Abstract
For HIV-infected patients, experiencing multiple traumas is associated with AIDS-related and all-cause mortality, increased opportunistic infections, progression to AIDS, and decreased adherence to therapy. The impact of intimate partner violence (IPV) on adherence and HIV outcomes is unknown. HIV-infected patients recruited from a public HIV clinic participated in this observational cohort study (n=251). Participants completed interviews evaluating IPV and covariates. CD4 count <200 (CD4<200), detectable HIV viral load (VL), and engagement in care (“no show rate” [NSR]) were the outcomes of interest. Medication adherence was not measured. Univariate and multivariate regression analyses were performed with covariates included if p<0.3 in the univariate phase. Seventy-four percent of the participants were male, 55% Caucasian, and 52.2% self-identified as “men who have sex with men.” IPV prevalence was 33.1% with no difference by gender or sexual orientation. In univariate analysis, IPV exposure predicted having a CD4<200 (p=0.005) and a detectable VL (p=0.04) but trended toward significance with a high NSR (p=0.077). Being threatened by a partner was associated with a CD4<200 (p=0.005), a detectable VL (p=0.011), and high NSR (p=0.019) in univariate analysis. In multivariate analysis, IPV predicted having a CD4<200 (p=0.005) and detectable VL (p=0.035). Being threatened by a partner predicted having a CD4<200 (p=0.020), a detectable VL (p=0.007), and a high NSR (p=0.020). Our results suggest IPV impacts biologic outcomes and engagement in care for HIV-infected patients. IPV alone predicts worse biologic outcomes, whereas the specific experience of being threatened by a partner was associated with all three outcomes in univariate and multivariate analyses.
Introduction
Intimate partner violence (IPV) is common in the United States and is known to affect health.1–6
Compared with people who have never experienced IPV, victims of IPV have increased rates of depression, smoking, drug and alcohol abuse, HIV-risk behaviors, sexually transmitted infections, stroke, self-reported poor health, and an increased risk of developing chronic diseases such as coronary artery disease and hypertension. 1,2,4–7 Experience of IPV has also been linked to increased symptom reporting, increased use of the healthcare system, and poorer adherence to medical therapy.4,6
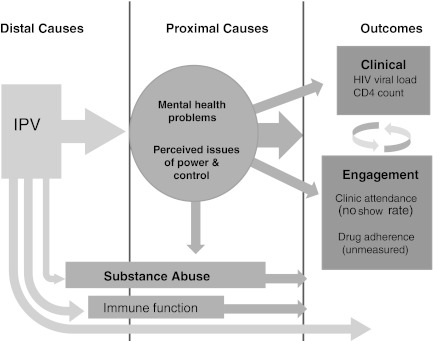
IPV may also impact human physiology through biologic mechanisms. In small studies, researchers have found significant differences in immune cell function, cytokine levels, hemoglobin, mean corpuscular volume of red blood cells, serum cortisol levels, and cell aging when comparing IPV-exposed with unexposed women.8–11 Based on these collective findings, we created a theoretical model of how IPV exposure, worse HIV disease outcomes, and engagement in care may interact (Fig. 1).
FIG. 1.
Theoretical model of how IPV may impact HIV-related outcomes.
In the United States, ∼25% of women and 7.6% of men experience IPV in their lifetimes.12 These numbers are startling in their magnitude; however, they are thought to be underestimates of the true prevalence. Limited data are available about IPV in males, both heterosexual and men who have sex with men (MSM), and in transgender populations. However, available estimates within populations of MSM suggest that 22–32.4% of MSM experience IPV in their adult lifetimes.13,14
Fewer investigations have evaluated IPV in the context of an HIV-positive population. Estimates of IPV prevalence range from 14% to 67% in HIV-positive women and up to 53.1% in a sample of HIV-positive men and women.15,16 Given the high prevalence of IPV in HIV-positive populations, it is important to define how IPV may affect HIV disease and outcomes.15
This article reports the epidemiology of IPV and its associations with disease outcomes and engagement in care in a clinic that serves a predominantly rural HIV-positive population at the University of Virginia (UVa). We hypothesized that people living with HIV (PLWH) who experience IPV in their adult lifetimes are more likely to have lower CD4+ cell counts (CD4 count), higher frequency of detectable viral load (VL), and poorer engagement in care. To our knowledge, this is the first report of IPV as a specific category of trauma and its relationship to engagement and outcomes for PLWH.
Methods
Sample and procedure
Between April 2010 and April 2011, we recruited 251 men and women into a cross-sectional cohort study to evaluate the relationships between IPV exposure and HIV outcomes. The participants were recruited from the UVa Ryan White clinic (RWC). The UVa RWC cares for >650 patients from 52 counties in rural western Virginia (nearly 24,000 sqare miles), with 19 of these counties designated as “underserved” by the Department of Health and Human Services. Some patients living in far southwestern and northwestern Virginia travel 6 h to medical appointments. Although the service area's population is 22% African-American, patients in the UVa RWC are 44% African-American. Of these patients, 33% are female and, among female patients, 50% are African-American. Poverty and unemployment rates are 20% higher in the clinic's catchment area than average for Virginia, and the median income is 25% lower. People in this region are undereducated, with only half of Caucasians and Hispanics and just over 25% of African-Americans completing high school. Thirty-one percent of the clinic patients have no health insurance.
The Principal Investigator (PI) selected and trained study personnel to obtain informed consent and to administer the interview tools. As part of the training, study staff observed the PI while interviewing participants, and then were supervised during their first three enrollments. Prior to data collection, the study protocol was approved by the UVa Institutional Review Board.
Inclusion in the study required HIV seropositivity, age ≥18, and the ability to speak and comprehend English. Patients were ineligible to participate if these criteria were not met or if they were currently incarcerated, or acutely ill and in need of urgent medical attention. Potential participants were approached while alone and in a private setting. Potential participants were not approached if in the company of another individual (such as a partner) in order to protect confidentiality and to minimize risk should the partner be a perpetrator of IPV. Written informed consent was obtained prior to the interview, which included descriptions of the following: study procedures (e.g., structured interview with the interviewer using computers to enter responses), subject areas and types of questions in the interview, ability of the participant to decline answering any question, reimbursement for participation ($5.00 gift card to retail outlet), and available resources should the participant need support or further counseling. Structured interviews were administered in a private setting using electronic forms created in Microsoft InfoPath (Microsoft Corporation, 2007). This format allowed immediate, real-time upload of data into a Microsoft Access database (Microsoft Corp, 2007), thereby decreasing risks of input errors. Following the interview, all participants were offered counseling, IPV victim resource information, and further referrals, if appropriate. All participants were also asked if they wanted any information from the interview shared with their providers and social workers, which was done if they desired.
Measures
Based on our theoretical model of how IPV could associate with HIV outcomes, we also assessed other key variables including: depression, socioeconomic status (SES), post-traumatic stress disorder (PTSD), substance abuse, and lifetime stressors (Fig. 1).
IPV, traumatic events, and PTSD
We used the Women's Experiences with Battering (WEB) to estimate the adult lifetime prevalence of IPV exposure in the HIV clinic population and to identify the exposed and unexposed subjects. The WEB consists of 10 items derived from battered women focus group descriptions of their IPV experiences. The items address a participant's loss of power and control (e.g., “My partner makes me feel like I have no control over my life, no power, no protection.”) and use a Likert-type scale for degree of agreement. Based on sensitivity and specificity analysis performed by the instrument designers, a score of at least 20 constitutes a positive test for IPV (94.6% sensitivity and 96.1% specificity).17
For further characterization of the participants' experience of IPV, we used the Severity of Violence Against Women/Men Scales (SVAW). The SVAW evaluates the frequency of specific IPV behaviors in three dimensions: threat, violence, and sexual aggression. Items address discrete behaviors (e.g., “How often has your partner threatened to hurt you? How often has your partner threatened to kill you?”) and their frequency of occurrence during the last 12 months of a participant's most recent relationship. Chronbach's α for men and women were equally high (α=0.95). Because all three subscales of this measure demonstrated non-normality of distribution using a visual inspection as well as a high skewness (>1.6), the final subscale score was converted to a binary categorical measure of positive or negative.18,19 Language used in all questions was gender neutral in order to include men, women, homosexual, and heterosexual subjects. We did not collect data on the specific timing of abuse.
In order to assess cumulative lifetime stressors, we used the Life Stressor Checklist (LSC), a 30 item tool that includes queries about episodes of violence as well as other nonviolent events (e.g., illness, natural disaster, incarceration).20 This tool is scored both for number of life stressors as well as a weighted score for the participant's subjective experience of stress. PTSD was assessed using the PTSD Civilian Checklist (PCL), a 17 item tool derived from the PTSD screen used for combat veterans. 21
Psychological covariates
The Center for Epidemiologic Studies Depression scale (CESD) is a 20 item assessment using validated questions for depression. Scores are calculated from points per item, with higher points given for higher frequency of depressive symptoms.22 The Addiction Severity Index (ASI) is a multifaceted tool designed to assess patients entering care for substance abuse. In its entirety, it evaluates seven domains of life: medical, employment and social support, drug and alcohol use, legal history, family history, family and social relationships, and psychiatric history. For the purposes of our study, we used only the section addressing substance use, and calculated the developer-recommended composite scores to assess relative severity of alcohol and drug use.23
Sociodemographic covariates
Age, sex, race, education level, sexual orientation, and insurance status were recorded from participant self-report. SES was determined by UVa pay scale data in the participant's medical record. UVa subsidizes healthcare for patients with low incomes. The institution categorizes individuals into six groups based on documented income and assets. Each group pays a different percentage of their medical costs ranging from 0 to 100%. For analysis, we evaluated the following categories: low SES (no co-pay for the patient), middle SES (5–70% co-pay for the patient), and high SES (100% co-pay for the patient).
Outcomes
The primary outcome variables were CD4 count, HIV VL, and engagement in care, defined as clinic “no show rate” (NSR). Laboratory results and visit attendance were abstracted from the medical record. NSR was calculated as the number of medical visits not attended (nor canceled or rescheduled by the participant) divided by the total number of scheduled visits during an 8–12 month period. These variables were all highly non-normally distributed and are typically used in binary format for clinical application (e.g., CD4<200 or CD4 ≥200, HIV VL detectable or undetectable). NSR was classified into two categories (“High NSR, ≥33% missed visits” and “Low NSR, <33% missed visits”) based on the visualized distribution of participants.
Statistical analysis
IBM SPSS software (version 19.0) was used for all statistical analyses. Patient characteristics were reported as frequency with percentage for categorical and mean with±SD for continuous data. Pearson's χ2 test for goodness of fit statistic was applied to univariate analyses of categorical predictors of each of three outcomes including CD4<200, detectable VL and NSR. Odds ratios (OR) with 95% confidence intervals (CI) were reported for 2×2 dichotomous comparisons. The Student's paired sample t test was applied to univariate analyses of continuous predictors of each outcome. Variables were included in multivariate logistic regression models if they met a conservatively set p≤0.30 inclusion criterion. Backwards elimination was used to eliminate non-significant predictors.
Results
Demographics and prevalence
A total of 251 patients were enrolled between April 13, 2010 and April 28, 2011. Although 87% of patients approached were willing to participate, the final participation rate (defined as all those completing study instruments divided by all those approached) from June 10, 2010 until April 28, 2011 was 57% (data on response were not collected prior to June 2010). Patients who expressed interest in the study but deferred participation cited time and illness as the primary reasons for postponing. Demographic characteristics of the sample are described in Table 1. The sample was mostly male (74.5%), between the ages of 41 and 45, and predominantly white (55%). Thirty-nine percent of the participants were African-American. Of the male participants, 70% (n=131) self-identified as MSM (52.2% of total sample). The demographics of our sample matched the overall profile of the clinic population where 69% of the patients are male, 49% are between the ages of 49 and 64, 43% are black/African American, and 45% of patients in clinic self identify as MSM. Eighty-three (33.1%) participants reported experiencing IPV during their adult lifetimes, as defined by a score ≥20 on the WEB. Pearson's χ2 analysis showed no association between IPV and sex or sexual orientation.
Table 1.
Participant Demographics and Clinical Characteristics
| Characteristic | Overall sample (n=251) |
|---|---|
| Age, years (n[%]) | |
| 18–25 | 12 (4.8) |
| 26–30 | 9 (3.6) |
| 31–35 | 26 (10.3) |
| 36–40 | 36 (14.3) |
| 41–45 | 46 (18.3) |
| 46–50 | 48 (19.0) |
| 51–55 | 34 (13.5) |
| 56–60 | 26 (10.3) |
| 61–65 | 9 (3.6) |
| 66–70 | 0 (0) |
| 71–75 | 1 (0.4) |
| 76–80 | 0 (0) |
| 81–85 | 1 (0.4) |
| Gender (n[%]) | |
| Male | 187 (74.5) |
| Female | 64 (25.5) |
| Race (n[%]) | |
| White | 138 (55.0) |
| African-American | 99 (39.4) |
| Pacific/other | 10 (4.0) |
| Native American | 2 (0.8) |
| Unknown | 1 (0.4) |
| Declined to answer | 1 (0.4) |
| Sexual orientation (n[%]) | |
| MSM | 131 (52.2) |
| HSM | 50 (19.9) |
| HSF | 56 (22.3) |
| WSW | 7 (2.8) |
| Declined to answer | 7 (2.8) |
| Education (n[%]) | |
| Did not complete 12th grade | 41 (16.3) |
| Completed 12th grade | 210 (83.7) |
| Socioeconomic status (SES)a (n[%]) | |
| Low SES | 105 (41.8) |
| Mid-range SES | 35 (13.9) |
| High SES | 110 (43.8) |
| Health insurance (n[%]) | |
| Public | 111 (44.2) |
| Private | 78 (31.1) |
| None | 59 (23.5) |
| Military | 2 (0.8) |
| Unknown | 1 (0.4) |
| Median CD4 count, cells/mm3 (range) | 551 (3–1927) |
| Undetectable viral load (n[%]) | 117 (46.4) |
| Clinic no show rate (NSR) (n[%]) | |
| <33% | 214 (85.3) |
| ≥33% | 37 (14.7) |
| Travel time to emergency medical care, minutes (Median [IQR]) | 12.00 (8.00–20.00) |
| Measures of IPV | |
| History of IPV (defined by WEB) (n[%]) |
83 (33.1) |
| Partner threat in last 12 months of relationship (SVAW) (n[%]) | 172 (68.8) |
| Positive depression screena (n=246) | 116 (46.2) |
| Positive PTSD screena (n=248) | 57 (22.7) |
| Lifetime traumatic experiencesa (Median[IQR]) (n=246) | 11.00 (8.00–14.25) |
| Participants using alcohol in past 30 daysa (n[%]) (n=245) | 138 (55.0) |
| Participants using alcohol to intoxication in past 30 daysa (n[%]) (n=245) | 61 (24.3) |
| Participants using drugs in past 30 daysa.b (n[%]) (n=246) | 67 (26.7) |
| Participants with a history of IVDUa (n[%]) (n=245) | 25 (10.2) |
| Heroin | 8 (3.2) |
| Cocaine | 11 (4.4) |
| Amphetamines | 6 (2.4) |
Data for some variables were not available for the entire group.
Includes: cocaine, amphetamines, cannabis, and inhalants.
MSM, men who have sex with men; HSM, heterosexual males; HSF, heterosexual women; WSW, women who have sex with women; IQR, interquartile range; IPV, intimate partner violence; WEB, womens' experiences with battering; SVAW, Severity of Violence Against Women/Men Scales; PTSD, post-traumatic stress disorder; IVDU, intravenous drug use.
In addition to assessing gross IPV prevalence, we also measured experience of threats by a partner (partner threat) using the SVAW. Sixty-nine percent (n=172) of participants had experienced partner threat during the last 12 months of the most recent relationship (Table 1). Separate models were used for IPV exposure (defined as a positive WEB) and partner threat.
IPV and HIV disease outcomes
The median CD4 count for this sample was 551 cells/μL and 46.4% (n=117) of the participants had an undetectable VL (Table 1). These figures mirror the larger clinic population from which the sample was drawn; in comparison, the median CD4 count for the entire clinic is 510.5 cells/μL and 53% of patients have an undetectable VL. Univariate analyses were used to assess the predictive value of variables with respect to having a CD4<200 (Table 2). In multivariate analysis, IPV exposure and male sex were independent, significant predictors of having a CD4<200 (Table 3). These variables explained 12% (Nagelkerke R2) of the variance associated with having a CD4<200.
Table 2.
Univariate Results
|
All participants (n=251) |
Participants with CD4<200 (n=21) |
Participants with detectable VL (n=116) |
Participants with high NSR (≥33%)(n=37) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR(95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | ||||
| Age in years (n[%]) | |||||||||
| 18-45 | 13 (10.1) | 0.63 (0.25–1.57) | 0.312 | 69 (53.5) | 0.55 (0.33–0.90) | 0.017 | 20 (15.5) | 0.88 (0.44–1.78) | 0.726 |
| n=129 (51.4) | |||||||||
| 46-82 | 8 (6.6) | 47 (38.5) | 17(13.9) | ||||||
| n=122 (48.6) | |||||||||
| Gender (n[%]) | |||||||||
| Male | 17 (9.1) | 1.50 (0.49–4.64) | 0.466 | 103 (55.1) | 0.82 (0.46–1.44) | 0.482 | 28 (15.0) | 1.08 (0.48–2.42) | 0.859 |
| n=187 (74.5) | |||||||||
| Female | 4 (6.3) | 32 (50.0) | 9 (14.1) | ||||||
| n=64 (25.5) | |||||||||
| Race (n[%]) | |||||||||
| White | 9 (6.5) | — | 0.225 | 62 (44.9) | — | 0.038 | 16 (11.6) | — | 0.271 |
| n=138 (55.0) | |||||||||
| African-American | 9 (9.1) | 43 (43.4) | 19 (19.2) | ||||||
| n=99 (39.4) | |||||||||
| Other | 3 (21.4) | 11 (78.6) | 2 (14.3) | ||||||
| n=14 (5.6) | |||||||||
| Education (n[%]) | |||||||||
| Did not complete 12th grade | 3 (7.3) | 1.19 (0.33–4.23) | 0.787 | 18 (43.9) | 1.12 (0.57–2.19) | 0.745 | 9 (22.0) | 0.55 (0.24–1.27) | 0.173 |
| n=41 (16.3) | |||||||||
| Completed 12th grade | 18 (8.6) | 98 (46.7) | 28 (13.3) | ||||||
| n=210 (83.7) | |||||||||
| Sexual orientationa (n[%]) n=244 | |||||||||
| MSM | 9 (6.9) | — | 0.095 | 58 (44.3) | — | 0.695 | 19 (14.5) | — | 0.996 |
| n=131 (52.2) | |||||||||
| HSM | 8 (16.0) | 23 (46.0) | 7 (14.0) | ||||||
| n=50 (19.9) | |||||||||
| Women | 3 (4.8) | 32 (50.8) | 9 (14.3) | ||||||
| n=63 (25.1) | |||||||||
| Insurance (n[%]) | |||||||||
| Public | 14 (12.6) | — | 0.173 | 54 (48.6) | — | 0.777 | 18 (16.2) | — | 0.563 |
| n=111 (44.2) | |||||||||
| Private | 4 (5.1) | 34 (43.6) | 9 (11.5) | ||||||
| n=78 (31.1) | |||||||||
| None | 3 (5.1) | 26 (44.1) | 10 (16.9) | ||||||
| n=59 (23.5) | |||||||||
| Otherb | 0 | 2 (66.7) | 0 | ||||||
| n=3 (1.2) | |||||||||
| Payscalea (n[%]) n=250 | |||||||||
| No co-pay (low SES) | 10 (9.5) | — | 0.066 | 54 (51.4) | — | 0.114 | 21 (20.0) | — | 0.116 |
| n=105 (41.8) | |||||||||
| 5-70% Co-pay (mid-range SES) | 6 (17.1) | 11 (31.4) | 5 (14.3) | ||||||
| n=35 (13.9) | |||||||||
| 100% Co-pay (high SES) | 5 (4.5) | 50 (45.5) | 11 (10.0) | ||||||
| n=110 (43.8) | |||||||||
| Positive IPV (n[%]) n=83 (33.1) | 13 (15.7) | 3.71 (1.47–9.36) | 0.005 | 46 (55.4) | 1.74 (1.02–2.96) | 0.040 | 17 (20.5) | 1.91 (0.94–3.87) | 0.077 |
| Men | 9 (16.1) | 2.94 (1.07–8.08) | 0.038 | 32 (57.1) | 2.03 (1.07–3.82) | 0.028 | 13 (23.2) | 2.34 (1.03–5.31) | 0.045 |
| Women | 4 (14.8) | 1.17 (1.00–1.37) | 0.028 | 14 (51.9) | 1.14 (0.42–3.07) | 0.800 | 4 (14.8) | 1.11 (0.27–4.60) | 1.000 |
| Threatened by partner in last 12 months of relationship (n[%]) n=250 | |||||||||
| Never | 1 (1.3) | 10.13 (1.34–76.91) | 0.005 | 27 (34.6) | 2.03 (1.16–3.53) | 0.011 | 5 (6.4) | 3.21 (1.20–8.60) | 0.019 |
| n=78 (31.2) | |||||||||
| At least once | 20 (11.6) | 89 (51.7) | 31 (18.0) | ||||||
| n=172 (68.8) | |||||||||
| Depression screena (n[%]) n=246 | |||||||||
| Positive | 9 (7.8) | 0.83 (0.34–2.04) | 0.679 | 54 (46.6) | 1.02 (0.62–1.68) | 0.950 | 18 (15.5) | 1.22 (0.60–2.50) | 0.585 |
| n=116 (46.2) | |||||||||
| Negative | 12 (9.2) | 60 (46.2) | 17 (13.1) | ||||||
| n=130 (52.8) | |||||||||
| PTSD screena=(n[%]) n=248 | |||||||||
| Positive n=57 (22.7) | 5 (8.8) | 1.05 (0.37–3.01) | 0.925 | 22 (38.6) | 0.66 (0.36–1.21) | 0.178 | 9 (15.8) | 1.14 (0.50–2.59) | 0.758 |
| Negative n=191 (77.0) | 16 (18.4) | 93 (48.7) | 27 (14.1) | ||||||
| Alcohol use in past 30 daysa (total n=245) n=138 (55.0) | 9 (6.5) | 0.55 (0.22–1.36) | 0.195 | 70 (50.7) | 1.47 (0.89–2.45) | 0.134 | 20 (14.5) | 1.13 (0.54–2.35) | 0.751 |
| Alcohol to intoxication in past 30 daysa (total n=245) n=61 (24.3) | 6 (9.8) | 1.23 (0.46–3.32) | 0.688 | 34 (55.7) | 1.64 (0.91–2.93) | 0.096 | 8 (13.1) | 0.92 (0.39–2.15) | 0.842 |
| Illicit substance use in past 30 daysa,c (total n=246) n=67 (26.7) | 3 (4.5) | 0.42 (0.12–1.47) | 0.206 | 35 (52.2) | 1.38 (0.79–2.43) | 0.257 | 13 (19.4) | 1.81 (0.85–3.86) | 0.132 |
| CD4<200 | CD4≥200 | p Value | Detectable VL | Undetectable VL | p Value | NSR ≥33% | NSR <33% | p Value | |
| Travel time (min) to emergency care (Mean[SD]) (16.50 [12.46]) | 13.52 (9.02) | 16.77 (12.71) | 0.254 | 17.01 (12.80) | 16.06 (12.19) | 0.548 | 15.38 (15.218) | 16.69 (11.95) | 0.555 |
| Overall life stressor score (Mean[SD])a (n=246) (11.42[4.93]) | 10.81 (2.73) | 11.48 (5.09) | 0.555 | 12.02(5.13) | 10.90 (4.72) | 0.077 | 13.09 (4.31) | 11.15 (4.98) | 0.033 |
| Alcohol composite score (Mean[SD])a (n=244) | 0.11 (0.19) | 0.07 (0.11) | 0.213 | 0.10(0.14) | 0.06 (0.09) | 0.007 | 0.10 (0.15) | 0.07 (0.11) | 0.169 |
| Drug composite score (Mean[SD])a (n=244) | 0.05 (0.06) | 0.06 (0.07) | 0.565 | 0.06 (0.08) | 0.05 (0.07) | 0.480 | 0.06 (0.07) | 0.05 (0.07) | 0.921 |
Data for some variables were not available for the entire sample.
Other includes: military and “don't know”.
Includes: cocaine, amphetamines, cannabis, and inhalants.
VL, viral load; NSR, “no show” (at appointments) rate; MSM, men who have sex with men; HSM, heterosexual males; SES, socioeconomic status; IPV, intimate partner violence; PTSD, post-traumatic stress disorder.
Table 3.
Multivariate Results for IPV Model
| |
CD4 <200 |
Detectable viral load |
High NSR (>33%) |
|||
|---|---|---|---|---|---|---|
| Variable | RR(95% CI) | p Value | RR (95% CI) | p Value | RR (95% CI) | p Value |
| Intimate partner violence (WEB) | 3.97 (1.51–10.42) | 0.005 | 1.92 (1.05–3.54) | 0.035 | NS | |
| Men | 4.9 (1.18–20.5) | 0.028 | NS | NS | ||
| MSM | 0.35 (0.12–1.00) | 0.052 | NS | NS | ||
| Age | NS | 0.51 (0.30–0.88) | 0.015 | NS | ||
| Positive PTSD screen | NS | 0.31 (0.15–0.67) | 0.003 | NS | ||
| Overall life stressor score | NS | 1.07 (1.00–1.14) | 0.040 | 1.08 (1.01–1.16) | 0.035 | |
| Severity of alcohol use | NS | 19.40 (1.60–234.95) | 0.020 | NS | ||
IPV, intimate partner violence; NSR, “no show” (at appointments) rate; RR, relative risk; WEB, womens' experiences with battering; MSM, men who have sex with men; PTSD, post-traumatic stress disorder.
Partner threat also predicted having a CD4<200 (p=0.005; OR 10.13 [95% CI 1.34–76.91]) in univariate and multivariate analysis (p=0.020; relative risk [RR] 11.67 [95% CI 1.47–92.49]). The only other significant predictor of having a CD4<200 in this model was race other than white or African-American (Table 3). These variables explained 17.8% (Nagelkerke R2) of the variance associated with having a CD4<200.
Univariate analysis was also used to assess the predictive value of variables with respect to having a detectable HIV VL (Table 2). Age, race, IPV exposure, and alcohol abuse were all associated with having a detectable VL. Multivariate analysis identified IPV exposure, younger age, increased lifetime stressors, and alcohol abuse as independent predictors of having a detectable VL, whereas race was no longer significant (Table 3). Conversely, a positive PTSD screen was inversely associated with having a detectable VL (Table 3). These variables explained 14% (Nagelkerke R2) of the variance associated with having a detectable VL. Similarly, partner threat also predicted having a detectable VL (p=0.011; OR 2.03 [95% CI 1.16–3.53]) in univariate analysis. In multivariate analysis, partner threat, age, race other than white or African-American, and alcohol abuse, were all independent, significant predictors of having a detectable VL (Table 4). A positive PTSD screen and midrange SES were inversely related to having a detectable VL (Table 4). These variables explained 16.8% (Nagelkerke R2) of the variance associated with having a detectable VL.
Table 4.
Multivariate Results for Partner Threat Model
| |
CD4 <200 |
Detectable viral load |
High NSR (>33%) |
|||
|---|---|---|---|---|---|---|
| Variable | RR(95% CI) | p Value | RR (95% CI) | p Value | RR (95% CI) | p Value |
| Partner threat (SVAW) | 11.67 (1.47–92.49) | 0.020 | 2.33 (1.26–4.32) | 0.007 | 3.21 (1.20–8.60) | 0.020 |
| Race (not white or African-American) | 5.85 (1.17–29.22) | 0.031 | 4.14 (1.02–16.81) | 0.047 | NS | |
| Age | NS | 0.54 (0.32–0.94) | 0.029 | NS | ||
| 5-70% Co-pay for patient | NS | 0.39 (0.16–0.94) | 0.035 | NS | ||
| Positive PTSD screen | NS | 0.45 (0.23–0.87) | 0.018 | NS | ||
| Severity of alcohol use | NS | 15.54 (1.24–194.40) | 0.033 | NS | ||
NSR, “no show” (at appointments) rate; RR, relative risk; SVAW, Severity of Violence Against Women/Men Scales; NS, not significant; PTSD, post-traumatic stress disorder.
IPV and engagement in care
In univariate analysis (Table 2), the average NSR was significantly higher for people threatened by their partners in the last 12 months of their most recent relationship (p=0.019). For the entire sample, the risk of an NSR > 33% trended toward significance in participants with a history of IPV in univariate analysis (p=0.077). Univariate analysis for men only showed a significant relationship between IPV exposure and having a high NSR (p=0.045; OR 2.34 (95% CI 1.03–5.31)). Partner threat emerged as the only significant predictor of having a high NSR in multivariate analysis (p=0.020; RR 3.21 [95% CI 1.20–8.60]).
Discussion
In our full cohort, IPV, defined by the WEB instrument, negatively impacts important HIV disease markers for PLWH and trends toward significant impairment of engagement in care. The risk of poor engagement in care is particularly high in men who have experienced abuse. Both the experience of IPV and threats from a partner predict having a CD4<200 and a detectable VL. These relationships remained, independent of age, race, education, sexual orientation, SES, insurance status, PTSD, depression, cumulative life stressors, and substance abuse. These results augment previously published research showing strong relationships between cumulative trauma exposure (not exclusive to IPV) and poor health outcomes.1,2,4,5,10,24,25 Small foundational studies suggest the biologic plausibility of this finding by showing that people who experience IPV may have altered cortisol responses and inappropriate T-cell activation as well as early cell senescence.8,9,11 Furthermore, increased lifetime traumatic experiences and stress are associated with worse medication adherence, a factor that clearly impacts the biologic outcomes of PLWH.26,27 Interestingly, PTSD appears to decrease the risk of having a detectable VL, which could reflect the known association between PTSD and increased healthcare utilization.28–30 This finding could also reflect the previously described association between receiving treatment for HIV and development of post-traumatic stress symptoms.31
CD4 count and HIV VL have traditionally been the measures by which we predict HIV outcomes. However, engagement in care is emerging as a significant factor in the health of PLWH. Engagement has been described as a continuum of processes through which patients move: from ignorance of the HIV infection, to diagnosis but being out of care, then linkage to care, and finally to being fully engaged and retained in HIV care.32 Visit attendance and adherence to antiretroviral therapy are critical elements if patients are to reach and remain fully engaged in care. These measures are often used as surrogate markers of engagement, given the documented benefits of consistent medication use and appointment attendance on reducing VL, raising the CD4 count, and, ultimately, survival.26,33 Exposure to higher levels of lifetime trauma (including, but not limited to, physical and sexual abuse) is associated with nonadherence, a factor that could contribute to worse outcomes in an abused population.26,27 Recent analysis by Siemieniuk et al. suggests that PLWH who specifically experience abuse may be more likely to miss clinic visits.14
Whereas we show a trend toward a significant relationship between WEB-defined IPV and a higher NSR, our results indicate that the specific experience of being threatened by a partner significantly predicts having poorer engagement in care in our population. For individuals currently abused (including by threats), possible connections between IPV and not attending clinic visits include: resource deprivation (e.g., money or transportation) by one partner over another, fear of rejection or violent retribution by a partner in response to disclosure of HIV seropositivity, overall poor health, denial when asymptomatic, or an unidentified interaction of factors remaining to be determined.34,35 For people with both a current or past history of IPV, the perceived loss of power and control may be so deeply ingrained that distrust and self-isolation pervade.5
Limitations of our study include: a relatively small sample size, single study site design, and a low proportion of females. Additionally, we did not measure medication adherence as a component of engagement in care for our cohort. Other limitations of our study relate to the assessment tools used to detect and rate the severity of IPV. Neither the WEB nor the SVAW has been validated in MSM and the majority of our participants self-identify in this demographic. These instruments may under- or overestimate the IPV prevalence of our sample because the dynamics in a same-sex relationship may not be captured by the items. There is also the possibility of selection bias in either direction; the patients most likely to participate may be abused and more likely to seek contact with the healthcare environment. Conversely, the patients who declined to participate may have done so because they are abused and are fearful of disclosure. Additionally, we did not screen patients who attended their appointments with a partner or family member, which may inherently exclude people most at risk for being controlled or abused by others.
Our results add to the limited body of literature investigating the prevalence of IPV in males, specifically MSM who are also HIV positive. They highlight the need for greater resources and services for men who experience IPV. Additional research is needed to validate existing IPV screening tools for MSM as well as longitudinal studies to measure how IPV exposure prospectively impacts HIV outcomes and engagement.
There is currently a paucity of information about the HIV epidemic in rural HIV-positive populations in developed nations. Our work contributes to a greater understanding of epidemiology and disease outcomes in the non-urban southeast United States. Recent work by Meditz et al. suggests worse clinical outcomes for PLWH in the southern United States, particularly nonwhites and women, and emphasizes the need to improve our understanding of this population's differential HIV courses in order to better serve them.36 IPV appears to be an important determinant of HIV outcomes in this population, and should be identified through routine screening of patients in HIV clinics.
Acknowledgments
The authors acknowledge the participants in the study, as well as the support from the UVA Ryan White Clinic staff and faculty. This work was supported by the National Institutes of Health [# 5 T32 AI07046-34 to William Petti], Dr. Dillingham's K23 [K23 AI77339-01A1.], and the Virginia Department of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Coker AL. Davis KE. Arias I, et al. Physical and mental health effects of intimate partner violence for men and women. Am J Prev Med. 2002;23:260–268. doi: 10.1016/s0749-3797(02)00514-7. [DOI] [PubMed] [Google Scholar]
- 2.Coker AL. Smith PH. Bethea L. King MR. McKeown RE. Physical health consequences of physical and psychological intimate partner violence. Arch Fam Med. 2000;9:451–457. doi: 10.1001/archfami.9.5.451. [DOI] [PubMed] [Google Scholar]
- 3.Coker AL. Does physical intimate partner violence affect sexual health? A systematic review. Trauma Violence Abuse. 2007;8:149–177. doi: 10.1177/1524838007301162. [DOI] [PubMed] [Google Scholar]
- 4.Breiding MJ. Black MC. Ryan GW. Chronic disease and health risk behaviors associated with intimate partner violence–18 U.S. states/territories, 2005. Ann Epidemiol. 2008;18:538–544. doi: 10.1016/j.annepidem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Bonomi AE. Anderson ML. Rivara FP. Thompson RS. Health outcomes in women with physical and sexual intimate partner violence exposure. J Womens Health (Larchmt) 2007;16:987–997. doi: 10.1089/jwh.2006.0239. [DOI] [PubMed] [Google Scholar]
- 6.Resnick HS. Acierno R. Kilpatrick DG. Health impact of interpersonal violence. 2: Medical and mental health outcomes. Behav Med. 1997;23:65–78. doi: 10.1080/08964289709596730. [DOI] [PubMed] [Google Scholar]
- 7.Coker AL. Flerx VC. Smith PH. Whitaker DJ. Fadden MK. Williams M. Partner violence screening in rural health care clinics. Am J Public Health. 2007;97:1319–1325. doi: 10.2105/AJPH.2005.085357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constantino RE. Sekula LK. Rabin B. Stone C. Negative life experiences, depression, and immune function in abused and nonabused women. Biol Res Nurs. 2000;1:190–198. doi: 10.1177/109980040000100304. [DOI] [PubMed] [Google Scholar]
- 9.Griffin MG. Resick PA. Yehuda R. Enhanced cortisol suppression following dexamethasone administration in domestic violence survivors. Am J Psychiatry. 2005;162:1192–1199. doi: 10.1176/appi.ajp.162.6.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brokaw J. Fullerton–Gleason L. Olson L. Crandall C. McLaughlin S. Sklar D. Health status and intimate partner violence: A cross-sectional study. Ann Emerg Med. 2002;39:31–38. doi: 10.1067/mem.2002.117271. [DOI] [PubMed] [Google Scholar]
- 11.Humphreys J. Epel ES. Cooper BA. Lin J. Blackburn EH. Lee KA. Telomere shortening in formerly abused and never abused women. Biol Res Nurs. 2011 Mar 8; doi: 10.1177/1099800411398479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tjaden P. Thoennes N. Full Report of the Prevalence, Incidence, and Consequences of Violence Against Women. Washington, DC: United States Department of Justice; 2000. [Google Scholar]
- 13.Houston E. McKirnan DJ. Intimate partner abuse among gay and bisexual men: Risk correlates and health outcomes. J Urban Health. 2007;84:681–690. doi: 10.1007/s11524-007-9188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siemieniuk RA. Krentz HB. Gish JA. Gill MJ. Domestic violence screening: Prevalence and outcomes in a canadian HIV population. AIDS Patient Care STDS. 2010;24:763–770. doi: 10.1089/apc.2010.0235. [DOI] [PubMed] [Google Scholar]
- 15.Campbell JC. Baty ML. Ghandour RM. Stockman JK. Francisco L. Wagman J. The intersection of intimate partner violence against women and HIV/AIDS: A review. Int J Inj Contr Saf Promot. 2008;15:221–231. doi: 10.1080/17457300802423224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leserman J. Pence BW. Whetten K, et al. Relation of lifetime trauma and depressive symptoms to mortality in HIV. Am J Psychiatry. 2007;164:1707–1713. doi: 10.1176/appi.ajp.2007.06111775. [DOI] [PubMed] [Google Scholar]
- 17.Smith PH. Earp JA. DeVellis R. Measuring battering: Development of the women's experience with battering (WEB) scale. Womens Health. 1995;1:273–288. [PubMed] [Google Scholar]
- 18.Marshall LL. Development of the severity of violence against women scales. J Fam Violence. 1992;7:103–121. [Google Scholar]
- 19.Marshall LL. The severity of violence against men scales. J Fam Violence. 1992;7:189–203. [Google Scholar]
- 20.Kimerling R. Calhoun KS. Forehand R, et al. Traumatic stress in HIV-infected women. AIDS Educ Prev. 1999;11:321–330. [PubMed] [Google Scholar]
- 21.Blanchard EB. Jones–Alexander J. Buckley TC. Forneris CA. Psychometric properties of the PTSD checklist (PCL) Behav Res Ther. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 22.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 23.McLellan AT. Cacciola JC. Alterman AI. Rikoon SH. Carise D. The addiction severity index at 25: Origins, contributions and transitions. Am J Addict. 2006;15:113–124. doi: 10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- 24.Campbell JC. Health consequences of intimate partner violence. Lancet. 2002;359:1331–1336. doi: 10.1016/S0140-6736(02)08336-8. [DOI] [PubMed] [Google Scholar]
- 25.Leserman J. Petitto JM. Gu H, et al. Progression to AIDS, a clinical AIDS condition and mortality: Psychosocial and physiological predictors. Psychol Med. 2002;32:1059–1073. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- 26.Mugavero M. Ostermann J. Whetten K, et al. Barriers to antiretroviral adherence: The importance of depression, abuse, and other traumatic events. AIDS Patient Care STDS. 2006;20:418–428. doi: 10.1089/apc.2006.20.418. [DOI] [PubMed] [Google Scholar]
- 27.Mugavero MJ. Raper JL. Reif S, et al. Overload: Impact of incident stressful events on antiretroviral medication adherence and virologic failure in a longitudinal, multisite human immunodeficiency virus cohort study. Psychosom Med. 2009;71:920–926. doi: 10.1097/PSY.0b013e3181bfe8d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fogarty CT. Sharma S. Chetty VK. Culpepper L. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008;21:398–407. doi: 10.3122/jabfm.2008.05.070082. [DOI] [PubMed] [Google Scholar]
- 29.Cohen BE. Gima K. Bertenthal D. Kim S. Marmar CR. Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning iraq and afghanistan veterans. J Gen Intern Med. 2010;25:18–24. doi: 10.1007/s11606-009-1117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calhoun PS. Bosworth HB. Grambow SC. Dudley TK. Beckham JC. Medical service utilization by veterans seeking help for posttraumatic stress disorder. Am J Psychiatry. 2002;159:2081–2086. doi: 10.1176/appi.ajp.159.12.2081. [DOI] [PubMed] [Google Scholar]
- 31.Theuninck AC. Lake N. Gibson S. HIV-related posttraumatic stress disorder: Investigating the traumatic events. AIDS Patient Care STDS. 2010;24:485–491. doi: 10.1089/apc.2009.0231. [DOI] [PubMed] [Google Scholar]
- 32.Cheever LW. Engaging HIV-infected patients in care: Their lives depend on it. Clin Infect Dis. 2007;44:1500–1502. doi: 10.1086/517534. [DOI] [PubMed] [Google Scholar]
- 33.Giordano TP. Gifford AL. White AC, Jr, et al. Retention in care: A challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 34.Gielen AC. O'Campo P. Faden RR. Eke A. Women's disclosure of HIV status: Experiences of mistreatment and violence in an urban setting. Womens Health. 1997;25:19–31. doi: 10.1300/J013v25n03_02. [DOI] [PubMed] [Google Scholar]
- 35.Lichtenstein B. Domestic violence in barriers to health care for HIV-positive women. AIDS Patient Care STDS. 2006;20:122–132. doi: 10.1089/apc.2006.20.122. [DOI] [PubMed] [Google Scholar]
- 36.Meditz AL. MaWhinney S. Allshouse A, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis. 2011;203:442–451. doi: 10.1093/infdis/jiq085. [DOI] [PMC free article] [PubMed] [Google Scholar]