Abstract
The acute toxicity of organophosphates (OPs) has been studied extensively; however, much less attention has been given to the subject of repeated exposures that are not associated with overt signs of toxicity (i.e., subthreshold exposures). The objective of this study was to determine if the protracted spatial learning impairments we have observed previously after repeated subthhreshold exposures to the insecticide chlorpyrifos (CPF) or the alkylphosphate OP, diisopropylfluorophosphate (DFP) persisted for longer periods after exposure. Male Wistar rats (beginning at two months of age) were initially injected subcutaneously with CPF (10.0 or 18.0 mg/kg) or DFP (0.25 or 0.75 mg/kg) every other day for 30 days. After an extended OP-free washout period (behavioral testing begun 50 days after the last OP exposure), rats previously exposed to CPF, but not DFP, were impaired in a radial arm maze (RAM) win-shift task as well as a delayed non-match to position procedure. Later experiments (i.e., beginning 140 days after the last OP exposure) revealed impairments in the acquisition of a water maze hidden platform task associated with both OPs. However, only rats previously exposed to DFP were impaired in a second phase of testing when the platform location was changed (indicative of deficits of cognitive flexibility). These results indicate, therefore, that repeated, subthreshold exposures to CPF and DFP may lead to chronic deficits in spatial learning and memory (i.e., long after cholinesterase inhibition has abated) and that insecticide and alkylphosphate-based OPs may have differential effects depending on the cognitive domain evaluated.
Keywords: Organophosphate; Pesticide; Cholinesterase inhibitor; Chronic; Cognition, Cognitive Flexibility
1. Introduction
Organophosphate (OP)-based compounds comprise many of the highly toxic chemical warfare agents as well as the most common agricultural and commercial pesticides used worldwide. The acute toxicity of OPs to target and non-target organisms has been studied extensively and is believed to result from irreversible inhibition of cholinesterase enzymes and subsequent elevations in synaptic acetylcholine levels (reviewed, Ecobichon, 1991). Among the variety of deleterious effects observed, cognitive symptoms associated with acute and/or repeated exposures to OPs can linger for months to years after exposure and include deficits in reaction time, as well as impairments of information processing, attention, learning, and memory (Amr et al., 1997; Dassanayake et al., 2007; De Silva et al., 2006; Salvi et al., 2003, Singh and Sharma, 2000; Steenland et al., 1994 and Stephens et al., 1995). It should be noted, however, that prior to the last several years, most of the published human literature on OP exposure and cognition described the consequences of relatively high-level exposures that also resulted in overt symptoms of cholinergic toxicity. Considerably less attention has been given to the subject of chronic, “subthreshold” exposures (defined as exposure levels not associated with acute symptoms of toxicity) to OPs (especially in adults). In the studies that have looked at lower-level exposure to OPs, subjects with a previous history of acute poisoning have rarely been excluded (see Roldan-Tapia, 2005). In addition, a considerable amount of the data in humans was obtained via case reports or retrospective analyses and in many cases several pesticides (i.e., from different chemical classes) may have contributed to the neuropsychological effects.
Prospective animal studies have indicated that repeated subthreshold exposures to OPs can indeed result in cognitive deficits (e.g., deficits in delayed matching performance associated with the alkylphosphate diisopropylfluorophosphate, DFP, Bushnell et al.., 1991). Previous work in our laboratories have shown that repeated subthreshold exposures to the insecticide OP, chlorpyrifos (O,O-diethyl O-[3,5,6,-trichloro-2-pyridyl] phosphorothionate), can result in several (persistent) cognitive effects including deficits in sensorimotor gating, spatial learning, recognition memory, and sustained attention (Middlemore-Risher et al., 2010; Terry et al., 2003; 2007). In addition, we have provided some evidence that insecticide OPs when compared to alkylphosphate-based OPs can (in some circumstances) have differential effects depending on the cognitive domain evaluated. Notably, in studies where rats were repeatedly exposed to subthhreshold doses of the insecticide chlorpyrifos (CPF) or the alkylphosphate DFP and then evaluated behaviorally during a two week, OP-free washout period, both similar and disparate effects were observed depending on the type of behavioral task employed (Terry et al., 2007; 2011). Specifically, while both compounds were associated with spatial learning impairments in a water maze procedure, CPF, but not DFP was associated with impairments of prepulse inhibition of the acoustic startle response. Moreover, DFP, but not CPF, was associated with deficits in a spontaneous novel object recognition task (a rodent model of recognition memory).
One objective of the current study was to determine (in adult rats) if the spatial learning (i.e., water maze) impairments observed previously with both CPF and DFP during a 14 day OP-free washout period persisted for longer periods after OP exposure. Via a repeated acquisition version of the water maze task we also made a preliminary assessment of the effects of the OPs on cognitive flexibility. We also sought to determine if impairments would be observed in an appetitively motivated spatial task that employs components of working and short term memory (i.e., the 8-ram radial maze task). Again, we specifically focused on repeated, intermittent, and subthreshold exposures to the OPs. For the purposes of these studies, we have operationally defined “subthreshold exposures” as doses that do not produce overt signs of cholinergic toxicity such as muscle fasciculations, seizures, diarrhea, excessive urination, and salivation (see reviews, Rusyniak and Nanagas, 2004; Sungurtekin et al., 2006). We chose to evaluate a representative insecticide OP (CPF) and an alkylphosphate OP (DFP). CPF has been used extensively as an agricultural and commercial pesticide worldwide since its introduction in 1965 (reviewed, Eaton et al., 2008). DFP is a prototypical alkylphosphate OP (first described in 1941) that was originally synthesized by British researchers as a potential chemical warfare agent (see Saunders, 1957). It possesses a great deal of structural homology with other highly toxic nerve agents such as sarin and soman. The intermittent dosing regimen was used to provide a model for the types of environmental exposures that might be experienced by agricultural, industrial, or pest control workers, or individuals who live in and around areas where OP insecticides or nerve agents have been released (or by soldiers who are deployed in these areas).
2. Materials and Methods
2.1. Compound Formulation and Administration
Chlorpyrifos (CPF) was obtained from ChemService Inc. (Cat# PS-674, West Chester, PA, USA) and diisopropylflurophosphate was obtained from Sigma Aldrich (CAS 55-91-4, St. Louis, MO). All other chemicals except peanut oil (see below) were purchased from Fisher Scientific or Sigma Aldrich. CPF 10.0, 18.0 mg/kg, or vehicle (3% DMSO + 97% peanut oil (v/v) or DFP 0.25, 0.75, or vehicle (Kroger® Pure Peanut Oil, obtained locally, Augusta, GA, USA) were administered to rats by subcutaneous (s.c.) injection (N=8–12) in a volume of 0.7ml/kg every other day for 30 days, then they were given an extended washout period (i.e., 50 days) before behavioral testing (see below). Additional cohorts of animals (n=3–6) were administered the higher doses of the OPs for analysis of plasma and brain cholinesterase activity at various time points during a OP-free washout period. The dosing procedure was selected based on our previous studies (Terry et al., 2003; 2007; 2011) and was defined as subthreshold according to the definition provided above. Individual rats were weighed and monitored (in their home cages for a period of approximately 5 minutes each day) for visible cholinergic signs (diarrhea, excessive salivation or lacrimation, respiratory difficulties, muscle fasciculations) or other signs of distress throughout the study.
2.2. Test Subjects
Male albino Wistar rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN, USA)) approximately 2 months old were housed individually in a temperature controlled room (25°C), maintained on a 12:12h reverse light-dark cycle (lights off at 6am) with free access to water and food except during radial arm maze testing in the animal cohorts that were behaviorally tested (see below in the behavioral methodology section). All behavioral testing began 2 hours after the initiation of the dark cycle with a minimum of 30 minutes habituation to the light environment prior to testing. All procedures employed during this study were reviewed and approved by the Georgia Health Sciences University Institutional Animal Care and Use Committee and are consistent with AAALAC guidelines. Measures were taken to minimize pain and discomfort in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996.
2.3 Blood Collection, Brain Harvest, and Homogenization of Brain for Enzyme Assays
At the end of the washout period, rats were anesthetized with isoflurane and blood was collected via cardiac puncture using a 5.0 cc syringe fitted with a 18 G needle. 0.7 ml of blood was immediately added to a Microtainer® Plasma Separator Tube containing lithium heparin (BD catalog #365958). This tube was inverted eight times, and then centrifuged according to the BD protocol. The resulting plasma was aliquoted into 0.5 ml tubes, snap frozen in liquid nitrogen, and stored at −70° C until analyzed. Brains were then harvested and snap frozen in dry ice-chilled isopentane before storage at −70°C. Later, whole brains were homogenized in 4 volumes of a 0.1M Na/K Phosphate buffer pH 7.9 using a Wheaton Overhead Stirrer (Wheaton Industries, Inc., Millville, NJ) and Thomas USA B663 glass homogenization tubes (Thomas Scientific, Swedesboro, NJ). Homogenates were aliquoted into 0.5 ml tubes (20 μl/tube), stored at −20°C and later analyzed for total protein (BCA Assay Thermo Scientific Rockford, IL) and cholinesterase activity (see below).
2.4 Plasma and Brain Cholinesterase Activities
Cholinesterase activity in plasma samples and brain homogenates was measured according to Ellman et al. (1961) in a 96-well plate format at room temperature (see Gearhart et al. 2006 for additional details). Five microliters (5 μl) of plasma (100–130 μg protein/μl) or brain homogenate (20–50 μg protein/μl) were dispensed into the bottom of the wells of the 96-well plate (Fisher Scientific #12-565-501). An 8- or 12-channel pipeter was used to quickly add 310 μl of reaction mixture to the wells. The reaction mixture contained acetylthiocholine (0.48 mM; # D-8130, Sigma-Aldrich, Inc., St. Louis, MO) and dithiobisnitrobenzoic acid (0.52 mM; Acros # 102710050) in 0.1 M sodium phosphate buffer (pH 8.0). The microplate was shaken for ~30 seconds using a Jitterbug™ plate shaker (Boekel Scientific; Feasterville, PA), before placing the microplate in a µQuant™ Microplate Spectrophotometer (BioTek Instruments Inc.; Winooski, VT). The formation of reaction product (yellow color) was monitored by measuring absorbance at 412 nm every 2 min for 16 min. The cholinesterase-mediated reaction rate (moles/liter per min) was calculated by dividing the change in absorbance per minute by 13,600 (for details, see Ellman et al., 1961). Each plasma sample or brain homogenate was assayed in triplicate.
2.5. Behavioral Experiments
All rats evaluated in behavioral tests were handled beginning the day after arrival, and received a minimum of two weeks of daily handling prior to the initiation of behavioral testing. Behavioral experiments were conducted in rooms with ambient lighting of approximately 25–30 Lux (lumen/m2). To attenuate potential distraction associated with mechanical noise, radial arm maze testing (see below) was conducted in a room that was equipped with a white noise generator (San Diego Instruments, San Diego, CA) set to provide a constant background level of 70 dB. Test subjects were transferred (in their home cages) to the behavioral testing rooms each morning approximately 30 min before the beginning of experiments.
2.5.1 Radial Arm Maze (RAM) Procedure
RAM testing was initiated on day 50 of the OP-free washout. Beginning two weeks prior to habituation in the RAM apparatus, test subjects were food restricted to approximately 85% of normal ad libidum levels of consumption. RAM experiments were conducted in Med-Associates (MED-RAM-1R) computer-automated, 8-Arm Radial Mazes consisting of a central octagonal hub (arena) with automatic guillotine doors connected to aluminum arms radiating distally (45.7 cm long). IR-photo beam sensors were positioned at the entrance to each runway, and a food pellet receptacle and head entry detector was positioned at the end of each runway. The maze was positioned approximately 90 cm above the floor in a testing room with a number of extra-maze cues (composed of large geometrical shapes). This computer-automated method (used currently in our laboratory) has been previously published by us (see Terry et al., 2008).
Habituation Phase
Test subjects were given one 15 min free exploration (habituation) session on the Friday prior to the Monday in which the Win-Shift portion of testing was conducted. This was done so that the animals became acquainted with the radial arm maze apparatus, as well as the handling procedures associated with it. Reinforcement food pellets were scattered randomly around the entire maze area during this session.
Acquisition (Win-Shift Training)
After the habituation phase, subjects were trained in a win-shift procedure. A trial began when the experimenter placed the test subject into the central octagonal arena. After a 30 sec delay, all guillotine doors raised allowing access to all of the 8 arms. When the animal broke a photobeam in the pellet receptacle at the end of each runway a reward pellet was delivered once. When the rat moved back into the central arena all doors closed for 5 seconds and then reopened. All reentries into an arm that previously delivered a reward were scored as errors. All animals were trained in the win-shift task to meet a performance criterion of four consecutive days with ≤ 2 total errors. At this point, individual rats were moved to the Delayed non Match to Position task
Delayed non Match to Position (DNMTP)
DNMTP testing was similar to the acquisition mode except that two sessions were given in a trial block, with a predetermined delay imposed between the sessions. Testing began with an information (forced 4) session in which four of the eight arms are blocked, i.e. the animal could only investigate the four remaining open arms. This information session ended when all four arms were visited or when the trial timed out (15 min.). The animal remained in the testing room for the delay period. In the “free 8” (retention) session all eight arms were accessible; however, food reinforcement occurred only at the ends of the arms not visited in the previous information session. The test session continued until all four of the previously-blocked arms were visited, or until 15 min. elapsed. Any entry into an arm that had been visited in the information session (which then delivered no food reinforcement) or repeat visits to any arm during the second session was recorded as an error. Following the second (test) session in each trial block, the animal was returned to its home cage in the housing facility, until the next day's information session. Animals were initially trained with 15 min delays in the DNMTP task to achieve a criterion of ≤ 1 error for four consecutive sessions during the free eight sessions. Subsequently, longer delays of 1, 3, and 6 hr were presented at least twice in a pseudorandom fashion along with 15 min delays.
2.5.2 Water Maze Repeated Acquisition
Beginning on day 140 of OP washout, the subjects were evaluated for performance of a water maze spatial learning task using a modification of a procedure we have published previously (Terry et al., 2008).
Test Apparatus
These experiments were performed in a circular pool (diameter: 180 cm, height: 76 cm) made of black plastic and filled to a depth of 35 cm of water (maintained at 25.0±1.0°C). The pool was located in a large room with a number of extra-maze visual cues including geometric images (squares, triangles, circles etc.) hung on the wall, and black curtains used to hide the experimenter (visually) and the resting test subjects. Swimming activity of each rat was monitored via a television camera mounted overhead, which relayed information including latency to find the platform, total distance traveled, time and distance spent in each quadrant etc. to a video tracking system (Noldus EthoVision® Pro 3.1)
Hidden Platform Task
For hidden platform tests, an invisible (black) 10 cm × 10 cm square platform was submerged approximately 1.0 cm below the surface of the water and placed in the center of a quadrant (one-fourth of the total pool area defined via the tracking software). For each test session rats were given 4 trials per day for 5 consecutive days to locate and climb on to the hidden platform. A trial was initiated by placing the rat in the water directly facing the pool wall (i.e., nose approximately 2 cm from the wall) in one of the 4 quadrants. The daily order of entry into individual quadrants was pseudo-randomized such that all 4 quadrants were used once per day. For each trial, the rat was allowed to swim a maximum of 90 sec, in order to find the platform. When successful the rat was allowed a 30-sec rest period on the platform. If unsuccessful within the allotted time period, the rat was given a score of 90 sec and then physically placed on the platform and also allowed the 30-sec rest period. In either case the rat was given the next trial after an additional 1.5 min rest period (i.e., intertrial interval =2.0 min). After the last hidden platform test on day 5, the platform was switched to the opposite quadrant and a phase 2 of testing was conducted (i.e., an additional 4 trials per day for 5 consecutive days).
Visible Platform Task
After the last hidden platform test of phase 2, a visible platform test was performed as a general estimate of visual acuity. To accomplish this task, a highly visible (white) cover fitted with a small white flag was attached to the platform (dimensions with cover attached = 12 cm × 12 cm) which raised the surface approximately 1.0 cm above the surface of the water. Each rat was gently lowered into the water in the quadrant diametrically opposite to the platform quadrant and given one or more trials with a 90 sec time limit to locate and climb on to the platform. When a rat was successful (on its own accord without assistance) it was then given a series of 4 additional trials (with a 1.0 min intertrial interval) and the latency (in sec) to locate the platform was recorded. The platform was moved on each trial to a different quadrant (the subject was always entered from the opposite quadrant) until the test was conducted once in all 4 quadrants.
2.6. Statistics
All statistical analyses were performed using SigmaPlot Version 11 (SPSS Inc., Chicago, IL) or JMP™ version 5 (Cary, NC). Two- or three-way analysis of variance (with repeated measures) was used for treatment group comparisons. Student Newman Keuls multiple comparison procedures (SigmaPlot) and orthogonal t-tests (JMP) corrected for multiple comparisons via the method of Bonferroni were used to examine post hoc differences when indicated. Statistical significance was assessed using an alpha level of 0.05
3. Results
3.1. Cholinesterase Activity
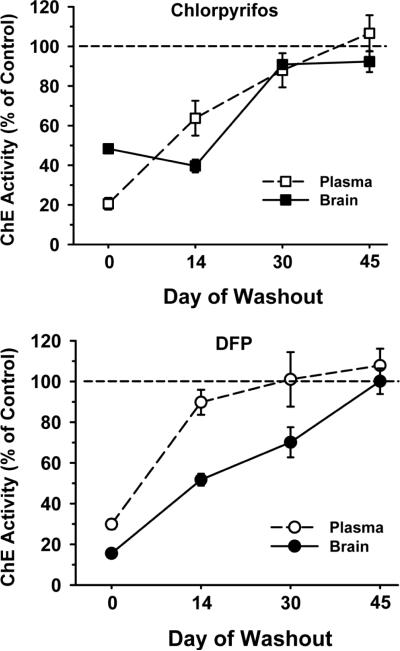
Plasma and brain cholinesterase activity was assessed at different time points of OP washout in separate cohorts of rats that were not behaviorally tested. The higher dose of each OP was evaluated (i.e., CPF 18.0 mg/kg and DFP 0.75 mg/kg). As indicated in Fig 1, on the last day of OP exposure (i.e., day 0 of the OP-free washout), plasma cholinesterase activity was inhibited to a roughly similar degree by each OP (i.e., to approximately 21% of control in the CPF-treated animals and to approximately 30% of control in the DFP-treated animals). In the brain, however, cholinesterase activity was inhibited by a much greater degree in the DFP-treated animals (i.e., to approximately 16% of control versus approximately 48% of control in the CPF-treated animals). Interestingly, cholinesterase activity was inhibited to a greater degree at the 14 day washout time point in the CPF-treated animals than at the day 0 time point. Otherwise, cholinesterase activity increased in a time-dependent manner with more extended washout periods (in both OP-treated groups) and it was not significantly different from control levels at the 45 day time point in brain or plasma.
Fig 1.
The effects of repeated exposures to CPF 18.0 mg/kg (Top) or DFP 0.75 mg/kg (Bottom) on cholinesterase activity in the plasma and brain at various time points during a 45 day OP-free washout period. Data (mean ± SEM) are presented as % of vehicle-matched control levels. (N=3–6).
3.2 Radial Arm Maze
CPF
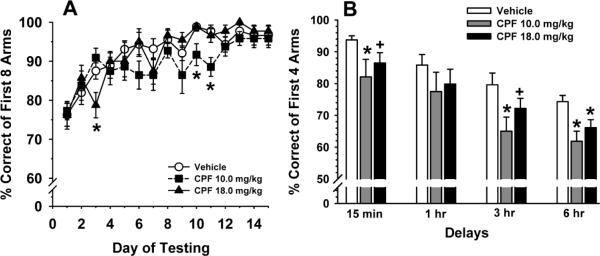
Fig 2A illustrates the effects of previous exposure to CPF (compared to vehicle-treated controls) on acquisition in an 8-arm radial maze win-shift task over 10 consecutive days of testing. There was a significant main effect of dose, F(2,33)=3.6, p<0.03; and day F(14,409)=14.9, p<0.001 (indicating that significant learning occurred over the 10 days of training), without a significant dose × day interaction, F(28,409)=1.3, p=0.15. Post hoc analysis indicated that both doses of CPF evaluated were associated with modest, but statistically significant (p<0.05) impairments of performance (compared to vehicle controls) on one or more days of testing. Fig 2B illustrates the effects of CPF on performance of a delayed non-match to position (DNMTP) task with delays. Again, there was a significant main effect of dose, F(2,31)=3.5, p<0.04; and delay F(3,83)=33.3, p<0.001 (indicating a decrement in performance with an increase in delay), without a significant dose × day interaction,, F(6,83)=0.2, p=0.96. Post hoc analysis indicated that both doses of CPF evaluated were associated with significant (p<0.05) or nearly significant (p<0.07) impairments of performance (compared to vehicle controls) at one or more of the delays imposed.
Fig 2.
Effects of prior exposure to CPF on radial arm maze performance conducted beginning on day 50 of an OP-free washout period. A. Win-shift acquisition over 14 consecutive days of testing as assessed by the % correct of the first 8 arms entered (mean ± S.E.M.). B. Delay dependent performance of the RAM delayed non-match to position (DNMTP) task as assessed by the % correct of the first 4 arms entered (mean ± S.E.M.). * = significantly (p<0.05) inferior performance when compared to vehicle control. + = nearly significant (p<0.07) performance deficit when compared to vehicle control.
DFP
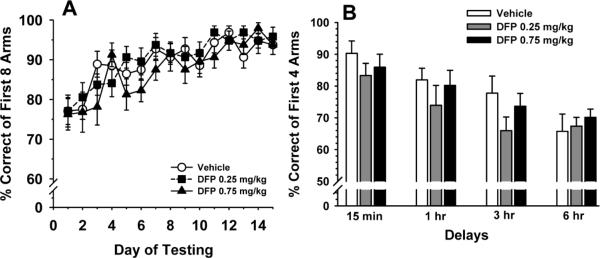
Fig 3A illustrates the effects of prior DFP exposure (compared to vehicle-treated controls) on acquisition in win-shift task over 10 consecutive days of testing. While there was a significant effect of day F(14,436)=12.9, p<0.001 (indicating significant learning over the 10 days of training), the main effect of dose and the dose × day interaction did not meet the required level of significance (i.e. p values were >0.05). Fig 3B illustrates the effects of DFP on performance of a delayed non match to position (DNMTP) task with delays. Again, there was a significant effect of delay F(3,88)=15.4, p<0.001 (indicating a decrement in performance with increasing delays), however, the main effect of dose and the dose × day interaction, did not meet the required level of significance (i.e. p values were >0.05).
Fig 3.
Effects of prior exposure to DFP on radial arm maze performance conducted beginning on day 50 of an OP-free washout period. A. Win-shift acquisition over 14 consecutive days of testing as assessed by the % correct of the first 8 arms entered (mean ± S.E.M.). B. Delay dependent performance of the RAM delayed non-match to position (DNMTP) task as assessed by the % correct of the first 4 arms entered (mean ± S.E.M.).
3.3. Water Maze
CPF
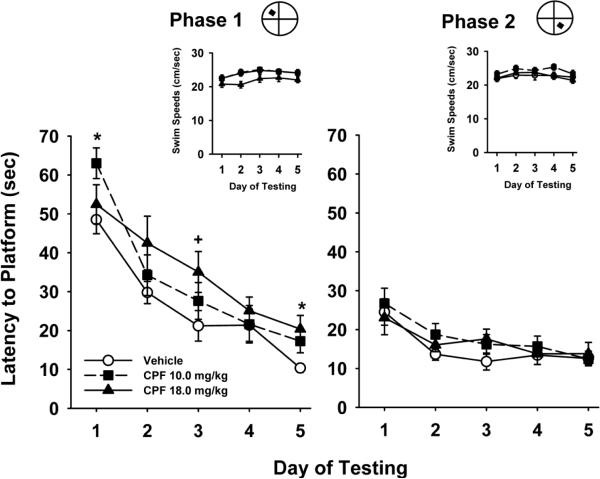
Fig 4 illustrates the acquisition curves for each experimental group to locate a hidden platform in two phases of water maze testing in the CPF study. The following statistical results were obtained, main effect of dose, F(2,33)=1.6, p=0.21, phase, F(1,277)=167.8, p<0.001, day, F(4,277)=58.6, p<0.001, dose × phase, F(2,277)=3.2, p=0.04, dose × day, F(8,277)=1.5, p=0.17, phase × day, F(4,277)=16.8, p<0.001, dose × phase × day, F(8,277)=0.78, p=0.62. Thus, under vehicle control conditions, rats progressively learned to locate the hidden platform with increasing levels of efficiency over the course of the 5 days in each testing phase as indicated by the decreasing slope of the acquisition curves. In the first phase of testing, there was evidence of modest levels of impairment associated with both doses of CPF depending on the day of testing. There was no evidence of impairment in phase two. Swim speeds were also evaluated (see insets to each phase in Fig 4) and generally ranged between approximately 21.0 and 24 cm/sec in the various treatment groups and were not significantly different.
Fig 4.
Effects of prior exposure to CPF on a water maze repeated acquisition procedure beginning on day 140 of an OP-free washout period. Phase 1 refers to the hidden platform tests that were conducted for the first 5 consecutive days. Phase 2 refers to the second 5 days of testing after the hidden platform was moved to a new quadrant location in the pool. Each point of the plotted curves represents the mean latency in seconds ± SEM for each testing day. Insets = swim speeds (cm/sec) plotted over the 5 days of testing in each phase. * = significantly (p<0.05) inferior performance when compared to vehicle control. + = nearly significant (p<0.07) performance deficit when compared to vehicle control.
Visible Platform Test
After the last hidden platform trial of phase 2, visible platform tests (4 trials per session per group) were conducted to ensure that the test subjects did not exhibit crude deficits in visual acuity that might have confounded the water maze hidden platform analyses. The latencies for the 3 groups to find the visible platform (i.e., the mean of the 4 trials per session) ranged from 10.5 to 12.1 sec. There were no significant treatment-related effects observed in this procedure (i.e., all p values were >0.05, data not shown).
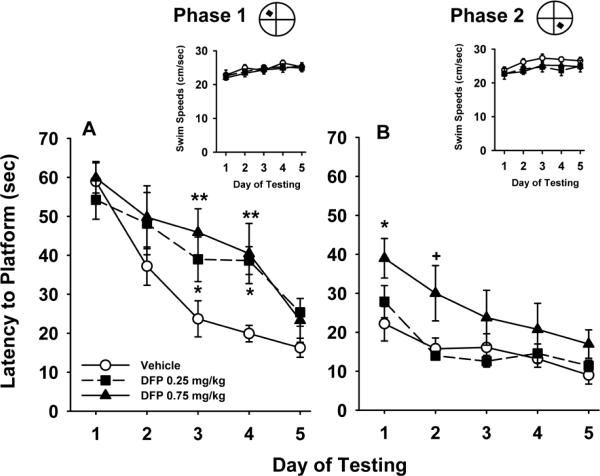
DPF
Fig 5 illustrates the acquisition curves for each experimental group to locate a hidden platform in two phases of water maze testing in the DFP study. The following statistical results were obtained, main effect of dose, F(2,29)=2.7, p=0.07, phase, F(1,2241)=167.2, p<0.001, day, F(4,241)=34.5, p<0.001, dose × phase, F(2,241)=3.3, p<0.04, dose × day, F(8,241)=0.7, p=0.72, phase × day, F(4,241)=4.8, p=0.001, dose × phase × day, F(8,241)=1.4, p=0.19. Again, under vehicle control conditions, rats progressively learned to locate the hidden platform with increasing levels of efficiency over the course of the 5 days during each testing phase as indicated by the decreasing slope of the acquisition curves. Both doses of DFP were associated significant levels of impairment in phase 1. In addition, the higher dose (0.75 mg/kg) was also associated with impairments in phase two. Swim speeds (see insets to each phase in Fig 5) generally ranged between approximately 22.0 and 28 cm/sec throughout the study in the various treatment groups and were not significantly different.
Fig 5.
Effects of prior exposure to DFP on a water maze repeated acquisition procedure beginning on day 140 of an OP-free washout period. Phase 1 refers to the hidden platform tests that were conducted for the first 5 consecutive days. Phase 2 refers to the second 5 days of testing after the hidden platform was moved to a new quadrant location in the pool. Each point of the plotted curves represents the mean latency in seconds ± SEM for each testing day. Insets = swim speeds (cm/sec) plotted over the 5 days of testing in each phase. ** = significantly (p<0.01) inferior performance when compared to vehicle control. * = significant (p<0.05), + = nearly significant (p<0.07) performance deficit when compared to vehicle control.
Visible Platform Test
The latencies for the 3 groups to find the visible platform (i.e., the mean of the 4 trials per session) ranged from 11.7 to 12.9 sec. There were no significant treatment-related effects observed in this procedure (i.e., all p values were >0.05, data not shown).
4 Discussion
As noted in the introduction, the primary objectives of this study were three fold: 1) to determine (in adult rats) if the protracted spatial learning impairments we have observed previously after subthhreshold exposures CPF and DFP persisted for longer periods of time, 2) to make a preliminary assessment of the effects of the OPs on cognitive flexibility via a repeated acquisition version of the water maze task, and 3) to determine if impairments of spatial working and short term memory would also be observed. For the later objective, the RAM procedure was selected since its performance relies on spatial working and short term memory (Levin et al., 1996; Olton and Papas, 1979), it is thought to reflect normal foraging strategies that rodents use in their natural environments (i.e., thus is ethologically relevant), and it is dependent on intact cholinergic function (reviewed, Decker and McGaugh, 1991), an important issue for this study. The water maze procedure was used to complement previous work by us and since it is also a visuospatial learning task that is sensitive to cholinergic alterations (McNamara and Skelton, 1993). In this context it is important to note that deficits in visuospatial processing have been identified as one of the negative outcomes in human patients previously exposed to OPs for chronic periods (Roldan-Tapia et al., 2005). As indicated in the results, rats previously exposed to CPF, but not DFP, were impaired in the RAM win-shift task and the DNMTP procedure, while subjects previously exposed to either OP demonstrated impairments in the acquisition of the water maze procedure (i.e., in Phase 1).
The basis of the differential effects of the OPs observed in the water maze and RAM is unclear, although we have encountered disparate (drug-related) effects on water maze and RAM performance previously (see Terry et al., 2008). As reviewed by Hodges (1996), a number of factors can potentially explain performance differences in water maze and RAM procedures including the free versus restrained search, differential availability and use of intra and extramaze cues, motivational issues (aversive search versus food reinforcement), and thus ostensibly similar spatial tasks may in fact be looking at different behavioral processes. It is also important to reiterate that the animals tested in the water maze task in our studies had 140 days of OP-free washout before the initiation of testing, whereas for the radial arm maze, testing began on the 50th day after OP termination. Another possible source of disparate effects of the OPs in the water maze and RAM could relate to their differential profiles of plasma and brain cholinesterase inhibition and recovery. Our selection of the doses of each OP to be evaluated in this study was based on their similar degrees of plasma cholinesterase inhibition observed in prior studies. For example, we previously observed that DFP 0.75 mg/kg and CPF 18.0 mg/kg resulted in approximately 80–90% inhibition in the plasma (without signs of overt toxicity) by day 7 of an alternate day dosing schedule (see Terry et al., 2007; 2011). In the current, study, the plasma and brain cholinesterase profiles in the OP-treated groups were somewhat different. Specifically, the degree of cholinesterase inhibition was greater in the brain than in the plasma on day 30 of DFP dosing and it recovered more slowly in the brain (compared to plasma) during the washout. Conversely, in the CPF-treated animals, cholinesterase inhibition in the brain was less pronounced than in the plasma on day 30 of dosing, and recovery during the washout period in the brain was biphasic (i.e., further inhibited on day 14 of washout, before improving toward control levels at subsequent time points). Plasma cholinesterase activity, however, did not exhibit the biphasic phenomenon of recovery and activity steadily increased over the course of the washout. This biphasic profile of cholinesterase inhibition and recovery from CPF in the brain could be related to its lipophilicity and tendency to accumulate in fatty tissues (e.g., the brain). CPF has an octanol-water partition coefficient (log Kow) of approximately 4.70 (Tomlin, 2006) and it has been observed (in rats) to accumulate in adipose tissues, but not in other tissue compartments (Bakke et al., 1976, see also review, Eaton et al., 2008). Thus it is possible, that its sustained effect on cholinesterase activity in the brain (i.e., for up to the 14th day of washout) could reflect its tendency to require additional time (compared to DFP) to redistribute out of fat stores. This argument is strengthened by the results of physiologically-based pharmacokinetic (PBPK) models indicating that DFP is cleared relatively rapidly from brain (Gearhart et al., 1994), while CPF sequesters in brain, especially after subcutaneous administration (Smith et al., 2009).
The differential effects in phase 2 of the water maze procedure (i.e., impairments after DFP, but not CPF exposure) were also intriguing. One potential interpretation of these data is that the greater degree of cholinesterase inhibition in the brain associated with DFP before washout might have resulted in some pathological process that was more persistent than that produced in the CPF-treated animals (although this would not explain the modest deficits observed in the CPF, but not the DFP-treated animals in the RAM). The DFP-related deficits in the second phase of water maze testing were suggestive of alterations in cognitive flexibility which is generally considered a subcomponent of executive function involving the shifting of strategies when the stimulus-response contingency changes (see Tanimura et al., 2008). Platform re-locating water maze tasks require cognitive flexibility and (given the importance of cognitive flexibility to human cognition) are suggested to be especially appropriate for extrapolation of learning and memory measures from rodent to human (Saab et al., 2011). Interestingly, deficits in cognitive (mental) flexibility were recently identified among the variety of neuropsychological performance deficits in a study of 127 agricultural workers with a history of low level exposure to organophosphate pesticides (Mackenzie Ross et al., 2010).
The mechanism of the protracted effects of OPs on cognition in this (and other) studies is unclear at present. While the inhibition of cholinesterase enzymes undoubtedly plays a key role in many aspects of the toxicology of OPs, there is considerable evidence that this mechanism cannot alone account for the wide range of disorders that have been reported (Duysen, et al., 2001, Pope, 1999). Not surprisingly (due to their highly reactive nature), OPs have been documented to alter the function of a variety other important enzymes and proteins (see reviews, Casida and Quistad, 2005 and Lopachin and Decaprio, 2005) and it has been suggested that interactions of OPs with non-cholinesterase targets may be particularly important to the more delayed and persistent effects associated with chronic exposure (see reviews, Costa, 2006; Lotti and Moretto, 2005). The list of non-cholinesterase targets for OPs (in environmentally relevant doses) includes neuropathy target esterase (Lush et al., 1998), acylpeptide hydrolase (Richards et al., 2000), M2 muscarinic receptors (Bomser and Casida 2001), fatty acid amide hydrolase (Quistad et al., 2001), cannabinoid CB1 receptors (Quistad et al., 2002), and albumin (Peeples et al., 2005). More recently, studies utilizing sophisticated mass spectrometric methods have indicated that in addition to their well-documented interactions with the active site serine in serine esterases and proteases, OPs can label a number of tyrosine and lysine-containing peptides (Grigoryana et al., 2009; Schopfer et al., 2010), i.e., interactions that could theoretically contribute to their deleterious effects.
We have provided evidence that repeated exposures to OPs can lead to persistent deficits in both anterograde and retrograde axonal transport (Terry et al., 2003; 2007). Additional studies in our laboratories (and our collaborator's laboratories) also indicated that OPs can disrupt kinesin-driven movement, covalently modify tubulin, and inhibit microtubule formation, i.e., factors that might contribute to the observed impairments in axonal transport (Gearhart et al., 2007; Grigoryan et al., 2008; Prendergast et al., 2007). We have also observed persistent OP-related alterations in neurotrophin receptors and cholinergic proteins (Terry et al., 2007; 2011) and have hypothesized that these effects might be related to the alterations in axonal transport. Finally, we have recently observed OP-related alterations in the dynamics and movement of mitochondria in rat cortical neurons (Middlemore-Risher et al., 2011). Collectively, the information provided here suggests that the mechanism of long term effects of OPs on cognition may be multifactorial, but could be related to covalent modifications of proteins that serve fundamental roles in neuronal function (axonal transport, mitochondrial dynamics, neurotrophin function, etc.).
There are some limitations to this study that should be discussed. As mentioned in the Introduction, our dosing paradigm in rats was designed to model subthreshold exposures to OPs that might be experienced by pesticide handlers/agricultural workers or individuals who live in and around areas where OP-nerve agents have been released. The maximal levels of plasma cholinesterase inhibition observed in our animal study (~70–80% for DFP and CPF, respectively) would likely be higher than that experienced by most of the aforementioned human individuals. In the more recent studies of agricultural workers conducted in the United States, the percentage of individual who handle pesticides detected with cholinesterase inhibition of greater than 40% has been relatively low (e.g., 4.6% in a study of 386 pesticide handlers in Washington State, see Furman et al., 2007). However, studies of pesticide handlers conducted around the world continue to detect some individuals with levels of cholinesterase inhibition of approximately 50% or greater who have not complained of acute symptoms. Examples include studies of sheep dippers in Australia (Dyer et al., 2001), farm workers in Ethiopia (Mekonnen and Ejigu, 2005) and cotton field workers in Egypt (Farahat et al., 2011). To our knowledge there have been no such studies to evaluate individuals who live in areas where OP-nerve agents have been released. Another potential limitation to our study is the route of administration of CPF. We chose the subcutaneous route due to the number of published animal studies for comparison and given the fact that OP exposure in humans often involves a combination of dermal, oral, and inhalation routes (i.e., routes that are difficult to model in rats, especially in combination). The relevance of the subcutaneous route for CPF administration in animal models has been challenged due to its unique pharmacokinetics (see Smith et al., 2009).
5. Conclusion
In conclusion, the results of this rodent study can be summarized as follows: 1) after an extended washout period (i.e., when cholinesterase activity had fully returned to normal in both plasma and brain), rats previously exposed to subthreshold doses of CPF, but not DFP, were impaired in a RAM win-shift task and a DNMTP procedure 2) subjects previously exposed to either OP demonstrated impairments in the acquisition of a water maze hidden platform task (i.e., in phase 1), however, only rats previously exposed to DFP demonstrated impairments in a second phase of water maze testing when the platform location was altered (i.e., suggestive if impairments in cognitive flexibility). These results thus support the premise that repeated, subthreshold exposures to commercial organophosphate pesticides like CPF as well as the alkylphosphate, DFP, may lead to protracted deficits in spatial learning and memory in the absence of cholinesterase inhibition. The observations are significant given the widespread use of OPs as pesticides worldwide as well as their potential employment in military and terrorist attacks. The results also further emphasize the need for additional (prospective) investigations to determine more definitively how subthreshold exposures to OPs of different classes may affect specific domains of cognition for extended periods of time.
Research Highlights
Subthreshold exposures to organophosphates lead to chronic deficits in spatial learning and memory.
Organophosphates-related cognitive deficits persist long after cholinesterase inhibition has abated.
Organophosphates may have differential effects depending on the cognitive domain evaluated.
Acknowledgments
The authors would like to thank Ms. Ashley Davis for her administrative assistance in preparing this article. This work was supported by The National Institute of Environmental Health Sciences (ES012241) to AVT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amr MM, Abbas EZ, El-Samra M, El Batanuoni M, Osman AM. Neuropsychiatric syndromes and occupational exposure to zinc phosphide in Egypt. Environ Res. 1997;73(1–2):200–6. doi: 10.1006/enrs.1997.3736. [DOI] [PubMed] [Google Scholar]
- Bakke JE, Price CE. Metabolism of O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in sheep and rats and of 3,5,6-trichloro-2-pyridinol in sheep. J Environ Sci Health B. 1976;11(1):9–22. doi: 10.1080/03601237609372022. [DOI] [PubMed] [Google Scholar]
- Bomser JA, Casida JE. Diethylphosphorylation of rat cardiac M2 muscarinic receptor by chlorpyrifos oxon in vitro. Toxicol Lett. 2001;119:21–6. doi: 10.1016/s0378-4274(00)00294-0. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Padilla SS, Ward T, Pope CN, Olszyk VB. Behavioral and neurochemical changes in rats dosed repeatedly with diisopropylfluorophosphate. J Pharmacol Exp Ther. 1991 Feb;256(2):741–50. [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Serine hydrolase targets of organophosphorus toxicants. Chem Biol Interact. 2005;157-158:277–83. doi: 10.1016/j.cbi.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Dassanayake T, Weerasinghe V, Dangahadeniya U, Kularatne K, Dawson A, Karalliedde L, et al. Cognitive processing of visual stimuli in patients with organophosphate insecticide poisoning. Neurology. 2007;68:2027–30. doi: 10.1212/01.wnl.0000264423.12123.f0. [DOI] [PubMed] [Google Scholar]
- Decker MW, McGaugh JL. The role of interactions between the cholinergic system and other neuromodulatory systems in learning and memory. Synapse. 1991;7:151–68. doi: 10.1002/syn.890070209. [DOI] [PubMed] [Google Scholar]
- De Silva HJ, Samarawickrema NA, Wickremasinghe AR. Toxicity due to organophosphorus compounds: what about chronic exposure? Trans.R.Soc.Trop.Med.Hyg. 2006;100:803–6. doi: 10.1016/j.trstmh.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Duysen EG, Li B, Xie W, Schopfer LM, Anderson RS, Broomfield CA, et al. Evidence for nonacetylcholinesterase targets of organophosphorus nerve agent: supersensitivity of acetylcholinesterase knockout mouse to VX lethality. J Pharmacol Exp Ther. 2001;299(2):528–35. [PubMed] [Google Scholar]
- Dyer SM, Cattani M, Pisaniello DL, Williams FM, Edwards JW. Peripheral cholinesterase inhibition by occupational chlorpyrifos exposure in Australian termiticide applicators. Toxicology. 2001 Dec 28;169(3):177–85. doi: 10.1016/s0300-483x(01)00509-1. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol. 2008;38(Suppl 2):1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Ecobichon DJ. Toxic effect of pesticides. In: Amdur MO, Doull J, Klaassen CD, editors. Cassarett and Doull's Toxicology. 4th ed. Pergamon; New York: 1991. pp. 565–622. [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Farahat FM, Ellison CA, Bonner MR, McGarrigle BP, Crane AL, Fenske RA, et al. Biomarkers of chlorpyrifos exposure and effect in Egyptian cotton field workers. Environ Health Perspect. 2011 Jun;119(6):801–6. doi: 10.1289/ehp.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman J. Cholinesterase Monitoring of Pesticide Handlers inAgriculture: 2007 Final Report. Washington State Department of Labor & Industries; Dec 24, 2007. [Google Scholar]
- Gearhart JM, Jepson GW, Clewell HJ, Andersen ME, Conolly RB. Physiologically based pharmacokinetic model for the inhibition of acetylcholinesterase by organophosphate esters. Environ Health Perspect. 1994 Dec;102(Suppl 11):51–60. doi: 10.1289/ehp.94102s1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart DA, Middlemore ML, Terry AV., Jr. ELISA Methods to Measure Cholinergic Markers and Nerve Growth Factor Receptors in Cortex, Hippocampus, Prefrontal Cortex, and Basal Forebrain from Rat Brain. Journal of Neuroscience Methods. 2006;150:159–73. doi: 10.1016/j.jneumeth.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Gearhart DA, Sickles DW, Buccafusco JJ, Prendergast MA, Terry AV., Jr. Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility. Toxicol Appl Pharmacol. 2007;218:20–9. doi: 10.1016/j.taap.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Grigoryan H, Schopfer LM, Thompson CM, Terry AV, Masson P, Lockridge O. Mass spectrometry identifies covalent binding of soman, sarin, chlorpyrifos oxon, diisopropyl fluorophosphate, and FP-biotin to tyrosines on tubulin: a potential mechanism of long term toxicity by organophosphorus agents. Chem Biol Interact. 2008 Sep 25;175(1–3):180–6. doi: 10.1016/j.cbi.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan H, Schopfer LM, Peeples ES, Duysen EG, Grigoryan M, Thompson CM, et al. Mass spectrometry identifies multiple organophosphorylated sites on tubulin. Toxicol Appl Pharmacol. 2009 Oct 15;240(2):149–58. doi: 10.1016/j.taap.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges H. Maze procedures: the radial-arm and water maze compared. Brain Res Cogn Brain Res. 1996;3:167–81. doi: 10.1016/0926-6410(96)00004-3. [DOI] [PubMed] [Google Scholar]
- Levin ED, Kim P, Meray R. Chronic nicotine working and reference memory effects in the 16-arm radial maze: interactions with D1 agonist and antagonist drugs. Psychopharmacology. 1996;127(1):25–30. doi: 10.1007/BF02805971. [DOI] [PubMed] [Google Scholar]
- Lopachin RM, Decaprio AP. Protein adduct formation as a molecular mechanism in neurotoxicity. Toxicol Sci. 2005;86(2):214–25. doi: 10.1093/toxsci/kfi197. [DOI] [PubMed] [Google Scholar]
- Lotti M, Moretto A. Organophosphate-induced delayed polyneuropathy. Toxicol Rev. 2005;24:37–49. doi: 10.2165/00139709-200524010-00003. [DOI] [PubMed] [Google Scholar]
- Lush MJ, Li Y, Read DJ, Willis AC, Glynn P. Neuropathy target esterase and a homologous Drosophila neurodegeneration-associated mutant protein contain a novel domain conserved from bacteria to man. Biochem J. 1998;332(part 1):1–4. doi: 10.1042/bj3320001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie Ross SJ, Brewin CR, Curran HV, Furlong CE, Abraham-Smith KM, Harrison V. Neuropsychological and psychiatric functioning in sheep farmers exposed to low levels of organophosphate pesticides. Neurotoxicol Teratol. 2010 Jul-Aug;32(4):452–9. doi: 10.1016/j.ntt.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Brain Res Rev. 1993;18:33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- Mekonnen Y, Ejigu D. Plasma cholinesterase level of Ethiopian farm workers exposed to chemical pesticide. Occup Med (Lond) 2005 Sep;55(6):504–5. doi: 10.1093/occmed/kqi088. [DOI] [PubMed] [Google Scholar]
- Middlemore-Risher ML, Buccafusco JJ, Terry AV., Jr. Repeated exposures to low-level chlorpyrifos results in impairments in sustained attention and increased impulsivity in rats. Neurotoxicology and Teratology. 2010;32:415–24. doi: 10.1016/j.ntt.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemore-Risher ML, Adam BL, Lambert NA, Terry AV. Effects of chlorpyrifos and chlorpyrifos-oxon on the dynamics and movement of mitochondria in rat cortical neurons. J Pharmacol Exp Ther. 2011 Jul 28; doi: 10.1124/jpet.111.184762. (In press) DOI:10.1124/jpet.111.184762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17(6):669–82. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Peeples ES, Schopfer LM, Duysen EG, Spaulding R, Voelker T, Thompson CM, et al. Albumin, a new biomarker of organophosphorus toxicant exposure, identified by mass spectrometry. Toxicol Sci. 2005;83:303–12. doi: 10.1093/toxsci/kfi023. [DOI] [PubMed] [Google Scholar]
- Pope CN. Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health B Crit. 1999;Rev 2:161–81. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Self RL, Smith KJ, Ghayoumi L, Mullins MM, Butler TR, et al. Microtubule-associated targets in chlorpyrifos oxon hippocampal neurotoxicity. Neuroscience. 2007;146(1):330–9. doi: 10.1016/j.neuroscience.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Casida JE. Fatty acid amide hydrolase inhibition by neurotoxic organophosphorus pesticides. Toxicol Appl Pharmacol. 2001;173(1):48–55. doi: 10.1006/taap.2001.9175. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Nomura DK, Sparks SE, Segall Y, Casida JE. Cannabinoid CB1 receptor as a target for chlorpyrifos oxon and other organophosphorus pesticides. Toxicol Lett. 2002;135(1–2):89–93. doi: 10.1016/s0378-4274(02)00251-5. [DOI] [PubMed] [Google Scholar]
- Richards PG, Johnson MK, Ray DE. Identification of acylpeptide hydrolase as a sensitive site for reaction with organophosphorus compounds and a potential target for cognitive enhancing drugs. Mol Pharmacol. 2000;58:577–83. doi: 10.1124/mol.58.3.577. [DOI] [PubMed] [Google Scholar]
- Roldan-Tapia L, Parron T, Sanchez-Santed F. Neuropsychological effects of long-term exposure to organophosphate pesticides. Neurotoxicol Teratol. 2005;27(2):259–66. doi: 10.1016/j.ntt.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Rusyniak DE, Nanagas KA. Organophosphate poisoning. Semin Neurol. 2004;24:197–204. doi: 10.1055/s-2004-830907. [DOI] [PubMed] [Google Scholar]
- Saab BJ, Saab AM, Roder JC. Statistical and theoretical considerations for the platform relocation water maze. J Neurosci Methods. 2011 May 15;198(1):44–52. doi: 10.1016/j.jneumeth.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Salvi RM, Lara DR, Ghisolfi ES, Portela LV, Dias RD, Souza DO. Neuropsychiatric evaluation in subjects chronically exposed to organophosphate pesticides. Toxicol Sci. 2003 Apr;72(2):267–71. doi: 10.1093/toxsci/kfg034. [DOI] [PubMed] [Google Scholar]
- Saunders BC. Some aspects of the chemistry and toxic action of organic compounds containing phosphorus and fluorine. University Press; Cambridge: 1957. p. 42. [Google Scholar]
- Schopfer LM, Grigoryan H, Li B, Nachon F, Masson P, Lockridge O. Mass spectral characterization of organophosphate-labeled, tyrosine-containing peptides: characteristic mass fragments and a new binding motif for organophosphates. J Chromatogr B Analyt Technol Biomed Life Sci. 2010 May 15;878(17–18):1297–311. doi: 10.1016/j.jchromb.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Sharma N. Neurological syndromes following organophosphate poisoning. Neurol India. 2000 Dec;48(4):308–13. [PubMed] [Google Scholar]
- Smith JN, Campbell JA, Busby-Hjerpe AL, Lee S, Poet TS, Barr DB, et al. Comparative chlorpyrifos pharmacokinetics via multiple routes of exposure and vehicles of administration in the adult rat. Toxicology. 2009 Jun 30;261(1–2):47–58. doi: 10.1016/j.tox.2009.04.041. [DOI] [PubMed] [Google Scholar]
- Steenland K, Jenkins B, Ames RG, O'Malley M, Chrislip D, Russo J. Chronic neurological sequelae to organophosphate pesticide poisoning. Am.J.Public Health. 1994;84:731–6. doi: 10.2105/ajph.84.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R, Spurgeon A, Calvert IA, Beach J, Levy LS, Berry H, et al. Neuropsychological effects of long-term exposure to organophosphates in sheep dip. Lancet. 1995 May 6;345(8958):1135–9. doi: 10.1016/s0140-6736(95)90976-1. [DOI] [PubMed] [Google Scholar]
- Sungurtekin H, Gurses E, Balci C. Evaluation of several clinical scoring tools in organophosphate poisoned patients. Clin. Toxicol (Phila) 2006;44:121–6. doi: 10.1080/15563650500514350. [DOI] [PubMed] [Google Scholar]
- Tanimura Y, Yang MC, Lewis MH. Procedural learning and cognitive flexibility in a mouse model of restricted, repetitive behaviour. Behav Brain Res. 2008 Jun 3;189(2):250–6. doi: 10.1016/j.bbr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Stone JD, Buccafusco JJ, Sickles DW, Prendergast MA. Repeated, Subthreshold Exposures to Chlorpyrifos in Rats: Hippocampal Damage, Impaired Axonal Transport and Deficits in Spatial Learning. J Pharmacol Exp Ther. 2003;305:375–84. doi: 10.1124/jpet.102.041897. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Beck WD, Truan JN, Middlemore ML, Williamson LN, et al. Chronic, Intermittent Exposure to Chlorpyrifos in Rats: Protracted Effects on Axonal Transport, Neurotrophin Receptors, Cholinergic Markers, and Information Processing. J Pharmacol Exp Ther. 2007;322:1117–28. doi: 10.1124/jpet.107.125625. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr., Warner SE, Vandenhuerk L, Pillai A, Mahadik SP, Zhang G, et al. Negative Effects of Chronic Oral Chlorpromazine and Olanzapine Treatment on the Performance of Tasks Designed to Assess Spatial Learning and Working Memory in Rats. Neuroscience. 2008;156:1005–16. doi: 10.1016/j.neuroscience.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Gearhart DA, Beck WD, Middlemore-Risher ML, Truan JN, et al. Repeated, intermittent exposures to diisopropylfluorophosphate in rats: protracted effects on cholinergic markers, nerve growth factor-related proteins, and cognitive function. Neuroscience. 2011;176:237–53. doi: 10.1016/j.neuroscience.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin CDS. The Pesticide Manual, A World Compendium. 14th ed. British Crop Protection Council; Alton, Hampshire, UK: 2006. pp. 186–7. [Google Scholar]