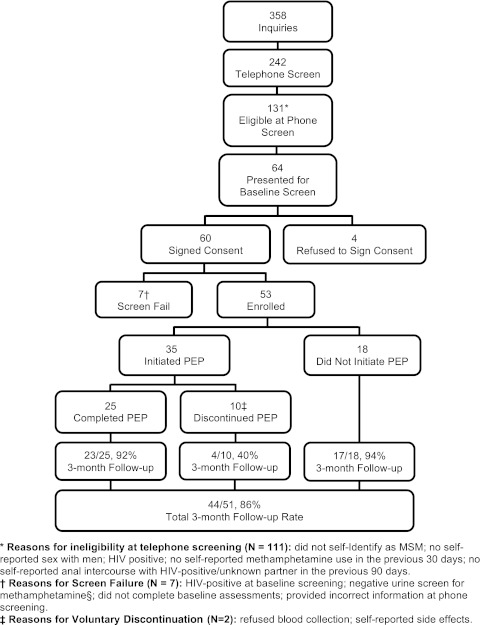
Between March 2009 and August 2010, 358 individuals inquired about the study on the basis of recruitment efforts, 64 presented for screening, and 53 participants enrolled in the study (Fig. 1).
FIG. 1.
Study progression and retention.
Participant baseline characteristics are presented in Table 1, and are consistent with the demographics of participants seen for methamphetamine treatment services collocated at the study facility.45
Table 1.
Participant Sociodemographic Characteristics (n=53)
| Characteristic | Mean (SD) or n (%) | |
|---|---|---|
| Age | Years | 36.1 (7.9) |
| Race/ethnicity | ||
| Caucasian/white | 29 (54.7%) | |
| African American/black | 5 (9.4%) | |
| Hispanic/Latino | 16 (30.2%) | |
| Asian/Pacific Islander | 1 (1.9%) | |
| Other/multiracial | 2 (3.8%) | |
| Sexual identity | ||
| Gay | 44 (83%) | |
| Bisexual | 8 (15.1%) | |
| Other | 1 (1.9%) | |
| Education | Years | 14.3 (2.3) |
| <HS diploma | 2 (3.8%) | |
| HS diploma/GED | 32 (60.4%) | |
| BA/BS | 14 (26.4%) | |
| Postgraduate degree | 5 (9.4%) | |
| Annual income | ||
| ≤$15,000 | 26 (49.1%) | |
| $15,001-$30,000 | 13 (24.5%) | |
| $30,001-$60,000 | 10 (18.9%) | |
| >$60,000 | 4 (7.6%) | |
| Living situation | ||
| Own home or rent apt | 32 (60.4%) | |
| Group housing/sober living | 4 (7.6%) | |
| With family/friends | 10 (18.9%) | |
| No current address | 7 (13.2%) | |
Methamphetamine use, dependency, and high-risk sexual behaviors
At baseline, participants self-reported a mean of 10.1 years (SD=8.2) of methamphetamine use and 2.7 years (SD=4.4) of self-reported “heavy use.” Most study participants (83%) met criteria for methamphetamine dependence on the DSM-IV diagnostic assessment. Additionally, participants reported high rates of lifetime use of recreational drugs and alcohol (Table 2).
Table 2.
Methamphetamine Usage and Sexual Behaviors
| |
|
Baseline (n=53) |
3-month follow-up (n=44) |
|
|---|---|---|---|---|
| Characteristics | Mean(SD) or n(%) | Mean(SD) or n(%) | Sig. | |
| Lifetime meth usage | Years | 10.1 (8.2) | NA | NA |
| Heavy meth usagea | Years | 2.7 (4.4) | NA | NA |
| Frequency of meth use | No. days in past month | 4.8 (5.5) | 1.6 (2.4) | g |
| Average meth use | No. times/day, past mo | 5.3 (6.3) | 1.1 (2.5) | g |
| Amount spent on methb | $ in past month | 172.4 (431.1) | 29.2 (63.8) | h |
| Meth abstinenced | Urinalysis results | 71.70% | 89.47% | h |
| DSM categorization | ||||
| Meth dependent | 44 (83%) | NA | NA | |
| Meth abuser | 3 (5.7%) | NA | NA | |
| Non-dependent/abusing | 6 (11.3%) | NA | NA | |
| Substances used w/meth (lifetime) | ||||
| Alcohol | 38 (71.7%) | NA | NA | |
| Marijuana | 28 (52.8%) | NA | NA | |
| Amyl Nitrite | 25 (47.2%) | NA | NA | |
| GHB | 20 (37.7%) | NA | NA | |
| Cocaine | 17 (32.1%) | NA | NA | |
| Ecstasy | 16 (30.2%) | NA | NA | |
| Viagra/Levitra/Cialis | 16 (30.2%) | NA | NA | |
| Ketamine | 12 (22.6%) | NA | NA | |
| Opiates (non-heroin) | 7 (13.2%) | NA | NA | |
| Crack | 5 (9.4%) | NA | NA | |
| Stimulants (pills) | 5 (9.4%) | NA | NA | |
| Heroin | 5 (9.4%) | NA | NA | |
| Speedballs | 5 (9.4%) | NA | NA | |
| No. of sexual partnerse | # in Past Month | 5.1 (4.8) | 3.1 (3.8) | h |
| No. episodes UAIf | # in Past Month | 6.5 (15.5) | 0.44 (1.4) | h |
| Sexually transmitted infections | ||||
| Hepatitis Bb | 3 (5.9%) | – | NA | |
| Syphilisb | 6 (11.8%) | 3 (6.8%) | NA | |
| Urethral NG | 1 (1.9%) | 0 (0%) | NA | |
| Rectal NG | 3 (5.7%) | 3 (6.8%) | NA | |
| Pharynx NGc | 3 (5.8%) | 5 (11.4%) | NA | |
| Urethral CT | 1 (1.9%) | 0 (0%) | NA | |
| Rectal CT | 3 (5.7%) | 2 (4.5%) | NA | |
Baseline n=50.
Baseline n=51.
Baseline n=52.
Follow-up n=38.
Baseline n=52 and follow-up n=32.
Baseline n=50 and follow-up n=25.
p<0.001; hp<0.05.
DSM, Diagnostic and Statistical Manual of Mental Disorders; UAI, unprotected anal intercourse; NG, Neisseria gonorrhoeae; CT, Chlamydia trachomatis.
Methamphetamine use decreased significantly from baseline to 3-month follow-up, by multiple parameters. Mean number of days (of the past 30) of methamphetamine use decreased from 4.8 to 1.6 (p<0.001), with mean number of uses per day decreased from 5.3 to 1.1 (p<0.001). Similarly, at baseline, participants reported spending a mean of $172.40 (SD=$431.10) on methamphetamine in the previous 30 days, which decreased to $29.20 (SD=$63.80; p<0.05) by 3-month follow-up. The percentage of participants who provided urine specimens free of methamphetamine metabolites (suggestive of methamphetamine abstinence for the 72 h prior to specimen provision) increased significantly over the course of the 3-month study period, from 71.7% at baseline to 89.47% at 3-month follow-up (p<0.05).
The mean number of self-reported sexual partners in the previous 30 days from baseline to 3-month follow-up decreased from 5.1 to 3.1 (p<0.05); additionally, participants reported significantly fewer mean episodes of UAI in the previous 30 days, 6.5 at baseline and 0.44 at 3-month follow-up (p=0.05).
STI
Prevalence and 3-month incidence rates of syphilis (11.8% and 6.8%, respectively), rectal gonorrhea (5.7% and 6.8%), pharyngeal gonorrhea (5.8% and 11.4%), and rectal chlamydia (5.7% and 4.5%) were high (Table 2), in contrast with reported decreases in sexual risk behavior.
PEP initiation, adherence, and adverse events
Thirty-five participants (66%) initiated PEP during the course of the study, with a median time from exposure to initiation of 37.8 h (range, 12.5–68.0 h; Table 3). Thirty (85.7%) participants were immediate initiators, and five were delayed initiators. There were no significant differences between immediate and delayed initiators in rates of medication adherence, medication possession, or course completion. Twenty-five (71.4%) participants completed the 28 dose course of PEP medication, with overall median medication adherence of 96% (IQR, 57–100%). Commonly reported adverse events are listed in Table 3. Most (78.1%) adverse events were graded as mild (grade 1 or 2), with one grade 3 adverse event (syncope) and one grade 4 adverse event (depression with suicidality); however, neither grade 3 or grade 4 adverse event was considered to be related to PEP medication. Of the four adverse events of any grade considered possibly related to the PEP medication (two cases of abdominal pain, one case of diarrhea, one case of flatulence), all resolved without the need for medication discontinuation.
Table 3.
Multivariate Associations of Critical PEP Parameters with Years of Heavy Methamphetamine Use and CM Drug Abstinence Induction (n=32)
| Outcome variable | Predictors | b (SE) or OR (SE) |
|---|---|---|
| PEP adherence | ||
| Years heavy meth usage | −0.03 (.02)a | |
| No. clean urine samples | 0.02 (.01)b | |
| Course completion | ||
| Years heavy meth usage | 0.75 (.13)a | |
| No. clean urine samples | 1.17 (.07)b | |
| Hours to initiation | ||
| Years heavy meth usage | 1.4 (1.0) | |
| No. clean urine samples | −0.03 (.34) | |
p≤0.1; bp≤0.01; All sig. tests 2-tailed.
PEP adherence
Exploratory multivariate analyses were performed to determine if lifetime or ongoing methamphetamine use (assessed by self-report and urine metabolite assay, respectively) influenced PEP adherence, course completion, and/or hours to PEP initiation (Table 3).
The number of clean urine samples provided by a participant significantly increased PEP adherence, with each additional clean urine sample increasing adherence by 2% (95% CI, +1–+3%; p=0.002). Self-reported years of heavy methamphetamine usage demonstrated a trend toward decreased PEP adherence; every 1 additional year of heavy usage decreased PEP adherence by 3% (95% CI, −7–+1%; p=0.095).
PEP course completion
The number of clean urine samples significantly increased the odds of course completion (OR, 1.17, 95% CI, 1.04–1.31; p=0.011). The number of cumulative years of heavy methamphetamine usage demonstrated a trend to decrease the odds of course completion (OR, 0.75, 95% CI, 0.53–1.05; p=0.099).
Exposure-to-dosing time
Cumulative number of years of heavy methamphetamine usage demonstrated a non-statistically significant increase in the number of hours to PEP initiation, with each additional year of heavy methamphetamine usage increasing the number of hours to PEP initiation by 1.4 (95% CI, −0.64–3.44; p=0.17).
Incident seroconversion
One incident seroconversion was observed during study conduct in a participant who discontinued PEP medication after 16 days of treatment for logistic reasons. The participant reported multiple repeat exposures subsequent to PEP treatment and had laboratory evidence of incident STIs at the time of serconversion.
Discussion
PEP has potential to be a powerful HIV prevention strategy, although historically, providers have been reluctant to initiate PEP with substance users out of concern for poor adherence, ability to present within the narrow window of post-exposure therapeutic efficacy, and the propensity for repeat exposures. Compelling data that substance-using MSM have high HIV prevalence and incidence rates underscore the urgency of finding effective HIV prevention interventions for this population. This pilot study explored an intervention that addressed these concerns by pairing CM with PEP to facilitate medication initiation, adherence, and completion, as well as to promote behavioral risk reduction; with the hope of creating combination prophylactic synergy.
During the course of the study, 35 (66%) of study participants reported a high-risk sexual exposure that met eligibility criteria for initiation of PEP. This finding corroborates the observation that methamphetamine-using MSM are an extremely high-risk population, and a population in whom biomedical HIV prevention strategies might be particularly beneficial with regard to reducing incident HIV infections, if the use of such strategies can be optimized.
One of the most critical parameters of PEP efficacy is exposure-to-dose time interval, with animal and human data suggesting diminished efficacy with incrementally delayed medication administration post-exposure.22,46 Previous PEP studies in non-occupational settings, which did not specifically target substance-using or substance-dependent MSM, have reported median exposure-to-dose times of 33 h.47 Although cross-study comparisons must be made with caution, our CM-facilitated PEP protocol found a median time from exposure to PEP initiation of 37.8 (range 12.5–68.0) h. Similarly, a PEP medication adherence rate of 76.3% and course completion rate of 71.4% compares favorably with other cohorts.30,47,48
Reductions in methamphetamine use and sexual risk behaviors including days of methamphetamine use, numbers of sexual partners, episodes of UAI, and drug use during sex (all with a 30-day horizon), as well as increases in rates of methamphetamine-free urine specimens, suggest that the CM intervention was successful and had potent methamphetamine-use and sexual risk behavior benefit. The absence of any additional behavioral intervention other than the CM suggests that the reduction in sexual risk behavior was largely mediated by reduced methamphetamine use, as has been seen in other studies.39 The durability of these benefits was not assessed past 3 months, but prior studies have demonstrated durability to 1-year follow-up evaluation.39,49 The high rate of incident STIs at the 3 month point tempers enthusiasm for the self-reported decrease in sexual risk behaviors, as it suggests underreporting of risk behavior and a high risk population in which even limited exposures are likely to result in STI acquisition. This situation suggests that these men transact parts of their sexual lives near the “core” of many epidemics, which reinforces the rationale of providing combination prevention to these MSM that includes efficacious interventions for both sex (PEP) and drug use (CM).
CM was considered the ideal behavioral intervention for this trial, given that the target population was out-of-treatment methamphetamine users. CM has been demonstrated to be the most efficacious intervention for promoting abstinence from stimulants during treatment,37,39 and, given that this was a non-treatment-seeking population it was unlikely that the participants would attend to a counselor-facilitated intervention. By providing a powerful reward for urine samples that were negative for methamphetamine metabolites, CM offered an external motivation to users who were not yet in the earliest stages of the change process.50
There was a concern that the early removal of a baseline positive urine-toxicology result could bias the sample to a less severely methamphetamide-affected study population; however, that concern was unfounded, as a full 83% met criteria for methamphetamine dependence on the baseline DSM-IV, rather than the lesser diagnostic classification of abuse. Increased abstinence from methamphetamine use during the intervention predicted increases in PEP adherence and the odds of course completion. Given that these findings persisted in multivariate analyses, future studies of combination HIV prevention strategies should focus on, or at minimum include, high-risk populations (including methamphetamine-using MSM), and include low-threshold substance use interventions, such as CM, to optimize the capacity of substance users to access the full benefits of the prevention package. The findings are consistent with previous observations that methamphetamine use reduces the ability to adhere to medical protocols, underscoring the need for behavioral interventions to be used in conjunction with PEP (or similar medical treatments) in methamphetamine-using populations, and suggesting that therapies that successfully reduce drug use may have significant independent HIV prevention potential.
We observed one incident of seroconversion in a participant who terminated PEP medication after 16 days and reported numerous high-risk exposures subsequent to PEP treatment, corroborated by concomitant incident STIs. He seroconverted with a multidrug-resistant (MDR) strain of HIV; however, the genotypic resistance profile and the absence of baseline and week 4–6 viremia exonerate incomplete PEP treatment as the etiology of the resistance. He most likely acquired an MDR strain primarily. Rather than a failure of the PEP strategy, this seroconversion underscores the high risk of HIV seroconversion in the face of repeat exposures.20
It is notable that despite high rates of both prevalent and incident STIs among the participants (Table 2), the overall screen failure rate for prevalent HIV positivity (Fig. 1), and incident HIV infections were limited. A plausible explanation is that HIV and other STIs may largely circulate in different social and/or sexual networks in this population.51,52 Although we demonstrated a decrease in sexual risk behavior among study participants, the overall high rates of STIs in this sample suggest a persistently high level of ongoing sexual risk behavior. These findings highlight the need for both regular STI testing and the importance of finding preventive synergies among interventions.
The current study had several limitations. The single-arm design and absence of a control group prevented the attribution of salutary rates of PEP-related parameters to CM (contrast structured study participation). A small sample size and a 66% rate of PEP initiation limited the power to detect associations between intensity of methamphetamine use and PEP-related outcomes. The relatively brief follow-up period (3 months) additionally limited the ability to assess durability of the intervention on drug and sexual risk behaviors, as well as potentially underestimated the number of HIV seroconversions. Additionally, allowing participants to initiate PEP at study entry or at a delayed exposure time point during study conduct (including subsequent to the 8 week CM intervention) compromises the ability to directly implicate concomitant CM as the reason for salutary PEP use parameters; however, studies of CM for methamphetamine abstinence have noted sustained effects out to 1-year post CM-induction, therefore covering the period of even delayed PEP initiators.
These pilot data demonstrate the feasibility, safety, and acceptability of HIV chemoprophylaxis in a sample of out-of-treatment, methamphetamine-using MSM, as demonstrated by exposure-to-dose times, adherence rates, and course completion rates comparable to non-methamphetamine-specific populations. With alarmingly high HIV prevalence and incidence rates among MSM methamphetamine users, the coupling of PEP with CM holds promise as a combination HIV prevention strategy. A randomized controlled trial of CM and a control behavioral condition for the facilitation of PEP use in stimulant users is currently enrolling participants.
Acknowledgments
This work was supported by the County of Los Angeles Department of Public Health, Office of AIDS Programs and Policy (contract number H-2702632 to Cathy J. Reback) and the National Institute of Drug Abuse at the National Institutes of Health (grant K23 DA 026308 to Raphael J. Landovitz).
Author Disclosure Statement
Raphael J. Landovitz reports being the site principal investigator for a Gilead Sciences-sponsored research protocol. No significant conflicts of interest exist for the other authors.
References
- 1.Hall HI. Song R. Rhodes P, et al. Estimation of HIV Incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Prevalence and awareness of HIV infection among men who have sex with men –– 21 cities, United States, 2008. MMWR Morb Mortal Wkly Rep 2010. 2010;59:1201–1207. [PubMed] [Google Scholar]
- 3.Plankey MW. Ostrow DG. Stall R, et al. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007;45:85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoptaw S. Reback CJ. Associations between methamphetamine use and HIV among men who have sex with men: A model for guiding public policy. J Urban Health. 2006;83:1151–1157. doi: 10.1007/s11524-006-9119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molitor F. Truax SR. Ruiz JD. Sun RK. Association of methamphetamine use during sex with risky sexual behaviors and HIV infection among non-injection drug users. West J Med. 1998;168:93–97. [PMC free article] [PubMed] [Google Scholar]
- 6.Purcell DW. Parsons JT. Halkitis PN. Mizuno Y. Woods WJ. Substance use and sexual transmission risk behavior of HIV-positive men who have sex with men. J Subst Abuse. 2001;13:185–200. doi: 10.1016/s0899-3289(01)00072-4. [DOI] [PubMed] [Google Scholar]
- 7.Stall R. Paul JP. Greenwood G, et al. Alcohol use, drug use and alcohol related problems among men who have sex with men: the urban men's health study. Addiction. 2001;96:1589–1601. doi: 10.1046/j.1360-0443.2001.961115896.x. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood GL. White E. Page–Shafer K, et al. Correlates of heavy substance use among young gay and bisexual men: the San Francisco young men's health study. Drug Alcohol Depend. 2000;61:105–112. doi: 10.1016/s0376-8716(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 9.Buchacz K. McFarland W. Kellogg TA, et al. Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS. 2005;19:1423–1424. doi: 10.1097/01.aids.0000180794.27896.fb. [DOI] [PubMed] [Google Scholar]
- 10.Colfax G. Coates TJ. Husnik MJ, et al. Longitudinal patterns of methamphetamine, popper (amyl nitrite), and cocaine use and high-risk sexual behavior among a cohort of San Francisco men who have sex with men. J Urban Health. 2005;82(1 Suppl 1):i62–70. doi: 10.1093/jurban/jti025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drumright L. Patterson T. Strathdee S. Club drugs as casual risk factors for HIV acquisition among men who have sex with men: a review. Subst Use Misuse. 2006;41:1551–1601. doi: 10.1080/10826080600847894. [DOI] [PubMed] [Google Scholar]
- 12.Garofalo R. Mustanski BS. McKirnan DJ. Jerrick A. Donenberg GR. Methamphetamine and young men who have sex with men: Understanding patterns and correlates of use and the association with HIV-related sexual risk. Arch Pediatr Adolesc Med. 2007;161:591–596. doi: 10.1001/archpedi.161.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoptaw S. Peck JA. Reback CJ. Rotheram–Fuller E. Psychiatric and substance dependence comorbidities, sexually transmitted diseases, and risk behaviors among methamphetamine-dependent gay and bisexual men seeking outpatient drug abuse treatment. J Psychoactive Drugs. 2003;35(Suppl 1):161–168. doi: 10.1080/02791072.2003.10400511. [DOI] [PubMed] [Google Scholar]
- 14.HIV Epidemiology Program Los Angeles County Department of Public Health. 2010 Annual HIV Surveillance Report. 2011. http://publichealth.lacounty.gov/hiv/ http://publichealth.lacounty.gov/hiv/
- 15.Rajasingham R. Mimiaga MJ. White JM. Pinkston MM. Baden RP. Mitty JA. A systematic review of behavioral and treatment outcome studies among hiv-infected men who have sex with men who abuse crystal methamphetamine. AIDS Patient Care STDS. 2012;26:36–52. doi: 10.1089/apc.2011.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardo DM. Culver DH. Ciesielski CA, et al. A case–control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337:1485–1490. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- 17.Smith DK. Grohskopf LA. Black RJ. Auerbach JD. Veronese F. Struble KA. Cheever L. Johnson M. Paxton LA. Onorato IM. Greenberg AE. Antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV in the United States: Recommendations from the U.S. Department of Health and Human Services. MMWR Recomm Rep. 2005;54:1–20. [PubMed] [Google Scholar]
- 18.Tsai CC. Follis KE. Sabo A, et al. Prevention of SIV infection in macaques by (R)–9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 19.Wade NA. Zielinski MA. Butsashvili M, et al. Decline in perinatal HIV transmission in New York State (1997–2000) J Acquir Immune Defic Syndr. 2004;36:1075–1082. doi: 10.1097/00126334-200408150-00011. [DOI] [PubMed] [Google Scholar]
- 20.Roland ME. Neilands TB. Krone MR, et al. Seroconversion Following Nonoccupational Postexposure Prophylaxis against HIV. Clin Infect Dis. 2005;41:1507–1513. doi: 10.1086/497268. [DOI] [PubMed] [Google Scholar]
- 21.Tsai CC. Emau P. Follis KE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinkin CH. Barclay TR. Castellon SA, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2006;11:185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celentano DD. Lucas G. Optimizing treatment outcomes in HIV-infected patient with substance abuse issues. Clin Infect Dis. 2007;45(Suppl 4):S318–S323. doi: 10.1086/522557. [DOI] [PubMed] [Google Scholar]
- 24.Ellis RJ. Childers ME. Cherner M, et al. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188:1820–1826. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- 25.Reback CJ. Larkins S. Shoptaw S. Methamphetamine abuse as a barrier to HIV medication adherence among gay and bisexual men. AIDS Care. 2003;15:775–785. doi: 10.1080/09540120310001618621. [DOI] [PubMed] [Google Scholar]
- 26.Patrick G OC. HIV post-exposure therapy for drug users in treatment. J Subst Abuse Treat. 2000;18:17–21. doi: 10.1016/s0740-5472(99)00015-x. [DOI] [PubMed] [Google Scholar]
- 27.Eaton L. A., Kalichman S. Risk compensation in HIV prevention: Implications for vaccines, microbicides, and other biomedical HIV prevention technologies. Curr HIV/AIDS Rep. 2007;4:165–172. doi: 10.1007/s11904-007-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbas U. Anderson R. Mellors JW. Potential effect of antiretroviral chemoprophylaxis on HIV-1 transmission in resource-limited settings. PLoS One. 2007;2:e875. doi: 10.1371/journal.pone.0000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schechter M. do Lago R. F., Mendelsohn A. B., Moreira R. L., Moulton L. H., Harrison L. H. Behavioral impact, acceptability, and HIV incidence among homosexual men with access to postexposure chemoprophylaxis for HIV. J Acqir Immune Defic Syndr 2004. 2004;35:519–525. doi: 10.1097/00126334-200404150-00010. [DOI] [PubMed] [Google Scholar]
- 30.Martin JN. Roland ME. Neilands TB, et al. Use of postexposure prophylaxis against HIV infection following sexual exposure does not lead to increases in high-risk behavior. AIDS. 2004;18:787–792. doi: 10.1097/00002030-200403260-00010. [DOI] [PubMed] [Google Scholar]
- 31.Donnell D. Mimiaga M. Mayer K. Chesney M. Koblin B. Coates T. Use of non-occupational post-exposure prophylaxis does not lead to an increase in high risk sex behaviors in men who have sex with men participating in the EXPLORE trial. AIDS Behav. 2010;14:1182–1189. doi: 10.1007/s10461-010-9712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott JD. Bolan RK. Factors associated with poor follow-up to hiv post-exposure prophylaxis [Abstract 71] 2nd International Workshop on HIV Transmission; Washington, DC: 2007. [Google Scholar]
- 33.Schepis TS. Duhig AM. Liss T, et al. Contingency management for smoking cessation: Enhancing feasibility through use of immunoassay test strips measuring cotinine. Nicotine Tob Res. 2008;10:1495–1501. doi: 10.1080/14622200802323209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volpp KG. John LK. Troxel AB. Norton L. Fassbender J. Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300:2631–2637. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volpp KG. Loewenstein G. Troxel AB, et al. A test of financial incentives to improve warfarin adherence. BMC Health Serv Res. 2008;8:272. doi: 10.1186/1472-6963-8-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volpp KG. Troxel AB. Pauly MV, et al. A randomized, controlled trial of financial incentives for smoking cessation. New Engl J Med. 2009;360:699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- 37.Rawson RA. McCann MJ. Flammino F, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101:267–274. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 38.Rawson RA. Huber A. McCann M, et al. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch Gen Psychiatry. 2002;59:817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- 39.Shoptaw S. Reback CJ. Peck JA, et al. Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug Alcohol Depend. 2005;78:125–134. doi: 10.1016/j.drugalcdep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Reback CJ. Peck JA. Dierst–Davies R. Nuno M. Kamien JB. Amass L. Contingency management among homeless, out-of-treatment men who have sex with men. J Subst Abuse Treat. 2010;39:255–263. doi: 10.1016/j.jsat.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanson T. Alessi SM. Petry NM. Contingency management reduces drug-related human immunodeficiency virus risk behaviors in cocaine-abusing methadone patients. Addiction. 2008;103:1187–1197. doi: 10.1111/j.1360-0443.2008.02216.x. [DOI] [PubMed] [Google Scholar]
- 42.Higgins ST. Budney AJ. Bickel WK. Hughes JR. Foerg F. Badger G. Achieving cocaine abstinence with a behavioral approach. Am J Psychiatry. 1993;150:763–769. doi: 10.1176/ajp.150.5.763. [DOI] [PubMed] [Google Scholar]
- 43.Higgins ST. Budney AJ. Bickel WK. Foerg FE. Donham R. Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- 44.Higgins ST. Delaney DD. Budney AJ, et al. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- 45.Reback CJ. Shoptaw S. Development of an evidence-based, gay–specific cognitive behavioral therapy intervention for methamphetamine–abusing gay and bisexual men. Addict Behav. 2011 doi: 10.1016/j.addbeh.2011.11.029. epub ahead of print November 25, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wade NA. Birkhead GS. Warren BL, et al. Abbreviated regimens of zidovudine prophylaxis and perinatal transmission of the human immunodeficiency virus. N Engl J Med. 1998;339:1409–1414. doi: 10.1056/NEJM199811123392001. [DOI] [PubMed] [Google Scholar]
- 47.Kahn JO. Martin JN. Roland ME, et al. Feasibility of postexposure prophylaxis (PEP) against human immunodeficiency virus infection after sexual or injection drug use exposure: The San Francisco PEP Study. J Infect Dis. 2001;183:707–714. doi: 10.1086/318829. [DOI] [PubMed] [Google Scholar]
- 48.Sonder GJ. Prins JM. Regez RM, et al. Comparison of two HIV postexposure prophylaxis regimens among men who have sex with men in amsterdam: adverse effects do not influence compliance. Sex Transm Dis. 2010;37:681–686. doi: 10.1097/OLQ.0b013e3181e2f999. [DOI] [PubMed] [Google Scholar]
- 49.Reback CJ. Peck JA. Dierst–Davies R. Nuno M. Kamien JB. Amass A. Contingency management among homeless, out-of-treatment men who have sex with men. J Subst Abuse Treat. 2010;39:255–263. doi: 10.1016/j.jsat.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prochaska JO. DiClemente CC. The Transtheoretical Approach: Crossing Traditional Boundaries of Therapy. Homewood IL: Dow Jones–Irwin; 1984. [Google Scholar]
- 51.Laumann EO. Youm Y. Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: A network explanation. Sex Transm Dis. 1999;26:250–261. doi: 10.1097/00007435-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Garnett GP. The transmission dynamics of sexually transmitted diseases. In: Holmes KK, editor; Sparling PF, editor; Mardh P-A, et al., editors. Sexually Transmitted Diseases. 4th. New York: McGraw Hill Professional; 2008. pp. 27–39. [Google Scholar]