Abstract
Libidibia ferrea has been used in folk medicine throughout Brazil, and this study evaluated the biological activities of crude extract (CE) as well as a partially purified fraction (F80) obtained from its pods. Results from the MTT assay revealed that only F80 inhibited NCI-H292 cell growth; however, neither CE nor F80 reduced HEp-2 cell growth or sarcoma 180 tumor weight with the in vivo assay. Acute oral toxicity of the extract and fraction was evaluated following the steps of Guideline 423, using female mice; LD50 for both preparations was determined as 2,500 mg/kg body weight. CE and F80 promoted a reduction of the leukocyte number and nitrite level in inflammatory exudates when the anti-inflammatory assay (carrageenan-induced peritonitis) was performed. CE and F80 inhibited writhing regarding antinociceptive activity (acetic acid-induced writhing response in mice). In conclusion, CE and F80 have no significant cytotoxic or antitumor activities in cell lines showing low toxicity and no action against tumors in vivo. Both preparations revealed anti-inflammatory and antinociceptive activities, corroborating the pharmacological basis of L. ferrea for ethnomedical use.
1. Introduction
Anticancer, pain relief, and anti-inflammatory drugs with weak adverse effects are some of the most important goals in modern research; natural products, especially plant-derivative substances, play a role in this search for an ideal treatment.
Libidibia ferrea var. parvifolia (Mart. ex Tul.) L. P. Queiroz (Leguminosae), whose basionym is Caesalpinia ferrea Mart. ex Tul. (Caesalpiniaceae) [1], is a tree that grows throughout Brazil, especially in the north and northeast regions [2, 3].
L. ferrea aqueous and alcoholic preparations are used popularly to treat a number of diseases such as diabetes, rheumatism, and cancer and also are said to litigate diarrhea, inflammation, and pain, among other symptoms [2, 4–7]. Some L. ferrea therapeutic properties have been studied including its antitumor effects [6, 8].
The cytotoxic and antitumor activities of an aqueous extract and a fraction obtained from the L. ferrea pods as well as preparation effects on the first moments of inflammation and nociception, using in vitro and in vivo assays, were investigated in light of its ethnomedical applications.
2. Materials and Methods
2.1. Plant Material
L. ferrea pods were collected from Ibimirim City, State of Pernambuco, Northeastern Brazil, in August (period of fruit abundance), 2006, and identified by A. Bocage. A sample of the collected material is archived as voucher specimen number 83566, IPA, at the herbarium “Dárdano de Andrade Lima” (Empresa Pernambucana de Pesquisa Agropecuária, Recife, Brazil).
2.2. CE and F80 Preparations
A crude, aqueous extract (CE) was prepared using pod powder in 0.9% NaCl (10% w/v) by gentle shaking for 16 h, at 4°C, passed through gauze, centrifuged (10000 × g) for 15 min, and filtered. Thereafter, proteins were precipitated over 4 h by 0–80% ammonium sulphate fractionation at room temperature; resuspended precipitate was dialyzed against distilled water followed by 0.9% NaCl (F80). Samples were stored at −20°C and subsequently lyophilized. The yield of total dried powder was 25% and 2.94% for CE and F80, respectively.
2.3. Animals
Swiss albino female and male mice (Mus musculus) weighing approximately 25 g (±50 days old) were obtained from the bioterium of the Departamento de Antibióticos, Universidade Federal de Pernambuco (UFPE) and maintained under constant conditions (temperature: 22 ± 2°C, humidity: 40–60%, 12 h light/12 h dark cycle). The mice were allowed access to standard rodent chow diet (Purina) and water ad libitum. These experiments were approved by the Comitê de Ética em Experimentação Animal, Centro de Ciências Biológicas (CEEA-UFPE), Brazil.
2.4. Cytotoxic Activity Evaluation
The cell lines used for the in vitro cytotoxicity assays were NCI-H292 (human lung mucoepidermoid carcinoma cells) and HEp-2 (human larynx epidermoid carcinoma cells) obtained from the Instituto Adolph Lutz (São Paulo, Brazil). The cells were maintained in Dulbecco's Modified Eagle's medium (DMEM) and supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C with 5% CO2.
Cell Viability —
Trypan Blue 0.4% (w/v) was used in sodium phosphate buffer (PBS). It penetrates easily into damaged cells staining them blue, while the intact ones remain colorless, allowing determination of living and dead cell percentages. Cells were counted with an inverted LEITZ microscope and a hemocytometer filled with a homogeneous cell suspension aliquot.
Cytotoxicity —
To determine cytotoxicity, a cell suspension (105 cells/mL) was prepared and distributed in wells of flat-bottom microtiter culture plates. They were incubated in a humidified 5% CO2 air atmosphere at 37°C for 24 h; following that, CE and F80 samples at different protein concentrations (50.0; 25.0; 12.5; 6.25 μg/mL) were added and incubated under the same conditions for 72 h [9]. Control wells received only 0.9% sterile NaCl solution; after that, 25 μL of 3-[4,5-dimethylthiazol-2-yl]-2,5-biphenyl tetrazolium bromide (MTT) in PBS (5 mg/mL, w/v) was added, and the plates were maintained at 37°C for 2 h. Culture medium with MTT was suctioned, and dimethylsulphoxide (DMSO) was added to dissolve formazan crystals [10]. MTT assay is based on the capability of living cells to reduce yellow tetrazolium salt into insoluble, purple formazan [11] which precipitates due to the mitochondrial enzyme succinate dehydrogenase active in living cells [12]. Optical density was carried out at 595 nm. Mean optical density (OD) of the test wells was compared to mean OD of control wells to determine IC50 (concentration that inhibits 50% of cell growth in relation to control). According to National Cancer Institute (NCI), values of IC50 ≤ 30 μg/mL, for nonpure products (e.g., extracts), are considered significant [13].
2.5. Acute Oral Toxicity
The extract and fraction were evaluated following the steps of Guideline 423 [14] using female mice. Animals were prevented by eating overnight prior to the experiment. Three animals for each preparation were orally given a single dose starting at 300 mg/kg (mg of product/kg of body weight) and observed for 14 days (during the first hour after treatment and then once per day). CE and F80 were dissolved in a 0.9% NaCl sterile saline solution (saline), and the calculation of the exact dose for each mouse was based on individual weights. This first step was repeated in the same way for confirmation of results, and a higher dose (2000 mg/kg) was administrated using different female mice.
2.6. Antitumor Activity
Studies were carried out in male mice, six animals per group, aiming to investigate the in vivo antitumor activity of CE and F80 against sarcoma 180.
CE, F80, and methotrexate (MTX) were calculated according to animal body weight index (100 mg/kg for CE and F80; 2.5 mg/kg for MTX). Malignant tumor cells (sarcoma 180) from donor animals with 8 days of implanting were used. All animals were previously hygienized in an experimental surgery room. Donor mice were anaesthetized for tumor suctioning, and the ascitic form of the tumor was introduced under the right axilla of the receptor animals. Treatment, by i.p. route, began 24 h after tumor implantation for 7 days. The negative control group received only saline, and the standard group (positive) received MTX as referential antitumor drug. The animals were sacrificed on the eighth day, by cervical dislocation; solid tumors were excised and weighed. Tumor inhibition was expressed as the mean of tumor weight for the treated animal group (T) in comparison to the untreated control group (C). The tumor inhibition was then calculated according to percentage tumor inhibition = [(C − T)/C] × 100. Animal experiments were performed according to the NCI protocol [13].
2.7. Anti-Inflammatory Assay—Carrageenan-Induced Peritonitis
Saline (control); the standard drugs: dexamethasone, piroxicam, and indomethacin (2 mg/kg, 3 mg/kg, and 10 mg/kg, resp.); CE; F80 (100 mg/kg for both) were administered by oral route to the correspondent groups (6 animals per group). After one hour, 0.25 mL carrageenan (1% in 0.9% NaCl), intraperitoneally injected, was used as a phlogistic agent. Four hours later, animals were sacrificed by cervical dislocation, and immediately the abdomen was opened [15]. The peritoneal cavity was washed with 2 mL of saline containing 3 mM EDTA. Exudates were collected, and the polymorphonuclear leukocytes (PMNLs) counting was performed in a Neubauer chamber after diluting the sample in Turk solution (0.01% crystal violet in 3% acetic acid).
2.8. Nitrite Analysis
Accumulated nitrite (NO2 −) in the peritoneal exudates was measured as an indicator of NO production according to a colorimetric assay based on Griess reaction [16]. The exudates (100 μL) were subjected to reaction with 100 μL Griess reagent (6 mg/mL) at room temperature for 10 min, and then NO2 − concentration was determined by measuring absorbance at 540 nm. A standard curve was constructed using known concentrations of sodium nitrite (NaNO2).
2.9. Analgesic Activity—Acetic Acid-Induced Writhing Response
The response to an i.p. injection of acetic acid solution exhibited as a contraction of the abdominal muscles and stretching of hind limbs was evaluated using a method adapted from Young et al. [17]. Animals (6 per group) were pretreated by i.p. with CE or F80 (100 mg/kg), vehicle (saline) or piroxicam (10 mg/kg), and dipyrone (150 mg/kg) as standard drugs. One hour later, a dose of 0.1 mL/10 g body weight of 1% acetic acid was injected via i.p. After 10 min, the number of writhings during the following 20 minute period was counted. Inhibition percentage was calculated through the decrease of total number of writhings in the treated groups against the control group.
2.10. Statistical Analysis
Data were expressed as mean ± SEM and statistically assessed using one-way ANOVA (origin 5.0). P values less than 0.05 were considered significant.
3. Results and Discussion
Natural products, known as secondary metabolites of plants or animals, continue to be an important segment of research into new drugs. In fact, many compounds frequently used in chemotherapy, such as vincristine, taxol, and camptothecins, were isolated from plants or derived from natural prototypes [18–20].
Additionally, the search for new drugs that effectively interfere with the inflammatory process and pain is currently of great relevance; plants traditionally used as well as their derivative substances have historically been valued as a source of anti-inflammatory agents and pain killers [21, 22].
3.1. Cytotoxicity
The assays revealed that, at 50.0, 25.0, and 12.5 μg/mL, F80 inhibited NCI-H292 cell growth by 25.6%, 14.3%, and 7.8%, respectively. A concentration of 6.25 μg/mL and HEp-2 cell line induced no growth inhibition. CE did not show significant cytotoxic activity for either cell line. IC50 up to 50.0 μg/mL used cell lines did not allow calculation of IC50. Therefore, both extract and fraction presented low cytotoxicity.
Only F80 showed some cytotoxic activity; CE did not present cytotoxicity against the cell lines used. Several previous studies have demonstrated that plant extracts and derivative compounds have an anticancer potential in vitro or in vivo. Nakamura et al. [6] tested distinct L. ferrea pod extracts using the in vitro Epstein-Barr virus early-antigen activation assay and found that the ethyl acetate extract exhibited the strongest inhibitory activity. Nozaki et al. [23], also working with L. ferrea, showed that pauferrol, a compound obtained from the stem, possessed a cell proliferation inhibitory activity through the induction of apoptosis in human leukemia HL60 cells.
3.2. Acute Oral Toxicity—LD50 Determination
The doses of 300 mg/kg and 2,000 mg/kg did not induce mice weight loss or death from either extract or fraction. LD50 cutoff of CE and F80 was determined as 2,500 mg/kg body weight; both concentrations used were considered as safe (category 5) [14].
Despite widespread use (herbal medicine is applied by up to 80% of the population in developing countries), few scientific studies have been undertaken to ascertain the safety and efficacy of traditional remedies [24]. The present investigation suggests that the aqueous extract of L. ferrea pods is practically nontoxic via oral route in mice using an acute single dose, indicating that the popular use of this kind of extract can be considered secure.
3.3. Antitumor Activity
F80 showed a tumor growth inhibition of 8.68%, but this value is not significant when compared with the control group. CE did not reveal antitumor activity (Figure 1); CE and F80 did not reduce tumor weight significantly. However, Nakamura et al. [8] isolated two constituents (gallic acid and methyl gallate) from L. ferrea pod and tested them against skin carcinogenesis in mice, finding a reduction in the average number of papillomas per mouse. Studying another Caesalpinia species, C. bonducella, Gupta et al. [18] evaluated the antitumor effect of methanol leaf extract against Ehrlich ascites carcinoma in mice; the extract promoted a significant increase of mean survival time of mice and a decrease of tumor volume, when compared with control group. Then, the antitumor effect of Caesalpinia spp. may vary according to extract (and/or isolated constituents) and tumor types used.
Figure 1.
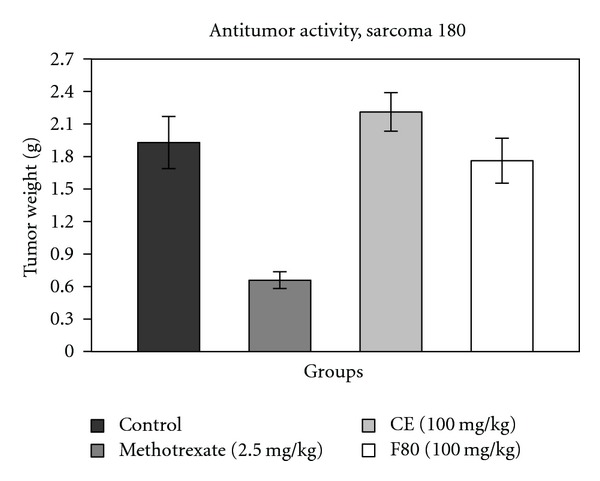
Effects of MTX (methotrexate), CE, and F80 on the growth of sarcoma 180 in Swiss albino male mice. Each column represents the mean of six animals, and vertical lines show the SEM. The asterisk denotes the significance level in comparison to the control value: *P < 0.05.
3.4. Anti-Inflammatory Assay
CE and F80 exhibited anti-inflammatory activity, reducing cell migration. CE and F80 showed significant reduction (40.9% and 38.2%, resp.) in the number of PMNL in the inflammatory exudate, similar to piroxicam (46.7%) (Figure 2 and Table 1).
Figure 2.
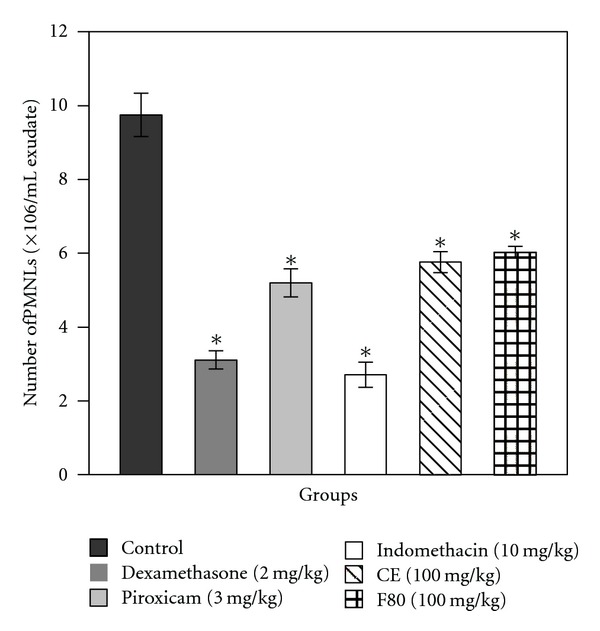
Effect of pretreatment with dexamethasone, piroxicam, indomethacin (standard drugs), CE, and F80 on migration of polymorphonuclear leukocytes (PMNLs) (number of PMNLs/mL exudate) in carrageenan-induced peritonitis in mice. Each column represents the mean of six animals, and vertical lines show the SEM. Asterisks denote the significance level in comparison to the control value: *P < 0.05.
Table 1.
Evaluation of anti-inflammatory activity of standard drugs (dexamethasone, piroxicam and indomethacin), CE, and F80 on carrageenan-induced peritonitis in pretreated mice.
| Compound | PMNL/mL exudate ± SEM (×106) | Anti-inflammatory activity (%) |
|---|---|---|
| Control | 9.7 ± 0.8 | — |
| Dexamethasone (2 mg/kg) | 3.1 ± 0.3* | 68.1 |
| Piroxicam (3 mg/kg) | 5.2 ± 0.5* | 46.7 |
| Indomethacin (10 mg/kg) | 2.7 ± 0.4* | 72.2 |
| CE (100 mg/kg) | 5.8 ± 0.3* | 40.9 |
| F80 (100 mg/kg) | 6.0 ± 0.1* | 38.2 |
n = 6. *P < 0.05 versus the control group.
Among the phlogistic agents available (such as dextran, bradykinin, β-glucan, etc.), carrageenan is perhaps the most commonly used and well studied [25] producing a maximal edema in 3 h. While the carrageenan model is typically associated with activation of the cyclooxygenase pathway and is sensitive to glucocorticoids and prostaglandin synthesis antagonists, the early phase of the carrageenan response is due to the release of serotonin and histamine [26]. Thus, the significant ameliorative activity of CE and F80 observed in the present study may be due to inhibition of inflammatory mediators such as histamine, serotonin, and prostaglandin and also due to a decrease in NO production.
According to Kelly et al. [27], there is a growing optimism that inhibition of leukocyte recruitment might prevent inappropriate inflammation. So, the search for drugs that act upon cell migration may be of great interest. Our study is unprecedented, since no other study has related the CE and F80 effects on cell migration.
In a previous approach, Carvalho et al. [28] determined the anti-inflammatory activity of a crude aqueous extract of L. ferrea pods at 60°C, using the carrageenan-induced paw edema method in mice. Assays revealed that the extract reduced the edema formation significantly from the first moments.
Several plant extracts present expressive anti-inflammatory activity by reducing leukocyte migration. Matos et al. [29], using the same experimental model applied in the present study, also observed a decrease in cell migration in mice treated with an aqueous fraction obtained from a leaf ethanolic extract of Spiranthera odoratissima. Gokhale et al. [30] working with ethanolic extracts from Saussurea lappa, Argyreia speciosa, and Achyranthes aspera discovered that all of them show anti-inflammatory activity using the carrageenan-induced paw edema and carrageenan-induced peritonitis models in rats and mice.
3.5. Nitrite Analysis
The nitrite content in the exudates was quantified using Griess reagent since nitric oxide (NO) synthesis by inducible nitric oxide synthase (iNOS) is increased in inflammation and leads to cellular injury. This assay is an indirect method to quantify NO, which rapidly reduces to nitrate and nitrite.
Nitric oxide derived from iNOS is involved in various pathological conditions such as inflammation and autoimmune diseases leading to tissue damage [31]. Thus, suppression of iNOS is closely linked with anti-inflammatory action [32].
CE and F80 promoted a high reduction in the content of nitrite, especially CE, which decreased it to a level smaller than those presented by the standard drugs, as shown in Figure 3. The strong reduction by CE of nitrite level in the exudate might be attributed to the presence of antioxidant compounds in this aqueous extract. It is likely that CE and F80 show their anti-inflammatory activity through the downregulation of NO production and reduction in leukocyte migration.
Figure 3.
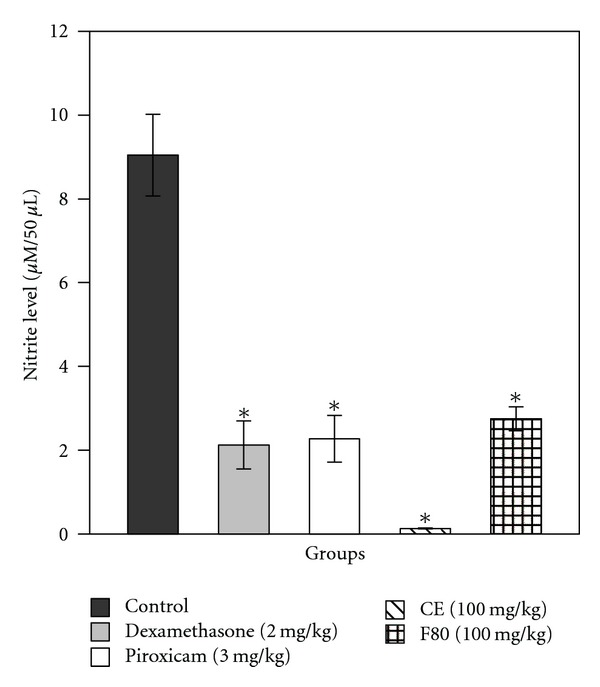
Inhibitory effects of the standard drugs, dexamethasone and piroxicam, CE, and F80 in relation to the control group for NO production. Each column represents the mean of six animals, and vertical lines show the SEM. Asterisks denote the significance level in comparison to the control value: *P < 0.05.
Plant extracts showing immunomodulatory activities have been extensively described, especially for their inhibitory effect upon the NO production by macrophages [33, 34]. Ahn et al. [32], investigating the ethanol extract and fractions of Gastrodia elata rhizomes, discovered that the extract and its n-butanol fraction decreased the nitrite content in the exudates obtained from the carrageenan-induced air-pouch model in mice. Koo et al. [35] also verified this reduction by testing two constituents (geniposide and genipin) obtained from the ethanol extract of Gardenia jasminoides fruit in rats.
3.6. Analgesic Activity—Acetic Acid-Induced Writhing Response
There are different routes to evaluate nociception; however, the assay using intraperitoneally acetic acid was the chosen approach. The animals were pretreated one hour before administration of phlogistic agent, then apparently, there was no influence of the administration effect. Other authors have also used parenteral route in their researches [36, 37].
It is well known that the intraperitoneal administration of agents that irritate serous membranes, such as acetic acid, causes a stereotypical behavior in mice characterized by abdominal contractions, movements of the body as a whole, twisting of dorsoabdominal muscles, and a decrease in motor activity and coordination [38]. Using the acetic acid-induced writhing response, which is the visceral pain model, the analgesic mechanism of abdominal writhing involves different nociceptive mechanisms, such as the process or release of arachidonic acid metabolites via cyclooxygenase and prostaglandin biosynthesis, opioid mechanisms, local peritoneal receptors and mediators related to acetylcholine and histamine, and sympathetic system mediators [35, 39, 40].
Acetic acid causes algesia by releasing endogenous substances, which then excite the pain nerve endings; the abdominal constriction is related to sensitization of nociceptive receptors to prostaglandins [41]. Although this assay is nonspecific (e.g., anticholinergic, antihistaminic, and other agents also show activity), it is a very sensitive procedure that enables the detection of peripheral antinociceptive activity of compounds using animal protocols and is widely used for analgesic screening. This method is simple and reliable and affords rapid evaluation of peripheral analgesic action [42].
Dipyrone (metamizol) is a widely used nonsteroidal anti-inflammatory drug (NSAID). In addition to the well-known peripheral effects of NSAIDs—especially prostaglandin synthesis inhibition—and the fact that dipyrone is able to induce a significant antinociceptive effect in the absence of an anti-inflammatory response, it has been proposed that it produces antinociception at least partially by acting upon central nervous system structures [43, 44].
Since CE and F80 were confirmed to have anti-inflammatory activity in carrageenan-induced peritonitis (Figure 2 and Table 1), their analgesic activity was examined. As shown in Table 2 and Figure 4, both preparations protected mice against chemically induced noxious stimulus, causing an inhibition at the rate of 56.6% (for CE, higher than that promoted by piroxicam) and 72.7% (for F80, comparable to dipyrone) on the writhing response induced by acetic acid. These results indicate that CE and F80 showed analgesic action in addition to anti-inflammatory activity. Subsequently, it suggests that prostaglandin biosynthesis might be commonly involved in both activities of CE and F80 or that the mode of action of both preparations is related to sensitization of nociceptive receptors to prostaglandins.
Table 2.
Antinociceptive effect of standard drugs (piroxicam and dipyrone), CE, and F80 on acetic acid-induced writhing response test in mice.
| Compound | Dose | Medium ± SEM | Protection (%) |
|---|---|---|---|
| Control | — | 69.1 ± 7.8 | — |
| Piroxicam | 10 mg/kg | 46.5 ± 1.6* | 32.73 |
| Dipyrone | 150 mg/kg | 18.2 ± 1.9* | 73.60 |
| CE | 100 mg/kg | 30.0 ± 4.0* | 56.60 |
| F80 | 100 mg/kg | 18.9 ± 1.9* | 72.72 |
n = 6. *P < 0.05 versus the control group.
Figure 4.
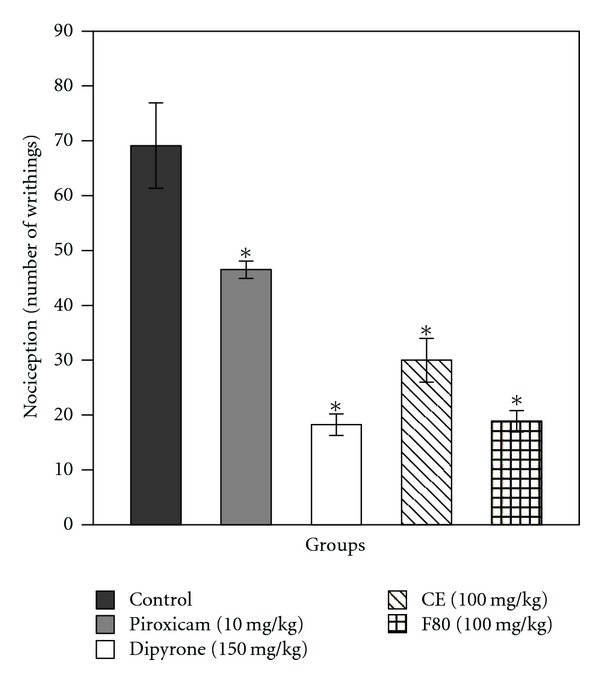
Effects of the standard drugs, piroxicam and dipyrone, CE, and F80 in relation to the control group on writhing induced in mice by intraperitoneal injection of acetic acid. Each column represents the mean of six animals, and vertical lines show the SEM. Asterisks denote the significance level in comparison to the control value: *P < 0.05.
Carvalho et al. [28] also evaluated the analgesic activity of an L. ferrea pod extract, using a variation of the method described here and the hot-plate test in mice. Our results corroborate theirs, both showing significant reduction in nociception in treated groups.
4. Conclusions
CE and F80 have neither significant cytotoxic nor antitumor activities over NCI-H292 and HEp-2 cell lines or the solid tumor (sarcoma 180) assayed. However, further studies are necessary to evaluate these activities using other cell lines and tumors to elucidate whether these preparations are effective against any of them, as this plant is used popularly for cancer prevention. On the other hand, their low toxicity allows the use of CE and F80 with a degree of safety in other situations. Furthermore, the antinociceptive and anti-inflammatory effects demonstrated in the present study contributed to the ethnomedical uses of L. ferrea. Further investigations are necessary to elucidate the precise mechanisms of action and the compounds responsible for these effects.
Acknowledgments
This work was financially supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The authors are also grateful for the technical assistance of Mrs. Maria Barbosa Reis da Silva and Mr. João Virgínio. Scott V. Heald, North American teacher at CIEC, bilingual school, is acknowledged for English review.
References
- 1. The International Plant Names Index, 2009, http://www.ipni.org/
- 2.Bragança LAR. Plantas Medicinais Antidiabéticas. Niterói, Rio de Janeiro, Brasil: EDUFF Press; 1996. [Google Scholar]
- 3.Lorenzi H. Árvores Brasileiras Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil. 4th edition. Nova Odessa, São Paulo, Brasil: Instituto Plantarum de Estudos da Flora; 2002. [Google Scholar]
- 4.Balbach A. As Plantas que Curam. Três, São Paulo, Brasil; 1972. [Google Scholar]
- 5.Hashimoto G, editor. Illustrated Cyclopedia of Brazilian Medicinal Plants. Kamakura, Japan: Aboc-sha; 1996. [Google Scholar]
- 6.Nakamura ELS, Kurosaki F, Arisawa M, et al. Cancer chemopreventive effects of constituents of Caesalpinia ferrea and related compounds. Cancer Letters. 2002;177(2):119–124. doi: 10.1016/s0304-3835(01)00708-x. [DOI] [PubMed] [Google Scholar]
- 7.Gomes M. As Plantas da Saúde—Guia de Tratamentos Naturais. 3rd edition. São Paulo, Brazil: Paulinas; 2003. [Google Scholar]
- 8.Nakamura ELS, Kurosaki F, Arisawa M, et al. Cancer chemopreventive effects of a Brazilian folk medicine, Juca, on in vivo two-stage skin carcinogenesis. Journal of Ethnopharmacology. 2002;81(1):135–137. doi: 10.1016/s0378-8741(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 9.Pereira EC, Nascimento SC, Lima RC, et al. Analysis of Usnea fasciat a crude extracts with antineoplastic activity. Tokai Journal of Experimental and Clinical Medicine. 1994;19(1-2):47–52. [PubMed] [Google Scholar]
- 10.Alley MC, Scudiero DA, Monks A, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Research. 1988;48(3):589–601. [PubMed] [Google Scholar]
- 11.Slater SF, Sawter R, Strauli U. Studies on succinate-tetrazolium reductase system—III. Points of coupling of different tetrazolium salts. Biochemical et Biophysica Acta. 1963;77:383–393. doi: 10.1016/0006-3002(63)90513-4. [DOI] [PubMed] [Google Scholar]
- 12.Hess R, Pearse AGE. Pathways of reduced pyridine nucleotide oxidation in rat-brain homogenate demonstrated by a tetrazolium method. Biochimica et Biophysica Acta. 1963;71:285–294. doi: 10.1016/0006-3002(63)91083-7. [DOI] [PubMed] [Google Scholar]
- 13.Geran RI, Greenber NH, Macdonald MM, Schumacher RI, Abbott BJ. Protocols for screening chemical agents and natural-products against tumors and other biological systems. Cancer Chemotherapy Reports. 1972;2(3, part 3) [Google Scholar]
- 14.OECD-Organization for Economic Cooperation and Development. OECD Guideline for Testing of Chemicals. 2001. (Guideline 423: Acute Oral Toxicity—Acute Toxic Class Method). [Google Scholar]
- 15.Gupta M, Mazumder UK, Sambath Kumar R, et al. Anti-inflammatory, analgesic and antipyretic effects of methanol extract from Bauhinia racemosa stem bark in animal models. Journal of Ethnopharmacology. 2005;98(3):267–273. doi: 10.1016/j.jep.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Sherman MP, Aeberhard EE, Wong VZ, Griscavage JM, Ignarro LJ. Pyrrolidine dithiocarbamate inhibits induction of nitric oxide synthase activity in rat alveolar macrophages. Biochemical and Biophysical Research Communications. 1993;191(3):1301–1308. doi: 10.1006/bbrc.1993.1359. [DOI] [PubMed] [Google Scholar]
- 17.Young HY, Luo YL, Cheng HY, Hsieh WC, Liao JC, Peng WH. Analgesic and anti-inflammatory activities of [6]-gingerol. Journal of Ethnopharmacology. 2005;96(1-2):207–210. doi: 10.1016/j.jep.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Gupta M, Mazumder UK, Kumar RS, Sivakumar T, Vamsi MLM. Antitumor activity and antioxidant status of Caesalpinia bonducella against ehrlich ascites carcinoma in Swiss albino mice. Journal of Pharmacological Sciences. 2004;94(2):177–184. doi: 10.1254/jphs.94.177. [DOI] [PubMed] [Google Scholar]
- 19.van der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R. The Catharanthus alkaloids: pharmacognosy and biotechnology. Current Medicinal Chemistry. 2004;11(5):607–628. doi: 10.2174/0929867043455846. [DOI] [PubMed] [Google Scholar]
- 20.Costa-Lotufo LV, Khan MTH, Ather A, et al. Studies of the anticancer potential of plants used in Bangladeshi folk medicine. Journal of Ethnopharmacology. 2005;99(1):21–30. doi: 10.1016/j.jep.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 21.Elisabetsky E, Arnador TA, Albuquerque RR, Nunes DS, Do CT Carvalho A. Analgesic activity of Psychotria colorata (Willd. ex R. and S.) Muell. Arg. alkaloids. Journal of Ethnopharmacology. 1995;48(2):77–83. doi: 10.1016/0378-8741(95)01287-n. [DOI] [PubMed] [Google Scholar]
- 22.Calixto JB, Campos MM, Otuki MF, Santos ARS. Anti-inflammatory compounds of plant origin—part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Medica. 2004;70(2):93–103. doi: 10.1055/s-2004-815483. [DOI] [PubMed] [Google Scholar]
- 23.Nozaki H, Hayashi KI, Kido M, et al. Pauferrol A, a novel chalcone trimer with a cyclobutane ring from Caesalpinia ferrea mart exhibiting DNA topoisomerase II inhibition and apoptosis-inducing activity. Tetrahedron Letters. 2007;48(47):8290–8292. [Google Scholar]
- 24.Veerappan A, Miyazaki S, Kadarkaraisamy M, Ranganathan D. Acute and subacute toxicity studies of Aegle marmelos Corr., an Indian medicinal plant. Phytomedicine. 2007;14(2-3):209–215. doi: 10.1016/j.phymed.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Garcia Leme J, Hamamura L, Leite MP, Rocha e Silva M. Pharmacological analysis of the acute inflammatory process induced in the rat’s paw by local injection of carrageenin and by heating. British Journal of Pharmacology. 1973;48(1):88–96. doi: 10.1111/j.1476-5381.1973.tb08225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Rosa M, Giroud JP, Willoughby DA. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. Journal of Pathology. 1971;104(1):15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- 27.Kelly M, Hwang JM, Kubes P. Modulating leukocyte recruitment in inflammation. Journal of Allergy and Clinical Immunology. 2007;120(1):3–10. doi: 10.1016/j.jaci.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Carvalho JCT, Teixeira JRM, Souza PJC, Bastos JK, Dos Santos Filho D, Sarti SJ. Preliminary studies of analgesic and anti-inflammatory properties of Caesalpinia ferrea crude extract. Journal of Ethnopharmacology. 1996;53(3):175–178. doi: 10.1016/0378-8741(96)01441-9. [DOI] [PubMed] [Google Scholar]
- 29.Matos LG, Santos LDAR, Vilela CF, et al. Atividades analgésica e/ou antiinflamatória da fração aquosa do extrato etanólico das folhas da Spiranthera odoratissima A. St. Hillaire (manacá) Revista Brasileira de Farmacognosia. 2003;13, supplement:15–16. [Google Scholar]
- 30.Gokhale AB, Damre AS, Kulkarni KR, Saraf MN. Preliminary evaluation of anti-inflammatory and anti-arthritic activity of S. lappa, A. speciosa and A. aspera . Phytomedicine. 2002;9(5):433–437. doi: 10.1078/09447110260571689. [DOI] [PubMed] [Google Scholar]
- 31.Singh VK, Mehrotra S, Narayan P, Pandey CM, Agarwal SS. Modulation of autoimmune diseases by nitric oxide. Immunologic Research. 2000;22(1):1–19. doi: 10.1385/IR:22:1:1. [DOI] [PubMed] [Google Scholar]
- 32.Ahn EK, Jeon HJ, Lim EJ, Jung HJ, Park EH. Anti-inflammatory and anti-angiogenic activities of Gastrodia elata Blume. Journal of Ethnopharmacology. 2007;110(3):476–482. doi: 10.1016/j.jep.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Rimbach G, Park YC, Guo Q, et al. Nitric oxide synthesis and TNF-α secretion in RAW 264.7 macrophages mode of action of a fermented papaya preparation. Life Sciences. 2000;67(6):679–694. doi: 10.1016/s0024-3205(00)00664-0. [DOI] [PubMed] [Google Scholar]
- 34.Ryu JH, Ahn H, Kim JY, Kim YK. Inhibitory activity of plant extracts on nitric oxide synthesis in LPS-activated macrophages. Phytotherapy Research. 2003;17(5):485–489. doi: 10.1002/ptr.1180. [DOI] [PubMed] [Google Scholar]
- 35.Koo HJ, Lim KH, Jung HJ, Park EH. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. Journal of Ethnopharmacology. 2006;103(3):496–500. doi: 10.1016/j.jep.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Woode E, Abotsi WK. Antinociceptive effect of an ethanolic extract of the aerial parts of Hilleria latifolia (Lam.) H. Walt. (Phytolaccaceae) Journal of Pharmacy and Bioallied Sciences. 2011;3(3):384–396. doi: 10.4103/0975-7406.84445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li P, Zhu S. Mutational analysis of the analgesic peptide DrTx(1–42) revealing a functional role of the amino-terminal turn. PLoS One. 2012;7(2, article e31830) doi: 10.1371/journal.pone.0031830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacological Reviews. 2001;53(4):597–652. [PubMed] [Google Scholar]
- 39.Collier HO, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. British Journal of Pharmacology. 1968;32(2):295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park YM, Won JH, Kim YH, Choi JW, Park HJ, Lee KT. In vivo and in vitro anti-inflammatory and anti-nociceptive effects of the methanol extract of Inonotus obliquus . Journal of Ethnopharmacology. 2005;101(1–3):120–128. doi: 10.1016/j.jep.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Chen Q. Methodology in Pharmacological Study on Chinese Materia Medica. Beijing, China: 7 People’s Medical Publishing House; 1993. [Google Scholar]
- 42.Shinde UA, Phadke AS, Nair AM, Mungantiwar AA, Dikshit VJ, Saraf MN. Studies on the anti-inflammatory and analgesic activity of Cedrus deodara (Roxb.) Loud. wood oil. Journal of Ethnopharmacology. 1999;65(1):21–27. doi: 10.1016/s0378-8741(98)00150-0. [DOI] [PubMed] [Google Scholar]
- 43.Hernández N, Vanegas H. Antinociception induced by PAG-microinjected dipyrone (metamizol) in rats: involvement of spinal endogenous opioids. Brain Research. 2001;896(1-2):175–178. doi: 10.1016/s0006-8993(01)02085-6. [DOI] [PubMed] [Google Scholar]
- 44.Hernández-Delgadillo GP, Cruz SL. Dipyrone potentiates morphine-induced antinociception in dipyrone-treated and morphine-tolerant rats. European Journal of Pharmacology. 2004;502(1-2):67–73. doi: 10.1016/j.ejphar.2004.08.032. [DOI] [PubMed] [Google Scholar]


