Abstract
Psoriasis is a hereditary disease elicited by chronic activation of cutaneous T cells. Delineating the mechanistic interplay of the cell subsets involved is key to developing the next generation of effective treatments. In this issue, Bovenschen et al. report that regulatory T cells maintain a fine balance between the transcription factors Foxp3 and RORγt. In patients with psoriasis, Tregs readily turn into IL-17-expressing cells, thus potentially perpetuating the inflammatory process that characterizes the disease. Results demonstrating that the histone/protein deacetylation inhibitor trichostatin A can block this conversion suggest that an epigenetic modification may underlie regulatory T-cell plasticity.
Psoriasis in history
References to psoriasis date to biblical times (Holubar, 2003). The condition is characterized by the relentless appearance of inflamed scaly patches of skin. The erythema associated with chronic plaques is the macroscopic manifestation of ongoing recruitment and activation of T cells that sustain a permanent inflammatory milieu, which ultimately stimulates keratinocytes into hyperproliferation and a vicious proinflammatory cycle (Nograles et al., 2010). The ultimate cause of psoriasis remains unknown, and a strict mitigating signal or single antigenic target has yet to be identified. Together, human and animal studies have led to the understanding that in patients genetically susceptible to psoriasis, some stimulus, perhaps infection, leads to a coordinated series of cytokine signaling events among keratinocytes, endothelial cells, T cells, macrophages, and dendritic cells, which, once initiated, sustains the cycle. Intervention at several points in this cycle results in clinical resolution; however, durable remissions and permanent clearance have not been achieved.
Can regulatory T cells become IL-17 producers in psoriasis spontaneously?
Th17 cells are a newly recognized lineage of T helper cells (Harrington et al., 2005; Park et al., 2005) that have been linked to pathogenic inflammatory processes and autoimmune diseases (Goodman et al., 2009; Rizzo et al., 2011). In their current article, Bovenschen et al. demonstrate that regulatory T cells (Tregs) in psoriatic patients can differentiate toward IL-17-expressing Tregs. The investigators suggest that the combination of psoriatic Treg dysfunction (Sugiyama et al., 2005) and a propensity for differentiating into IL-17-producing regulatory cells contributes to the perpetuation of chronic autoimmunity. Interestingly, the investigators demonstrate dermal IL17+ Foxp3+ CD4+ Tregs in psoriatic skin lesions, confirming the possibility that Treg cells in disease probably become pathogenic IL-17 producers.
Under normal conditions, Tregs suppress inflammation, but numerous publications have demonstrated a dysfunction of Tregs, particularly in autoimmune diseases (Bettini and Vignali, 2009). Bovenschen et al. have identified another mechanism behind the propensity of autoimmune diseases to alter the fine balance between regulatory and pathogenic T cells.
Balancing Foxp3/RORγt levels
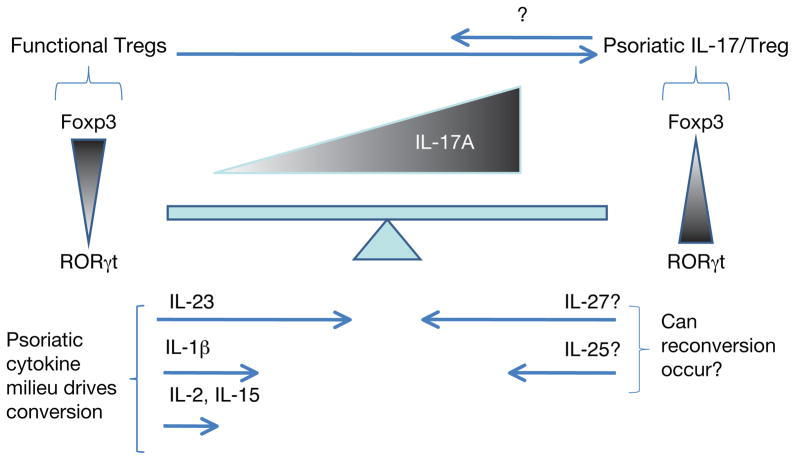
Bovenschen et al. (2011) demonstrate that psoriatic Tregs exhibit a propensity to alter levels of the master regulator Foxp3. They observed that upregulating the expression of RORγt in Tregs as modeled by the addition of cytokines representative of the psoriatic milieu (IL-2, IL-15, IL-1β, and IL-23), together with T cell receptor stimulation through anti-CD3/CD28, favors a concomitant decrease in Foxp3 and an increase of IL-17-producing Tregs. These investigators further demonstrate that the conversion from Treg to IL-17/Treg is not an all-or-nothing transition. Indeed, it is likely that a continuum of converting cells exists, as evidenced by Foxp3+ RORγt+ coexpression and a gradual loss of Foxp3.
Conversion to IL-17/Treg
This observation introduces a new concept in psoriasis research: a cell continuum in which pure Foxp3-expressing Tregs coexist in the vicinity of psoriatic lesions with dual Foxp3- and RORγt-expressing Tregs that ultimately converge into the IL-17-expressing Tregs, potentially becoming Th17 over time, driven by the psoriatic cytokine milieu (Figure 1). The data presented identify IL-23 as the cytokine primarily responsible for this conversion. Interestingly, this effect is not mediated by IL-23’s receptor, suggesting that IL-23 might promote this conversion through an alternative pathway. Indeed, IL-23 seems to play a pivotal role in downregulating expression of Foxp3, a key feat that IL-1β and IFN-γ are not able to achieve. Still open for debate is whether conversion is initiated by Foxp3low or Foxp3high Tregs.
Figure 1. The regulatory cell continuum mediates psoriatic pathogenesis.
Foxp3 T regulatory cells (Tregs) convert progressively to IL-17-producing regulatory cells driven by psoriatic cytokines, predominantly IL-23. The fine balance between the Treg master regulatory Foxp3 and RORγt controls the cellular fate of Tregs.
If, as the investigators propose, the conversion of Tregs into IL-17-producing Tregs is a second important concept in regulatory cell dysfunction in psoriasis, this conversion may represent a window of opportunity for clinical interventions aimed at delaying or halting the transition. Indeed, an examination of patients currently receiving therapeutics targeting IL-12/23p40 is called for in light of this new information. If these therapies prevent conversion, it may present a new mechanism of action for such biologics. Many questions arise from these observations. For example, is it possible to reverse the phenotype of IL-17-expressing Treg into Foxp3high Tregs? Are there drivers of the reconversion, such as IL-25 or IL-27, or is programmed cell death of Th17-expressing Tregs the only solution (Figure 1)?
Are epigenetic changes necessary for IL-17/Treg conversion?
Another observation is perhaps even more intriguing. Bovenschen et al. (2011) demonstrate in psoriatic Tregs, as they have previously shown in nonpsoriatic Tregs/IL-17 cells, that addition of the histone/protein deacetylase inhibitor trichostatin A can block the conversion of Foxp3+ Tregs into Foxp3–RORγt+ IL-17/Tregs. This implicates histone/protein deacetylation as a key factor in the conversion of regulatory T cells to potentially pathogenic effector T cells. This is intriguing given the report of increased levels of histone deacetylase 1 (HDAC1) in psoriatic tissue (Tovar-Castillo et al., 2007) and the proposed therapeutic use of HDAC inhibitors for autoimmune diseases on the basis of their anti-inflammatory properties (Dinarello et al., 2011).
Thus, the work presented by Bovenschen and colleagues reveals interesting results with clinical implications for improved therapeutic agents designed to inhibit the conversion of Tregs in psoriasis. The proposed use of HDAC inhibitors for autoimmune diseases such as systemic lupus erythematous, another autoimmune disease with a described defect in Tregs, might doubly benefit from agents that may restore the Foxp3/RORγt balance toward functional regulation by Tregs (Reilly et al., 2011).
Clinical Implications.
Psoriatic regulatory T cells (Tregs), influenced by a psoriatic cytokine milieu, have an enhanced propensity to secrete the proinflammatory cytokine IL-17.
Treg plasticity is directed by the balance of Foxp3 and RORγt signaling.
Inhibition of histone deacetylase (HDAC) activity blocks the conversion of Tregs to IL-17/Tregs, suggesting that HDAC inhibitors may have therapeutic benefit in treating psoriasis.
Acknowledgments
TSM is supported by grants P30 AR39750, P50 AR05508 and RO1 AR05498 from the National Institutes of Health as well as funds from the Murdough Family Center for Psoriasis at University Hospitals Case Medical Center and Case Western Reserve University.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Bettini M, Vignali DA. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr Opin Immunol. 2009;21:612–8. doi: 10.1016/j.coi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovenschen HJ, van de Kerkhof PC, van Erp PE, et al. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol. 2011;131:1853–60. doi: 10.1038/jid.2011.139. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Fossati G, Mascagni P. Histone deacetylase inhibitors for treating a spectrum of diseases not related to cancer. Mol Med. 2011;17:333–52. doi: 10.2119/molmed.2011.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WA, Levine AD, Massari JV, et al. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183:3170–6. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Holubar K. Psoriasis and parapsoriasis: since 200 and 100 years, respectively. J Eur Acad Dermatol Venereol. 2003;17:126–7. doi: 10.1046/j.1468-3083.2003.00622.x. [DOI] [PubMed] [Google Scholar]
- Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29:3–9. doi: 10.1016/j.sder.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly CM, Regna N, Mishra N. HDAC inhibition in lupus models. Mol Med. 2011;17:417–25. doi: 10.2119/molmed.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo HL, Kagami S, Phillips KG, et al. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol. 2011;186:1495–502. doi: 10.4049/jimmunol.1001001. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Gyulai R, Toichi E, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–73. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Castillo LE, Cancino-Diaz JC, Garcia-Vazquez F, et al. Under-expression of VHL and over-expression of HDAC-1, HIF-1alpha, LL-37, and IAP-2 in affected skin biopsies of patients with psoriasis. Int J Dermatol. 2007;46:239–46. doi: 10.1111/j.1365-4632.2006.02962.x. [DOI] [PubMed] [Google Scholar]