Abstract
Background
Tolerance to non-inherited maternal antigens (NIMA) has provided clinical advantage when kidney transplants are exchanged between siblings, but not when mother herself is the donor. This paradox prompted us to revisit the “two-way” hypothesis of transplant tolerance—that the immune status of both the organ recipient and the organ donor critically influences allograft outcome.
Methods
We obtained PBMC from 29 living donor-recipient pairs prior to transplant and used the trans-vivo DTH assay to measure immune regulation in both the recipient anti-donor and donor anti-recipient directions.
Results
We found pre-existing bidirectional regulation in all HLA-identical sibling pairs tested (7/7), and half (9/18) the HLA haploidentical pairs. No significant regulation was found in 4 control living unrelated (LUR) and 2 HLA haploidentical living-related (LR) donor recipient pairs, while unidirectional regulation was found in the remaining 7 haploidentical pairs. Of the 9 HLA haploidentical transplants with unidirectional or no pre-transplant regulation, 7 had an acute rejection episode, and 4 of these experienced graft loss. In contrast, of the 9 HLA haploidentical transplants with bidirectional regulation, only 1 had rejection. Renal function for the latter group was similar to HLA-identical kidney recipients at 3 years post-transplant. Significantly (p<0.05) lower mean sCr values in bidirectional regulators were noted as early as 4 months and this difference became more pronounced at 12 (p<0.005) and 36 months (p<0.0001).
Conclusions
Contrary to the belief that only the recipient’s immune status matters, the data indicate that pre-transplant immune status of both donor and recipient influence post transplant outcome.
Keywords: kidney transplantation, pre-transplant bidirectional regulation, prediction of outcome, indirect pathway
Introduction
Exposure to non-inherited maternal antigens (NIMA) in fetal and neonatal life has lifelong immunological consequences. Studies by Owen et al. (1) and Claas et al. (2) established that NIMA exposures induced partial B cell tolerance in offspring. We subsequently reported that 10-year survival of 1-haplotype-mismatched kidney allografts from a sibling donor was significantly better if the mismatched HLA haplotype was maternal (NIMA), as compared with paternal (NIPA, non-inherited paternal antigen)-mismatched donor-recipient pairs (3). A strong NIMA effect was also seen in patients receiving HLA haploidentical hematopoietic stem cell transplants from a sibling (4, 5). However, the most straightforward type of NIMA transplant, kidney replacement by mother herself, has never been shown to provide longer term graft survival in offspring than a kidney donated by father, and indeed may have worse outcome (6, 7). This “NIMA paradox” has never been resolved.
Van Halteren et al. found that the majority of parous women were primed to respond strongly to minor H antigens (miHA) of their sons, as indicated by HY-specific CTL generation, lack of regulation to HY allopeptides, and predominance of CD8 “tetramer-bright” effector T cells (8). In the majority of healthy sons tested, however, NIMA miHA-specific CTLA4+CD8 “tetramer-dim” T regulatory cells (TR), were found to predominate in PBMC, similar to CD8 TR found in a case of tolerance to a NIMA miHA-mismatched, HLA-ID sibling kidney transplant (9). These disparate results in mothers and offspring highlight the asymmetry of regulation caused by fetal-maternal cell exchange.
We recently reported that pre-transplant evaluation of indirect pathway TR cells in NIMAd-exposed, H2b-homozygous F1 backcross mice using the trans-vivo DTH (tvDTH) assay predicts tolerance to a NIMA-expressing, H2d-mismatched heart allograft from a naïve DBA/2 donor (10). To follow up this discovery we utilized a similar tvDTH assay to test the hypothesis that pre-existing regulation is a common feature of human living related donor-recipient pairs prior to transplant. Our data suggest that this hypothesis is correct, and surprisingly, that the immune status of the kidney donor toward recipient critically influences subsequent renal allograft outcome.
Results
Table 1 shows the demographics of 29 patients with ESRD and their living donors. There were no significant ethnic, age, or gender differences at transplant between the HLA identical (HLA-ID), HLA haploidentical (Haplo-ID) and living unrelated (LUR) transplant recipients or donors, except that there were more female donors in the Haplo-ID group vs. other two groups. Induction therapy (Table 1) and immunosuppression (IS) regimen are shown in detail in Supplementary Table 1. Only 1 out of 7 patients in the HLA-ID group received an induction therapy (Simulect) whereas for Haplo-ID and LUR groups a strongly depleting regimen (Campath, or Campath/Rituximab) was used with several variations, in trials designed to avoid CNI toxicity while preserving kidney allograft function.
TABLE 1.
Demographics of 29 patients and living donors
| HLA Identical | HLA Haploidentical | Living Unrelated | ||
|---|---|---|---|---|
| Recipients: | ||||
| n | 7 | 18 | 4 | |
| Gender | Male | 3 | 11 | 3 |
| Female | 4 | 7 | 1 | |
| Age at transplant | 42.7 ± 11.4 | 40 ± 16.2 | 46 ± 14.4 | |
| Ethnicity | Caucasian | 7 | 17 | 4 |
| Hispanic | 0 | 1 | 0 | |
| Induction therapy: | ||||
| Simulect | 1 | 3 | 0 | |
| Campatha | 0 | 8 | 2 | |
| Campath/Rituximabb | 0 | 6 | 2 | |
| No Induction | 6 | 1 | 0 | |
| Donors: | ||||
| Gender | Male | 4 | 4 | 3 |
| Female | 3 | 14 | 1 | |
| Age at donation | 42.5 ± 10.4 | 46.6 ± 10.2 | 39.1 ± 12.7 | |
| Ethnicity | Caucasian | 7 | 17 | 4 |
| Hispanic | 0 | 1 | 0 | |
2 of the10 patients in this group were part of a ITN-013ST study of CNI withdrawal to sirolimus monotherapy.
Patients enrolled in a trial of calcineurin-inhibitor-free immunosuppression. All other patients received either cyclosporin A or tacrolimus as maintenance therapy. See Supplemental Table 1 for details.
Baseline regulation is strongly influenced by family relationship
To examine pre-existing regulation in individuals with ESRD and in healthy controls, we analyzed peripheral blood from 75 subjects. These included 25 living related donor-recipient pairs and 4 LUR pairs (1 day prior to transplant, n=58 subjects) and an additional 13 ESRD subjects and 4 healthy non-donor family members.
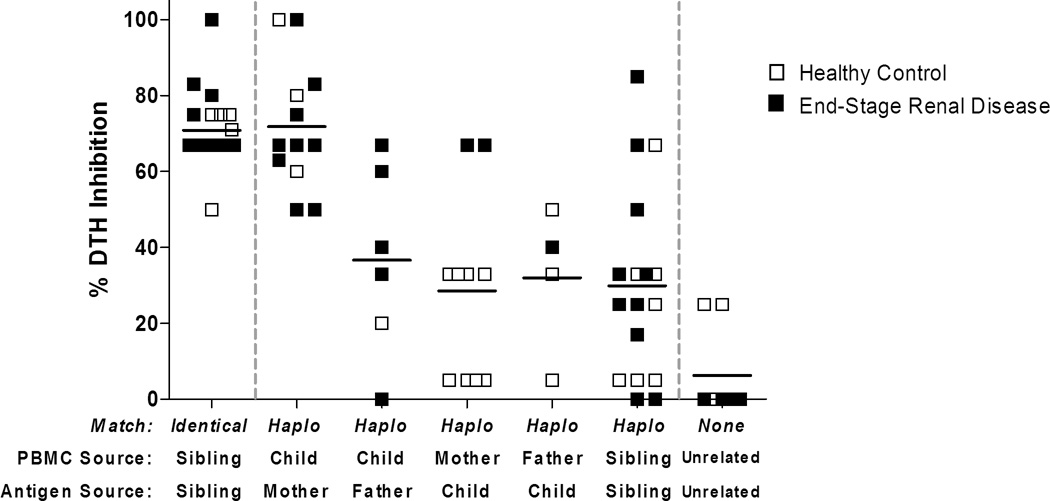
Figure 1 shows regulation expressed as percent inhibition of a recall antigen (EBV or TT) response in the presence of cell sonicate-derived alloantigen. Responses were grouped based on alloantigen source. As expected, we found no evidence of pre-transplant regulation (mean inhibition value 6.2 ± 11.6%) in the indirect pathway alloresponses of living unrelated (LUR) pairs. In contrast, all HLA identical siblings exhibited uniformly strong (≥50%) regulation to each other’s alloantigens, with an average inhibition of 70.9 ± 9.4%. Such a strong regulatory bias between HLA-ID siblings was most likely caused by exposure to maternal non-inherited minor H antigens, since a) identical twins do not exhibit any evidence of immune regulation to each other (Haynes, L.D. et al. manuscript submitted), and b) regulation to the minor H NIMA is a common feature of healthy offspring (8). Indeed we found a consistently high suppression of recall responses (by at least 50%) in all sons’ and daughters’ PBMC tested with maternal antigen. The average percentage of tvDTH inhibition was 71.8 ± 16.6%, similar to that observed in the HLA-ID sibling group. Importantly, unlike the bidirectional regulation seen in the HLA-ID siblings, only 2/10 mothers (both with ESRD) exhibited a strongly (>50% inhibition) regulated response to inherited paternal antigens (IPA) expressed by her offspring. Of the healthy mothers tested, 4 had moderate (33%) regulation to IPAs, while 4 had no regulation.
Figure 1. Pre transplant immune regulation to familial antigens in healthy controls and in patients with end-stage renal disease (ESRD).
PBMC from donor and recipients pairs obtained on day -1 were tested in the trans-vivo DTH assay. Subjects were grouped based on antigen source [x axis]: HLA-ID sibling (familial minor H antigens); mother or father (NIMA, NIPA); Offspring antigen presented to mother (IPA), or father (IMA); one HLA-haplotype matched siblings; and living unrelated donor (LURD). Healthy subjects and patients with ESRD are indicated by open and closed symbols, respectively. Solid horizontal lines are mean % inhibition values for all patients in a given relationship category.
Other living-related Haplo-ID pairs showed a mixed pattern: for example, 2/6 offspring had a strong regulatory response in the presence of the father’s NIPA. Pre-transplant regulation was seen also between some Haplo-ID sibling pairs. Regulation in this group was highly variable: the mean inhibition was 29.9%, similar to that observed in the NIPA, IPA and IMA (father vs. son or daughter) response groups, but the standard deviation was high (25.1%).
Kidney transplant outcome is influenced by regulation status of both donor and recipient
Table 2 details the donor and recipient pre-transplant regulation scores and renal function at 3 years post-transplant for all 29 recipients. Within each group, transplants are listed in order of the combined (Recipient + Donor) tvDTH percent inhibition score. All 7 HLA-ID recipient-donor pairs exhibited bidirectional regulation (mean combined inhibition= 142 ± 9.2%). As expected, none of the patients in this group experienced any episode of allograft rejection, and renal function was excellent at 3 years follow-up, with a mean serum creatinine (sCr) 1.26 ± 0.29 mg/dL and a mean GFR 59.0±18.3 mL/min/1.73m2.
TABLE 2.
Summary of pre-transplant TV-DTH results and 3 yr graft outcomes
| % DTH Inhibition (Pre-Transplant) | Outcomes at 36 Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Identifier | Donor:Recipient Relationship |
Donor Mismatch |
Recipient Cells: Donor Ag |
Donor Cells :Recipient Ag |
Combined % Inhibition |
Serum Creatinine (mg/dL) |
GFR (mL/min/1.73m2) |
Rejection Episodes |
DSA Class I/ Class II |
| HLA Identical | HLA-ID 40 | brother:brother | Minor H | 83 | 75 | 158 | 1.3 | 53 | - | NT |
| HLA-ID 37 | sister:brother | Minor H | 100 | 50 | 150 | 1.2 | 71 | - | NT | |
| HLA-ID 33 | brother:sister | Minor H | 67 | 75 | 142 | 0.9 | 72 | - | NT | |
| HLA-ID 44 | sister:sister | Minor H | 67 | 75 | 142 | 1.8 | 30 | - | NT | |
| HLA-ID 35 | brother:brother | Minor H | 67 | 67 | 134 | 1.1 | 84 | - | NT | |
| HLA-ID 34 | brother:sister | Minor H | 67 | 67 | 134 | 1.1 | 58 | - | NT | |
| HLA-ID 36 | sister:sister | Minor H | 67 | 67 | 134 | 1.4 | 45 | - | NT | |
| Mean ± SD | 74.0 ± 12.9 | 68.0 ± 8.9 | 142.0 ± 9.2 | 1.26 ± 0.29 | 59.0 ± 18.3 | n=0 | ||||
| HLA Haploidentical: Bidirectional Regulationa | Haplo-ID 49 | daughter:mother | IPA | 67 | 100 | 167 | 1.1 | 54 | + | +/− |
| Haplo-ID 67 | daughter:mother | IPA | 67 | 60 | 127 | 1.1 | 53 | - | - | |
| Haplo-ID 45 | sister:sister | Haplo | 85 | 33 | 118 | 1.3 | 45 | - | - | |
| Haplo-ID 62 | mother:son | NIMA | 83 | 33 | 116 | 1.2 | 80 | - | - | |
| Haplo-ID 58 | sister:sister | Haplo | 25 | 67 | 92 | 1.1 | 57 | - | +/−c | |
| Haplo-ID 63 | mother:daughter | NIMA | 50 | 33 | 83 | 1.0 | 66 | - | - | |
| Haplo-ID 50 | mother:son | NIMA | 50 | 33 | 83 | 1.3 | 84 | - | - | |
| Haplo-ID 59 | father:son | NIPA | 40 | 33 | 73 | 1.5 | 54 | - | - | |
| Haplo-ID 51 | sister:sister | Haplo | 33 | 33 | 66 | 1.2 | 47 | - | - | |
| Mean ± SD | 55.6 ± 21.3 | 47.2 ± 23.9 | 102.8 ± 32.1 | 1.20 ± 0.15 | 60.0 ± 13.9 | n=1 | n=2 | |||
| HLA Haploidentical: One-way or No Regulationb | Haplo-ID 56 | mother:daughter | NIMA | 67 | 0 | 67 | 4.8 | 12 | + | +/+ |
| Haplo-ID 43 | mother:son | NIMA | 67 | 0 | 67 | 2.2 | 55 | + | +/+ | |
| Haplo-ID 55 | mother:son | NIMA | 63 | 0 | 63 | 1.6 | 57 | + | −/+ | |
| Haplo-ID 61 | son:father | IMA | 40 | 20 | 60 | 5.4 | 12 | + | - | |
| Haplo-ID 40 | sister:brother | Haplo | 33 | 25 | 58 | 1.8 | 43 | - | +/+ | |
| Haplo-ID 54 | brother:brother | Haplo | 50 | 0 | 50 | 2.0 | 39 | + | - | |
| Haplo-ID 66 | father:son | NIPA | 0 | 50 | 50 | 5.5 | 14 | + | - | |
| Haplo-ID 60 | sister:brother | Haplo | 25 | 0 | 25 | 10.0 | 6 | + | +/+ | |
| Haplo-ID 46 | sister:brother | Haplo | 0 | 0 | 0 | 1.4 | 54 | - | - | |
| Mean ± SD | 38.3 ± 26.3 | 10.6 ± 17.8 | 48.9 ± 22.4 | 3.86 ± 2.86 | 32.4 ± 21.2 | n=7 | n=5 | |||
| All HLA Haploidentical | Mean ± SD | 46.9 ± 24. 9 | 28.9 ± 27.8 | 75.8 ± 38.6 | 2.50 ± 2.40 | 46.2 ± 22.4 | n=8 | n=7 | ||
| Living Unrelated Donor | LUR 2 | sister-in-law:recipient | MHC | 0 | 25 | 25 | 1.4 | 57 | - | - |
| LUR 3 | friend:recipient | MHC | 0 | 25 | 25 | 3.0 | 26 | - | +/+ | |
| LUR 4 | friend:recipient | MHC | 0 | 0 | 0 | 1.2 | 65 | + | - | |
| LUR 5 | husband:recipient | MHC | 0 | 0 | 0 | 1.0 | 55 | - | - | |
| Mean ± SD | 0.0 | 12.5 ± 14.4 | 12.5 ± 14.4 | 1.65 ± 0.91 | 50.8 ± 17.1 | n=1 | n=1 | |||
Bidirectional Regulation is defined as percentage of tvDTH inhibition >0 for both Recipient and Donor and by combined percentage of DTH inhibition ≥66%
One-Way or No Regulation is present when the percentage of tvDTH inhibition is 0 for donor or recipient or combined percentage of tvDTH inhibition is ≥66%
Class I DSA developed at 3 yr post tx
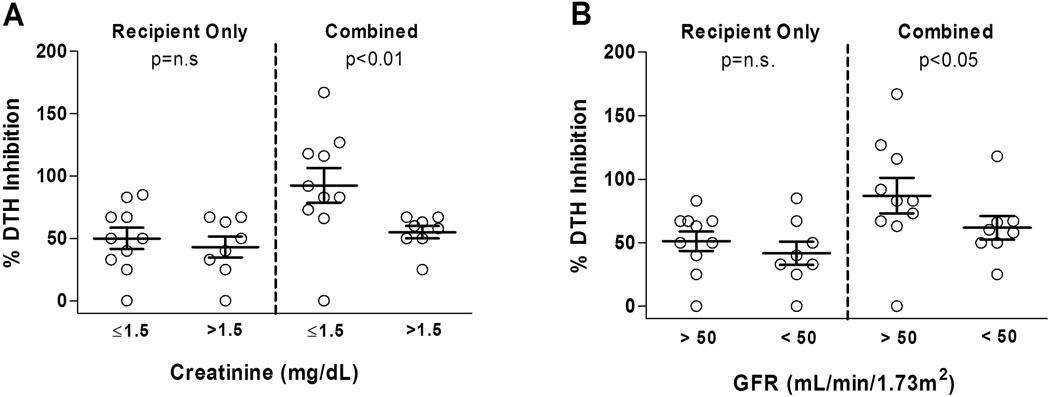
Analysis of pre-transplant regulation vs. outcomes in the 18 Haplo-ID LR kidney transplants revealed significant differences between patients. When we compared patients with favorable outcomes, (sCr ≤1.5 mg/dl or GFR> 50 mL/min/1.73 m2 at 3 years post-transplant) and those with less favorable outcomes, we found no difference in pre-transplant % inhibition scores if we only considered the recipient response to the donor (Figure 2 left panels; p=N.S.). However, when we considered the pre-transplant combined % inhibition (recipient anti-donor plus donor anti-recipient) we found significantly higher scores in patient-donor pairs with favorable outcome (sCr, p<0.01; GFR, p<0.05; Figure 2 right panels).
Figure 2. Analysis of 3 year graft outcome in 18 HLA Haplo-ID patients, in relation to pre-transplant regulation.
Recipient/donor pairs were scored for regulation to each other one day before transplantation [y axis]. Patients were grouped by renal function: serum creatinine (A) and GFR (B) at 3 years post transplant [x axis]. “Recipient only” (recipient vs. donor antigen) pre-transplant regulation did not differ between groups [left]. “Combined” % (recipient vs. donor plus donor vs. recipient Ag) trans-vivo DTH inhibition values were significantly higher in patients with sCr ≤1.5 mg/dL, GFR> 50 mL/min/1.73m2 vs. those with sCr >1.5 mg/dL, GFR< 50 mL/min/1.73m2. P values for comparison of pre-transplant recipient-only, or combined (donor + recipient) % inhibition values by Mann-Whitney U test are shown.
When Haplo-ID recipients were sorted by the combined pre-transplant inhibition scores, half (9/18) of Haplo-ID donor-recipient pairs exhibited pre-transplant bidirectional regulation as defined in Methods (Table 2). Only 1 recipient in this group (Haplo-ID 49) had any rejection episode (d.8, antibody-mediated) following Campath/Rituximab induction. This patient, and another in this group (Haplo-ID 58) developed class I donor-specific antibody (DSA) without any class II DSA. The mean renal function values at 3 years for the bidirectional subgroup (sCr 1.2 ± 0.1 mg/dL, GFR 60.0 ± 13.9 mL/min/1.73 m2) were similar to those of the HLA-ID sibling transplants. In the remaining Haplo-ID “non-bidirectional” cases, overall outcomes were surprisingly poor: 5/9 had sCr levels above 2.0 mg/dL, and 4 of these had a graft loss (GFR <15 mL/min/1.73 m2 at 3 years); 7 experienced acute rejection and 5 developed class II DSA; 4/5 also formed class I-DSA. The mean sCr and GFR values at 3 years follow-up in this subgroup were significantly different from both HLA-ID siblings (sCr, p=0.001; GFR, p=0.03) and from the bidirectional regulator Haplo-ID subgroup (sCr, p<0.0001; GFR, p=0.02).
The LUR group had outcomes intermediate between these extremes: 1/4 (LUR 3) had elevated sCr and decreased GFR (3.0 mg/dL and 26 mL/min/1.73m2) with DSA by 3 years, and one (LUR 4) recently died with a functioning graft at year 6, having experienced acute rejection episodes shortly after transplant, but otherwise excellent renal function (Table 2).
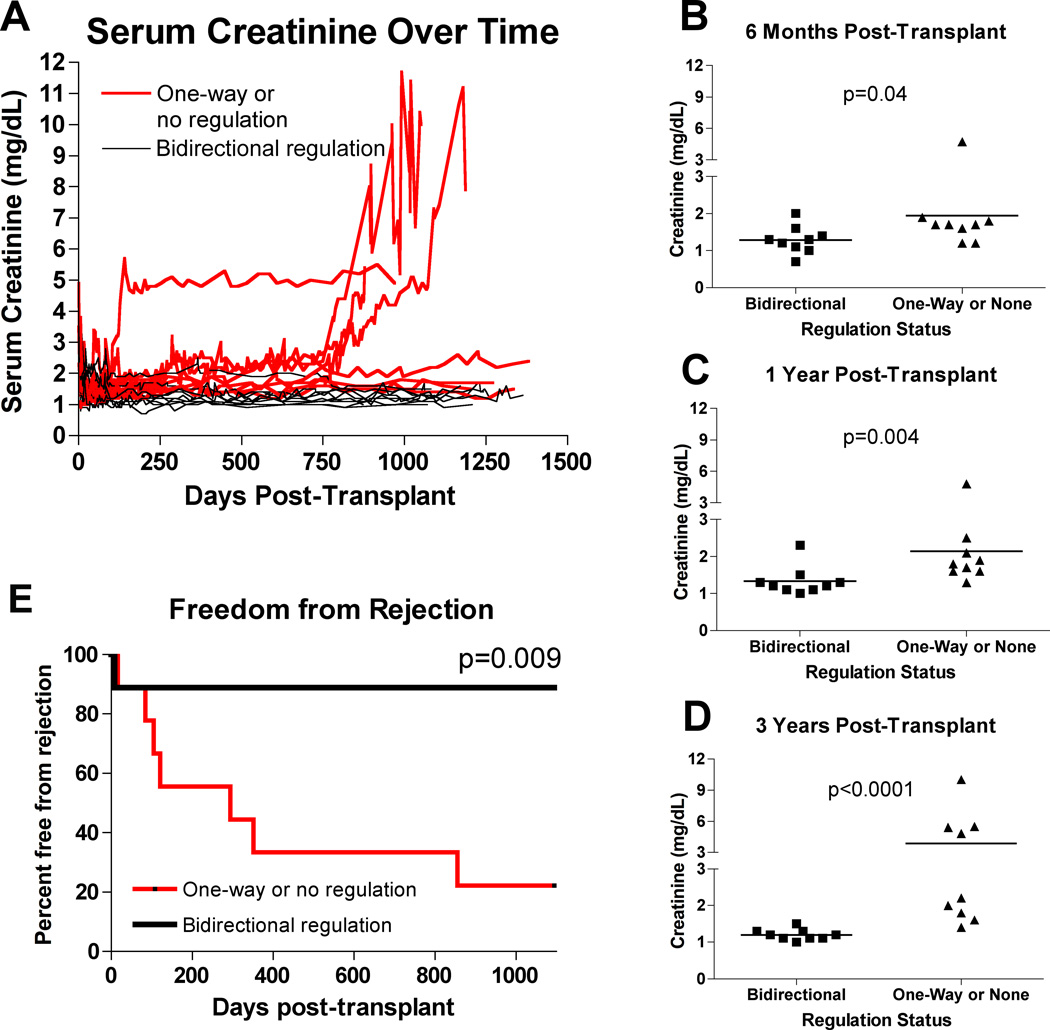
To determine if the impact of bidirectional regulation could be seen at time points earlier than 3 years, we compared renal function and times to first acute rejection episode in the 2 Haplo-ID subgroups. As shown in Figure 3A, bidirectional regulators consistently experienced lower sCr values. The differences were significant at 4 months (data not shown), 6 months (Figure 3B, both p=0.04), 12 months (Figure 3C, p=0.004) and 36 months (Figure 3D, p<0.0001) post transplant. As shown in Figure 3E, there was a significantly higher incidence of acute rejection episodes in the one-way or no regulation subgroup with 4/9 experiencing a rejection by 6 months and 6/9 by 12 months. By month 36, 4 patients in the one-way or no regulation group had sCr >4 mg/dL (Figure 3A), 7 had experienced at least one rejection episode, 3 had lost their graft due to rejection, and one (Haplo-ID 61) had a graft loss due to BK virus infection.
Figure 3. Serum creatinine and acute rejection episodes in HLA Haplo-ID recipients: bidirectional vs. unidirectional or non-regulators.
(A) Serum creatinine values for all HLA Haplo-ID patients over time. Red lines are patients in the one-way or no regulation group, while black lines are patients in the bidirectional regulator group. In general, patients who exhibited bidirectional regulation had lower sCr values with smaller slopes than patients exhibiting one-way or no regulation. (B) Serum creatinine values for all HLA Haplo-ID patients at 6 months post-transplant. Bidirectional regulators had lower sCr values (p=0.04) when groups were compared using the Mann-Whitney U test. (C) Serum creatinine values for all HLA Haplo-ID patients at 1 year post-transplant. Bidirectional regulators had lower sCr values at this timepoint (p=0.004), though one patient in this group had a value above the mean of the one-way or no regulation group. (D) Serum creatinine values for all HLA Haplo-ID patients at 3 years post-transplant. Bidirectional regulators had much lower sCr levels (p<0.0001), with four patients in the one-way or no regulation group exhibiting creatinine values over 4.5 mg/dL. (E) Acute rejections over time. The red line represents patients in the one-way or no regulation group, while the black line represents patients in the bidirectional regulator group.
We were unable to detect differences in end-stage renal disease, donor age, donor gender, recipient age at transplant, gender mismatch, or IS regimens between the LRD transplants with pre-existing bidirectional versus one-way or no regulation that could explain the striking differences in outcomes we observed (Supplemental Table 1).We cannot rule out the possibility that the presence of 3 HLA homozygous donors (Haplo-ID 45, 63, & 67; Table 2) in the bidirectional regulation subgroup may have contributed to successful outcome, since in these transplants, the donor kidney lacked HLA differences required to induce a DSA response in the recipients. However, a statistically significant difference in sCr values between the subgroups remained (Mann-Whitney p<0.003) even when these 3 subjects were excluded from the analysis.
Discussion
We showed previously that prediction of tolerance to an organ allograft in a mouse model is possible, based solely on pre-transplant assessment of NIMA-specific TR cells in the recipient (10). Using a very similar tvDTH approach for assessing regulation in a group of 29 recipients and their living donors, we now provide proof of principle that assessment of pre-transplant regulation status in human renal transplantation between family members is indeed possible (Figure 1 and Table 2). Remarkably, not only did pre-transplant regulation analysis appear to provide a means for predicting outcomes, but it also revealed a previously unsuspected role of donor immune status in kidney transplantation. While regulation toward the prospective donor’s alloantigens may indeed identify “tolerance-prone” family members, we show here that the recipient response to the donor alone is not enough (Figure 2) to predict outcome. Rather, it appears that the immune status of the donor actively synergizes with, or conversely, may undermine, pre-transplant regulation in the recipient. Because the regulation detected occurs via the indirect pathway, the results presented here implicate both tissue APC and T cells among donor passenger leukocytes as critical players in determining allograft outcome. However we cannot rule out the participation of other elements within the donor kidney such as endothelial, or epithelial cells.
Although our study was not confined to transplants which differed at known NIMA loci, the intra-familial regulation patterns revealed by tvDTH (Figure 1) are entirely consistent with the profound developmental impact of maternal cells on formation of human allo-specific TR in utero (11). This was most clearly evident in the uniform regulation of offspring to maternal cell lysates containing all HLA and miHA NIMAs. Given the strong regulation to NIMA on the indirect pathway of alloreactivity, previously undetectable using direct pathway-biased in vitro assays (12–14), the uniform regulation seen between HLA-ID siblings is readily explained on the basis of differential inheritance of maternal miHAs amongst siblings, each regulating to the non-inherited miHA peptides seen in the other’s cell lysate, and presented on a common HLA platform (see Model, Supplemental Figure 1). Furthermore, the asymmetry of regulation between mothers and offspring noted previously (8) was clearly evident in Figure 1 (see also Supplemental Figure 1), and appears to have clinical consequences. Mothers’ immune responses toward paternal antigens of their offspring ranged from strongly regulatory (interestingly, the only 2 mothers with ESRD and both transplants had excellent outcome), to moderately regulatory, to non-regulatory (Figure 1). In all 6 cases where mother was the donor, the son or daughter’s PBMC regulated strongly to donor NIMAs prior to transplant, but 3 years outcomes were worse when regulation was not reciprocated on the maternal donor side (compare Haplo-ID 56, 43, & 55 - 0% regulation to Haplo-ID 62, 63, & 50–33% regulation: Table 2). Overall, 4 graft losses occurred in renal allografts from a Haplo-ID sibling, parent, or offspring when unidirectional or no regulation was present prior to transplant. Serum creatinine values at 3 years in the remaining 5 patients, while acceptable, was still worse than that of transplants between bidirectional regulators (mean sCr 1.8 ± 0.3 vs.1.2 ± 0.1 mg/dL, p=0.002). Mean estimated GFR values were also lower (49.6 ± 8.1 vs. 60 ± 13.9 mL/min/1.73 m2) however this difference did not reach statistical significance.
Since the introduction of the two-way model of transplant tolerance by Starzl (15, 16), there has been much controversy about the role of microchimerism (17, 18) with relatively little attention paid to the core principle that the donor organ, including its passenger leukocyte component, markedly impacts the immunologic trajectory of the transplant (19). A separate but related principle of the two-way model is that the proper integration of the transplanted organ into the host—the gradual formation of a stable chimera—requires a balance between the host-versus-graft and graft-versus-host tendencies of the T cells on either side. These principles were derived from analysis of the immunobiology of liver transplantation, in which the primary mode of tolerance is mutual clonal exhaustion/deletion, where donor and recipient T cell apoptosis appears to play a major role (20).
In contrast to the liver transplant, renal transplant tolerance appears to rely primarily on immune-regulatory cell accumulation within the allograft—primarily TR (21, 22), but also B cells (23) and anergy (18), but not exhaustion/deletion mechanisms (23). However, this does not mean that the two-way model of tolerance no longer applies. Indeed, although all the patients in the current study were taking standard IS, we could clearly discern differences in outcomes between bidirectional regulators compared with all other patients. HLA-ID sibling kidney recipients at our center are routinely given corticosteroids for <2 weeks, and are maintained on a CNI plus mycophenolate, whereas the vast majority of HLA Haplo-ID LR and LUR kidney transplant recipients receive continuous corticosteroids as part of their maintenance IS, which may or may not include CNI therapy (see Supplementary Table 1). Indeed the transplants monitored in this study occurred at a time (2005–2006) when we were experimenting with depletional induction approaches using anti-CD52 monoclonal antibody (Alemtuzimab) + or − anti-CD20 immunotoxin (Rituximab) in order to reduce reliance on CNI inhibitors like cyclosporine or tacrolimus.
While the role of passenger leukocytes in immunizing or tolerizing the host is widely accepted in transplant immunology (19, 24–28), a critical role for donor-derived TR in allotolerance is a novel concept, but has some basis in the literature. MHC class II-matched kidney (but not heart) allografts in partially inbred miniature swine given a short course of post-transplant cyclosporine treatment are accepted indefinitely (29) and combined kidney-heart allografts are also accepted. Furthermore, the pro-tolerogenic effect of the kidney was abolished by prior x-irradiation of the donor, suggesting a radiosensitive kidney passenger leukocyte was responsible (27). Although the identity of this cell has yet to be determined, it is at least formally possible that a TR specific for NIMA miHA in the partially inbred swine were responsible.
In contrast to the findings reported here, a recent study by Mathew et al. (30) found a low incidence of regulation between HLA-ID siblings using a tvDTH footpad assay, with only 1/9 demonstrating bystander suppression. One possible explanation for this discordant observation is that the Mathew study included a significantly higher proportion of individuals of Hispanic ethnicity compared to >95% Caucasian families in the present study. The one Hispanic haploidentical sibling pair we tested (Table 2: Haplo-ID 60) showed no pre-transplant regulation in either direction, developed both class I and class II DSA, frequent acute rejection episodes, and ultimately lost the transplant by 36 mos. As in different mouse F1 backcrosses (31), immune responses to NIMA exposure may be highly variable in individuals of different ethnicity.
Conclusion
If the findings in this single-center pilot study can be confirmed in a wider multi-center trial, several novel possibilities are suggested for improved kidney transplant outcome and lower IS burden. One is the potential to identify before transplantation those donor-recipient pairs within a family who will be optimal candidates for either post-transplant IS withdrawal, CNI avoidance trials, or tolerance trials. A second is the opportunity to avoid poor outcomes seen in donor-recipient pairs with unbalanced regulation, an area of concern based on the poor results in these LR transplants. First generation assays such as the tvDTH and newer methods currently under development for detection of allopeptide-specific indirect pathway T regulatory cells for the clinical lab may guide the selection of a more suitable living kidney donor in cases where unbalanced regulation is a risk for graft failure. Finally, our findings may be relevant to deceased donor transplants, since they suggest the possibility of ex-vivo biologic manipulation of the donor organ to infuse TR or remove T effector cells to improve transplant outcomes.
Materials and Methods
Human Subjects
Immunological monitoring was performed on samples obtained from healthy control and end-stage renal disease (ESRD) patients according to informed consent procedures, subject to human subjects Institutional Review Board approval at the University of Wisconsin-Madison.
PBMC Isolation
Fresh blood from healthy family members, living related donors, and allograft recipients was obtained one day prior to transplant by sterile venipuncture into ACD tubes (Becton-Dickinson, Franklin Lakes, NJ). PBMC were further purified by Ficoll-density centrifugation (Cellgro; Mediatech, Inc., Herndon, VA) according to company protocol.
Mice
CB.17 SCID mice were bred at the University of Wisconsin-Madison and were housed and treated in accordance with guidelines outlined by the institutional Animal Care and Use Committee and the National Institutes of Health.
Analysis of Indirect Pathway Regulation using the Trans-Vivo DTH assay
Fresh human PBMC [7–9×106] were mixed with either donor or recipient cell-sonicate derived antigen or recall (tetanus toxoid/TT or EBV) antigen, or with a combination of donor or recipient plus recall antigen, and injected into CB.17 SCID mouse footpads as previously described (32, 33). Footpad thickness was measured using a spring-loaded caliper at time 0 and 24 hours. Net swelling was determined by subtracting “background” swelling from a control injection of PBMC alone. Inhibition of recall responses in the presence of donor or recipient antigens was determined by comparing the net swelling of each injection using the formula
to measure indirect pathway donor antigen-specific regulation (32, 33).
The combined % inhibition score was calculated as the sum of percent inhibition scores for both recipient anti-donor and donor anti-recipient tests. Regulation was considered bidirectional when the combined % inhibition score was ≥ 66% and regulation on each side was >0%.
Statistical analysis
Data were analyzed using the Mann-Whitney U test and Mantel-Haenzel log rank test. Statistical significance was established at a two-sided α level of 0.05 using Prism 5 software (GraphPad Software, La Jolla, CA).
Supplementary Material
In this hypothetical family, two sons are HLA identical, but mismatched for minor antigens HA-1 and HA-8. Parental HLA haplotypes are represented by the A locus only. CD8 TR cells, generated in each sibling by exposure to NIMA HA-1H (sib1) and HA-8R (sib 2) will recognize their target miHA present in sonicates prepared from the opposite sibling, triggering bystander suppression of TV-DTH. In the absence of any other strong antigenic differences, the NIMA-specific CD8 TR cells would predominate and the result would be bidirectional regulation. A similar model could be constructed for CD4 TR cells recognizing DR/DQ-restricted minors.
NOTE:1) A1, A2, A3, A24 indicate the HLA-A for the 4 parental haplotypes. 2) HA-1, HA-8, and HY are all HLA-A2-restricted minor Hags.
Acknowledgements
The authors would like to acknowledge Glen Leverson for statistical analysis, Drs. Melanie Dart, Qingyong Xu, and Partha Dutta for their helpful comments on the manuscript, and Dr. Dixon Kaufman for encouragement and support for this work. The research was supported by R01AI066219-04 (WJB) and 1 UL1RR025011 (JDM).
Sources of support: National Institute of Health Grant R01 AI066219 (WJB), and Grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (NCRR), National Institutes of Health (JDM).
Abbreviation list
- APC
antigen-presenting cells
- ESRD
end stage renal disease
- IMA
inherited maternal antigens
- IPA
inherited paternal antigens
- miHA
minor H antigen
- NIMA
non-inherited maternal antigens
- LR
living related
- LUR
living unrelated
- TR
T regulatory cells
- tvDTH
Trans-vivo delayed type hypersensitivity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Ewa Jankowska-Gan: Research design, writing, data analysis, and performance of research
- Adam Sheka: Writing and data analysis
- Hans W. Sollinger: Data analysis
- John Pirsch: Data analysis
- R. Michael Hofmann: Data analysis
- Lynn D. Haynes: Research design and data analysis
- Michael Armbrust: Data analysis
- Joshua Mezrich: Data analysis
- William J. Burlingham: Research design, writing, and data analysis
No author has any conflict of interest associated with the publication of this manuscript.
References
- 1.Owen RD, Wood HR, Foord AG, Sturgeon P, Baldwin LG. Evidence for actively acquired tolerance to Rh antigens. Proc.Natl.Acad.Sci.U.S.A. 1954;40:420. doi: 10.1073/pnas.40.6.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claas FH, Gijbels Y, van Der Velden-de Munck J, van Rood JJ. Induction of B cell unresponsiveness to noninherited maternal HLA antigens during fetal life. Science. 1988;241:1815. doi: 10.1126/science.3051377. [DOI] [PubMed] [Google Scholar]
- 3.Burlingham WJ, Grailer AP, Heisey DM, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998;339(23):1657. doi: 10.1056/NEJM199812033392302. [DOI] [PubMed] [Google Scholar]
- 4.Ichinohe T, Uchiyama T, Shimazaki C, et al. Feasibility of HLA-haploidentical hematopoietic stem cell transplantation between noninherited maternal antigen (NIMA)-mismatched family members linked with long-term fetomaternal microchimerism. Blood. 2004;104(12):3821. doi: 10.1182/blood-2004-03-1212. [DOI] [PubMed] [Google Scholar]
- 5.van Rood JJ, Loberiza FR, Jr, Zhang MJ, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99(5):1572. doi: 10.1182/blood.v99.5.1572. [DOI] [PubMed] [Google Scholar]
- 6.Opelz G, Study ftCT. Analysis of the 'NIMA effect' in renal transplantation. In: Terasaki PI, editor. Clinical Transplants 1990. Los Angeles, CA: UCLA Tissue Typing Laboratory; 1990. p. 63. [PubMed] [Google Scholar]
- 7.Miles CD, Schaubel DE, Liu D, Port FK, Rao PS. The role of donor-recipient relationship in long-term outcomes of living donor renal transplantation. Transplantation. 2008;85(10):1483. doi: 10.1097/TP.0b013e3181705a0f. [DOI] [PubMed] [Google Scholar]
- 8.van Halteren AG, Jankowska-Gan E, Joosten A, et al. Naturally acquired tolerance and sensitization to minor histocompatibility antigens in healthy family members. Blood. 2009;114(11):2263. doi: 10.1182/blood-2009-01-200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai J, Lee J, Jankowska-Gan E, et al. Minor H Antigen HA-1-specific Regulator and Effector CD8+ T Cells, and HA-1 Microchimerism, in Allograft Tolerance. J Exp Med. 2004;199(7):1017. doi: 10.1084/jem.20031012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutta P, Dart M, Roenneburg DA, Torrealba JR, Burlingham WJ. Pretransplant immune-regulation predicts allograft tolerance. Am J Transplant. 2011;11(6):1296. doi: 10.1111/j.1600-6143.2011.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mold JE, Michaelsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322(5907):1562. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadley GA, Phelan DL, Duffy BF, Mohanakumar T. Lack of T-cell tolerance of noninherited maternal HLA antigens in normal humans. Hum.Immunol. 1990;28:373. doi: 10.1016/0198-8859(90)90032-k. [DOI] [PubMed] [Google Scholar]
- 13.Roelen DL, Van Bree SPMJ, Van Beelen E, van Rood JJ, Claas FHJ. No evidence of an influence of the non-inherited maternal HLA antigens on the alloreactive T cell repertoire in healthy individuals. Transplantation. 1995;59:1728. doi: 10.1097/00007890-199506270-00015. [DOI] [PubMed] [Google Scholar]
- 14.van den Boogaardt DE, van Miert PP, Koekkoek KM, et al. No in vitro evidence for a decreased alloreactivity toward noninherited maternal HLA antigens in healthy individuals. Hum Immunol. 2005;66(12):1203. doi: 10.1016/j.humimm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance [see comments] Hepat. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 16.Starzl TE, Demetris AJ, Trucco M, et al. Systemic chimerism in human female recipients of male livers. Lancet. 1992;340:876. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood KJ, Sachs DH. Chimerism and transplantation tolerance: cause and effect. Immunol.Today. 1998;17:584. doi: 10.1016/s0167-5699(96)10069-4. [DOI] [PubMed] [Google Scholar]
- 18.Burlingham WJ, Grailer AP, Fechner JHJ, et al. Microchimerism linked to cytotoxic T lymphocyte functional unresponsiveness (clonal anergy) in a tolerant renal transplant recipient. Transplantation. 1995;59:1147. [PubMed] [Google Scholar]
- 19.Ko S, Deiwick A, Jager MD, et al. The functional relevance of passenger leukocytes and microchimerism for heart allograft acceptance in the rat. Nat Med. 1999;5(11):1292. doi: 10.1038/15248. [DOI] [PubMed] [Google Scholar]
- 20.Bishop GA, Sun J, Sheil AG, McCaughan GW. High-dose/activation-associated tolerance: a mechanism for allograft tolerance. Transplantation. 1997;64:1377. doi: 10.1097/00007890-199711270-00001. [DOI] [PubMed] [Google Scholar]
- 21.Torrealba JR, Katayama M, Fechner JH, Jr, et al. Metastable tolerance to rhesus monkey renal transplants is correlated with allograft TGF-beta 1+CD4+ T regulatory cell infiltrates. J Immunol. 2004;172(9):5753. doi: 10.4049/jimmunol.172.9.5753. [DOI] [PubMed] [Google Scholar]
- 22.Xu Q, Lee J, Jankowska-Gan E, et al. Human CD4+CD25low adaptive T regulatory cells suppress delayed-type hypersensitivity during transplant tolerance. J Immunol. 2007;178(6):3983. doi: 10.4049/jimmunol.178.6.3983. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Cordoba S, Hu M, et al. Spontaneous acceptance of mouse kidney allografts is associated with increased Foxp3 expression and differences in the B and T cell compartments. Transpl Immunol. 2011;24(3):149. doi: 10.1016/j.trim.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Lafferty KJ, Cooley MA, Woolnough J, Walker KZ. Thyroid allograft immunogenicity is reduced after a period in organ culture. Science. 1975;188(4185):259. doi: 10.1126/science.1118726. [DOI] [PubMed] [Google Scholar]
- 25.Lechler RI, Batchelor JR. Immunogenicity of retransplanted rat kidney allografts. Effect of inducing chimerism in the first recipient and quantitative studies on immunosuppression of the second recipient. J Exp Med. 1982;156(6):1835. doi: 10.1084/jem.156.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan WF, Perez-Diez A, Razavy H, Anderson CC. The ability of natural tolerance to be applied to allogeneic tissue: determinants and limits. Biol Direct. 2007;2:10. doi: 10.1186/1745-6150-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mezrich JD, Yamada K, Lee RS, et al. Induction of tolerance to heart transplants by simultaneous cotransplantation of donor kidneys may depend on a radiation-sensitive renal-cell population. Transplantation. 2003;76(4):625. doi: 10.1097/01.TP.0000079926.80833.42. [DOI] [PubMed] [Google Scholar]
- 28.Steinmuller D. Immunization with skin isografts taken from tolerant mice. Science. 1967;158:127. doi: 10.1126/science.158.3797.127. [DOI] [PubMed] [Google Scholar]
- 29.Rosengard BR, Kortz EO, Guzzetta PC, et al. Transplantation in miniature swine: analysis of graft-infiltrating lymphocytes provides evidence for local suppression. Hum Immunol. 1990;28(2):153. doi: 10.1016/0198-8859(90)90012-e. [DOI] [PubMed] [Google Scholar]
- 30.Mathew JM, Ciancio G, Burke GW, et al. Immune "tolerance profiles" in donor bone marrow infused kidney transplant patients using multiple ex vivo functional assays. Hum Immunol. 2010;71(6):566. doi: 10.1016/j.humimm.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molitor-Dart ML, Andrassy J, Haynes LD, Burlingham WJ. Tolerance induction or sensitization in mice exposed to noninherited maternal antigens (NIMA) Am J Transplant. 2008;8(11):2307. doi: 10.1111/j.1600-6143.2008.02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burlingham WJ, Jankowska-Gan E. Mouse strain and injection site are crucial for detecting linked suppression in transplant recipients by trans-vivo DTH assay. Am J Transplant. 2007;7(2):466. doi: 10.1111/j.1600-6143.2006.01627.x. [DOI] [PubMed] [Google Scholar]
- 33.VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, et al. Human allograft acceptance is associated with immune regulation. J Clin Invest. 2000;106(1):145. doi: 10.1172/JCI9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In this hypothetical family, two sons are HLA identical, but mismatched for minor antigens HA-1 and HA-8. Parental HLA haplotypes are represented by the A locus only. CD8 TR cells, generated in each sibling by exposure to NIMA HA-1H (sib1) and HA-8R (sib 2) will recognize their target miHA present in sonicates prepared from the opposite sibling, triggering bystander suppression of TV-DTH. In the absence of any other strong antigenic differences, the NIMA-specific CD8 TR cells would predominate and the result would be bidirectional regulation. A similar model could be constructed for CD4 TR cells recognizing DR/DQ-restricted minors.
NOTE:1) A1, A2, A3, A24 indicate the HLA-A for the 4 parental haplotypes. 2) HA-1, HA-8, and HY are all HLA-A2-restricted minor Hags.