Abstract
Osteocytes, the cells residing within the bone matrix and comprising 90% to 95% of the all bone cells, have long been considered quiescent bystander cells compared to the osteoblasts and osteoclasts whose activities cause bone gain and loss, and whose dysfunction lead to growth defects and osteoporosis. However, recent studies show that osteocytes play a crucial, central role in regulating the dynamic nature of bone in all its diverse functions. Osteocytes are now known to be the principal sensors for mechanical loading of bone. They produce the soluble factors that regulate the onset of both bone formation and resorption. Osteocytes regulate local mineral deposition and chemistry at the bone matrix level, and they also function as endocrine cells producing factors that target distant organs such as the kidney to regulate phosphate transport. Osteocytes appear to be the major local orchestrator of many of bone’s functions.
Keywords: Osteocytes, Bone formation, Bone resorption, Mineral regulation, Mechanical sensing, Signaling, Osteoporosis
Introduction
Osteocytes, the cells residing within the bone matrix, make up between 90% to 95% of the cellular component in skeletally mature adult bone tissue. This cell type is derived from the osteoblast lineage and is morphologically characterized by distinct dendritic processes that emanate from the cell body and connect with other osteocytes, cells at the bone surface, and nearby blood vessels. The cell body is located in an ellipsoidal space called a lacuna, while the dendritic processes reside in tiny cylindrical channels called canaliculi (~ 250–300 nm in diameter) [1]. At larger length scales, it is the highly regular and ubiquitous distribution of these cells, and their interconnecting processes, in the mineralized matrix that characterize the osteocyte. Furthermore, it is this feature that makes this network of cells the perfect candidate to serve as a sensory network that can respond to the effects of external influences (hormonal/mechanical, etc.) imposed on bone. It is beginning to emerge that osteocytes play a crucial role in regulating the dynamic nature of bone; it may even be said that this is a major function of these cells.
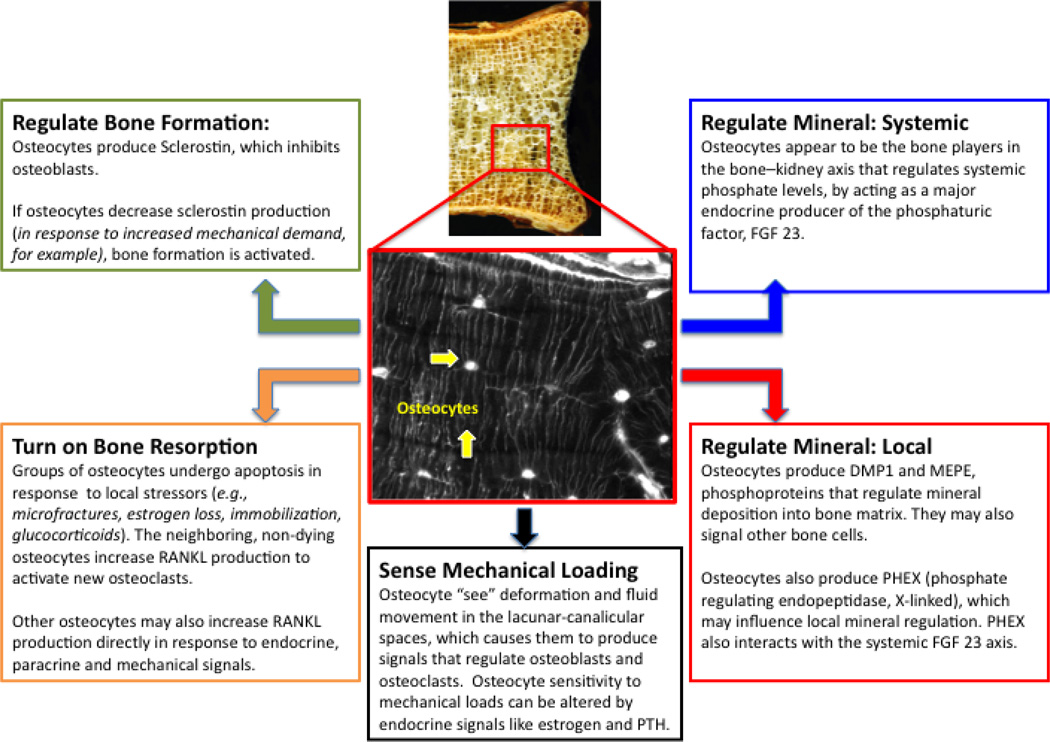
Osteocytes are derived from the osteoblast cell lineage. The transformation from plump collagen-producing osteoblasts to smaller dendritic osteocytes is a dramatic one that requires extensive restructuring of the cytoskeletal and intracellular machinery. For example, having undergone the transition osteocytes typically have lost the apical and basolateral plasma membrane polarization normally seen in osteoblasts [2]. While the mechanisms that cause certain osteoblasts to differentiate into osteocytes are not fully understood, it is known that those that do not make that transition either undergo apoptosis or become bone-lining cells. Until recently, constitutive molecular markers for osteocytes were limited to low collagen and alkaline phosphatase production, high casein kinase II and osteocalcin protein expression, and high CD44 [1]. However, more recently the osteocyte has been shown to produce a range of proteins including dentin matrix protein 1 (DMP1), phosphate-regulating neutral endopeptidase on chromosome X (PHEX), matrix extracellular phosphoglycoprotein (MEPE), sclerostin, fibroblast growth factor 23 (FGF-23), osteoprotegerin (OPG) and, in certain situations, receptor activator of nuclear factor-κB ligand (RANKL). Expression of these factors by osteocytes suggests that these cells play an integral role in the regulation of a wide range of bone remodeling and mineral regulatory functions. Specifically, the role of osteocyte signaling in skeletal metabolism can be considered in terms of how it contributes to 1) regulation of local mineralization; 2) regulation of systemic mineralization; 3) bone formation by osteoblasts; 4) bone resorption by osteoclasts; and 5) sensing mechanical loads on bone (Fig. 1).
Figure 1. Recent insights in osteocyte signaling and regulation in bone function.
DMP1—dentin matrix protein 1; FGF 23—fibroblast growth factor 23; MEPE—matrix extracellular phosphoglycoprotein; PHEX—phosphate-regulating neutral endopeptidase on chromosome X; PTH—parathyroid hormone; RANKL—receptor activator of nuclear factor-κB ligand.
Osteocyte Signaling and Regulation of Local Mineralization
Since the osteocyte, by definition, is located within the dense calcified matrix of bone it is reasonable to suggest that some part of the cell repertoire involves the development and maintenance of that matrix. While this seems intuitive and logical today, it is only in relatively recent years that osteocytes have been considered capable of this rather than simply being inert cells buried in the bone matrix. Three proteins have been identified as having some role in the mineralization process: DMP1, PHEX, and MEPE. DMP1 is an acidic noncollagenous protein that is expressed during the initial stages of mineralized matrix formation in bone and dentin. It is also specifically expressed along and in the canaliculi of osteocytes in the bone matrix [3, 4]. As a highly phosphorylated protein it is thought that DMP1 may be involved in the regulation of hydroxyapatite formation. More specifically, transmission electron microscopy analyses have shown that DMP1 is localized in the gap region between collagen type 1 fibrils during the early phases of mineralization [4]. PHEX is thought to play a similar role, and likely interacts extensively with DMP1; deletion of either gene in mice results in a similar, if not identical, phenotype [3]. PHEX, a type 1 cell-surface zinc metalloprotease, was originally described on the plasma membrane of osteoblasts and osteocytes [5] and loss-of-function mutations in this gene resulted in X-linked hypophosphatemic rickets [6]. Although the precise function of this factor is not yet known, it clearly plays a significant role in phosphate homeostasis and bone mineralization.
MEPE, along with DMP1, belongs to the SIBLING (small integrin-binding ligand N-linked glycoprotein) family and is thought to be involved in the regulation of matrix mineralization [7]. It has been shown to contain a particularly highly phosphorylated region (called the ASARM region), which is a potent inhibitor of mineralization in vitro [8]. Furthermore, in the X-linked rickets mouse model, high ASARM production by osteocytes was correlated with an osteomalacia-like phenotype (ie, defective mineralization). In other studies, MEPE was deleted from mice, which resulted in increased bone formation and resistance to age-related bone loss, leading the authors to speculate that MEPE is a marker of mature osteocytes that act directly on osteoblasts to inhibit their bone formation activity [9].
Osteocyte Signaling and Regulation of Systemic Mineralization
The transport of inorganic minerals to and from the skeletal tissues necessarily requires interaction with, and has effects on, other organs—particularly the kidneys. One factor that is central to the regulation of the bone–kidney axis is the phosphaturic factor FGF-23. High circulating levels of FGF-23 can cause disorders that are characterized by hypophosphatemia, decreased production of 1,25 dihydroxyvitamin D, and osteomalacia. By contrast, low circulating levels can be associated with hyperphosphatemia, increased production of 1,25 dihydroxyvitamin D, soft tissue calcification, and hyperostosis [10].
A fascinating feature of this protein is its predominant production by osteocytes and its regulation by PHEX, DMP1, and MEPE [10–12]. The interrelationship between these factors can be demonstrated in the case of DMP1 deficiency, where matrix mineralization is impaired due to the loss of this mineralization-promoting extracellular protein [3]. Osteocytes in this situation increase FGF-23 expression, which results in increased phosphate excretion from the kidneys to match the decreased mineralization of bone. Based on these associations it is reasonable to posit that osteocytes are capable of coordinating osteoblast-mediated bone formation with renal regulation of systemic phosphate homeostasis through the regulated expression of sclerostin, FGF-23, and possibly other factors [10].
Osteocyte Signaling and Bone Formation by Osteoblasts
In addition to their role in local and systemic mineral homeostasis, osteocyte signaling also has a direct effect on the bone formation activities of osteoblasts. One product in particular that has this effect is called sclerostin, which is a protein that is encoded by the SOST gene. This factor is highly expressed in osteocytes and appears to directly inhibit bone formation [13]. Individuals with impaired, or prematurely terminated, function of the SOST gene can develop a rare, recessively inherited high bone mass disorder called sclerosteosis [14, 15]. By presenting the opportunity to tap directly into a potential bone-building pathway, SOST has become an exciting gene target that many believe could yield novel anabolic therapeutic agents to combat diseases such as osteoporosis. Recently, mouse models have been generated, with the SOST gene deleted, to facilitate the study of this pathway in more detail. Like the condition in humans, SOST knockout mice display a high bone mass phenotype demonstrating evolutionary conservation of this protein’s function as a negative regulator of bone formation [16].
To fully characterize and understand the function of osteocytes, it is advantageous to carry out experiments in tightly controlled cell-culture conditions. However, the isolation of primary osteocytes is a challenging task and successful yields become smaller as a function of age and mineralization of the tissue. Thus, investigators have attempted to establish osteocyte cell lines in recent years. One such model of the early osteocyte is the HOB-01-C1 [17], and another model of that transitional phase is the MLO-A5 [18]; however; by far the most commonly used osteocyte-like cell line is the MLO-Y4 [19]. These cells exhibit many of the properties that osteocytes in situ are known to possess such as the characteristic dendritic morphology, low alkaline phosphatase expression, and high expression of connexin 43 and E11. These cells provide a unique opportunity to probe the effects of external stimuli, such as fluid flow, on the activation of osteocyte-specific pathways and on the release of signaling factors and cytokines [20]. Furthermore, they also have been used to investigate apoptosis, autophagy, primary cilia, hemichannels, gap junctions, hypoxia, osteoblast activity, and osteoclast recruitment and activation [21•, 22–24, 25•, 26, 27••, 28].
One of the first descriptions of the relationship between osteocytes and osteoclast activity was from in vitro studies by Tanaka et al. [29], who reported that isolated avian osteocytes could support osteoclast formation in the absence of any other osteotropic factors. This confirmed that the necessary cellular machinery exists in osteocytes to produce cytokines that are relevant in osteoclastogenesis. While it is of utmost importance to analyze and characterize this system under culture conditions, it is also critical to have the ability to probe osteocytes in situ. It is now understood that osteocytes are capable of modulating the formation and resorption of living bone [30]. However, understanding the workings of this system in vivo requires in-depth knowledge of the physiological microenvironment in bone, and in particular the lacunar-canalicular system. Interstitial fluid movement within this system is caused by extravascular pressure and also cyclical mechanical loading generated during habitual usage. It is thought that these forces generate shear stresses on the cell body, which is likely the way that osteocytes are informed of their mechanical environment [31, 32]. In live bone tissue, mechanical strain is a key regulator that influences bone modeling and remodeling via the osteocyte network.
As noted above, osteocytes make up 90% to 95% of bone cells in the mature adult skeleton at any one time, with osteoblasts and osteoclasts comprising the remainder (4% to 6% and 1% to 2%, respectively). This fact alone may suggest intuitively that osteocytes would in some way be involved in sensing changes in the mechanical environment, and responding to them. This idea is further reinforced by the high degree of interconnectivity within the lacunar-canalicular system. Various in vivo studies on small animals have demonstrated load-related responses in osteocytes. For example, within minutes of mechanical loading, various markers of cell metabolism including GAPDH are increased in osteocytes and bone-lining cells [33–35]. Other factors such as DMP1 have been shown to change in studies of orthodontic tooth movement [36], and also in the ulnar loading models of bone formation [37, 38, 39••]. E11, a factor that is related to the formation of the characteristic osteocyte dendrites, is also increased following loading, not only in cells near the surface but also in those residing deeper within the matrix [40].
While a mechanosensory role for osteocytes seems intuitive and likely, and is supported by the data from these studies, key questions pertaining to the precise mechanism of osteocyte signal sensation, and how these signals are conveyed to other non-sensing cells, remain to be answered. In recent years, various theories have been proposed that suggest that deformation of the cell surface, resulting from interstitial fluid flow, is critical to the osteocyte mechanosensory function [32, 41, 42]. However, strains that were required to elicit the expected response from cells under culture conditions were too high to be applicable in living bone tissue. Thus, Weinbaum et al. [32] proposed a strain amplification model of the osteocyte process that attempted to reconcile those disparities.
In that model an osteocyte dendrite, within its canaliculus, is assumed to be tethered to the surrounding matrix by some structural component such as CD44, laminins, and other as-yet unknown structural proteins. A second requirement of this model is the presence of hexagonal actin bundles within the cell process. The model predicts that fluid flow in the system, and the resulting interaction between those structural components, result in osteocyte wall shear stresses in the range of 8 to 30 dynes/cm2 [32]. To complement these theoretical proposals, experimental studies have successfully measured lacuno-canalicular solute transport in vivo, via analyses of dye diffusion at depths of up to 50 µm in mouse bone [43]. In these studies, the diffusion coefficient for a specific molecule in bone was found to be 62% of its value in water. Furthermore, that diffusion coefficient was like that of a similarly sized molecule in cartilage, suggesting the presence of a pericellular matrix around osteocytes with a structure resembling that proposed for the endothelial glycocalyx [44]. These data have provided some valuable insights that have been beneficial in building a more complete picture of mechanisms of molecular transport within the lacuno-canalicular microenvironment.
Osteocyte Signaling and Bone Formation by Osteoclasts
Of particular utility in probing the mechanosensory role of osteocytes is the study of their response to localized, noninflammatory micro-injury. This stems from the unique relationship between microdamage in bone and the intrinsic tissue repair processes. Microdamage is known to accumulate in normal human bone as a result of daily wear and tear [45, 46]. Those damaged areas are subsequently targeted for repair by basic multicellular units comprised of osteoclasts (removal) and osteoblasts (replacement). Existing in vivo loading models have been developed to the point where microdamage can be induced in highly localized regions. These can then be studied in terms of their in situ cell response relative to nearby sites of microdamage. One of the early studies of this kind was performed by Bentolila et al. [47], in which in vivo cyclic loading was used to induce microdamage in the rat ulnar cortex and whole-limb stiffness loss was used to monitor the accumulation of microdamage. These studies showed that cortical bone does indeed undergo intracortical remodeling specifically in response to microdamage and that resorption is associated with areas of microdamage after 10 to 14 days. Furthermore, and perhaps most importantly, the resorptive response in these experiments was also found to co-localize with regions of altered osteocyte integrity.
In subsequent studies from the same group, the nature of that observed altered osteocyte integrity near microdamage was investigated. Verborgt et al. [37] hypothesized that changes in osteocyte integrity resulted from the initiation of apoptosis (regulated cell death) in those cells near the microdamage locus, which subsequently would undergo resorption. Using the same loading model described above, the authors assessed osteocyte integrity histomorphometrically from terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) stained sections to detect cells undergoing DNA fragmentation associated with apoptosis. Ten days after loading large numbers of TUNEL-positive osteocytes were observed in areas near microdamage and surrounding intracortical remodeling spaces. These studies confirmed the hypothesis that microdamage in cortical bone results in osteocyte apoptosis, which is restricted to cells near the original damage site. Furthermore, they showed that the subsequent resorption also co-localizes with microdamage and apoptotic osteocytes after a period of 10 to 14 days. By identifying the strong association between each event, these studies reinforce the hypothesis that osteocyte apoptosis plays a key role in the activation of signaling mechanisms by which osteoclasts target bone for removal after the occurrence of microdamage.
While the relationship between microdamage, osteocyte apoptosis, and osteoclastic resorption had been clarified, the problem of identifying the controlling mechanisms for osteocyte apoptosis in vivo remained. To address this issue Verborgt et al. [48] performed a related study that probed the expression of BAX (a pro-apoptotic gene product) and Bcl-2 (an anti-apoptotic gene product) in osteocytes from rat ulnae that were fatigue loaded to induce microdamage in the same way as described previously. Once again, apoptotic osteocytes (BAX+) were concentrated at the microdamage locus, in a similar distribution to the TUNEL+ cells in the previous study. Importantly, the pattern of Bcl-2+ osteocytes as a function of distance from microdamage was very different, with peak levels observed some distance (1–2 mm) away from the damaged tissue. These data suggest that osteocytes near microcracks are sufficiently damaged to initiate an apoptotic response; however, those farther away from the insult do not undergo apoptosis but activate a protective mechanism to prevent from apoptosis. Effectively the zone of apoptotic osteocytes near damage is “walled-in” by a population of surviving osteocytes that are actively producing Bcl-2. This penumbra-like phenomenon of non-apoptotic cells surrounding those undergoing apoptosis has also been reported in other focal injury systems such as ischemic brain and heart [49, 50].
To establish a causal relationship between microdamage and osteoclastogenesis, a study in which osteocyte apoptosis in fatigued bone could be inhibited was required. Cardoso et al. [39••] designed such a study, by using a pan caspase inhibitor quinolyl-valyl-O-methylaspartyl-[-2, 6-difluorophenoxy]-methylketone (QVD) to block the apoptotic response in fatigue-loaded rat ulnae in vivo. This experiment was designed to test whether the physical presence of microcracks alone, or perhaps some other heretofore undefined pathway, possessed the potential to initiate osteoclastogenesis. The results from these studies showed that complete inhibition of fatigue-induced apoptosis blocks the activation of osteoclastic resorption. This work demonstrated that apoptosis is indeed necessary to initiate intracortical bone remodeling following fatigue-induced microdamage. Supporting in vitro experiments were also performed to ensure that the pharmacological agent used did not have a direct effect on osteoclast initiation. Accordingly, formation of TRAP-positive multinucleated cells by total rat marrow was unaffected by QVD at concentrations at least equivalent to the estimated serum concentration used in vivo.
While it had been demonstrated that apoptosis in osteocytes is a critical stage in the initiation of resorption, whether or not those same dying cells actually produce signaling factors to induce osteoclast differentiation has not yet been established. To achieve this, it would be necessary to probe the system for specific pro-osteoclastogenic proteins in the region around microdamage and to then localize their expression, thus identifying the cellular source. In terms of the characterization of pro-osteoclastogenic proteins, the identification of the RANK/RANKL/OPG pathway as the dominant final mediator of osteoclastogenesis represented a major advance in understanding the bone remodeling process [51]. RANKL, a factor that is expressed on the surface of pre-osteoblastic/stromal cells, binds to its cognate receptor RANK on the surface of pre-osteoclastic cells; this interaction is crucial for differentiation, activation, and survival of osteoclasts. OPG, constitutively expressed by mature osteocytes, serves as a decoy receptor and blocks the RANK/RANKL interaction, and has the effect of preventing osteoclastogenesis. The RANKL/OPG ratio can be used as an indicator of the level of osteoclastogenic activity in a system.
In a recent development of this work, Kennedy et al. [52••] showed that apoptotic osteocytes near microdamage do not upregulate pro-osteoclastogenic markers following fatigue-induced microdamage. Here, again using the same ulnar loading model, the authors induced microdamage in mid-diaphyseal cortical bone and then examined the damaged tissue segments at two time points in the pre-osteoclastic phase (3 and 7 days post fatigue). They used gene expression analyses to confirm the upregulation of pro-osteoclastogenic factors in tissue segments containing microdamage. Subsequently, and perhaps most importantly, the cellular sources of those signals were localized using a novel double-staining immunohistochemistry technique. This permitted simultaneous localization of apoptotic osteocytes and osteocytes expressing pro-osteoclastogenic signals relative to microdamage sites. Apoptotic osteocytes and those staining for osteoclast regulatory signals exhibited different spatial distributions, with the former concentrated in the damage region and declining as a function of distance from the microdamage focus. Cells expressing RANKL peaked at a greater distance from the damage site, then returned to baseline beyond this distance. OPG staining was also reduced markedly in osteocytes immediately surrounding microdamage and also changed as a function of distance. However, in this case cells expressing this factor increased, as OPG is constitutively expressed by normal healthy osteocytes. These results show that dying and signaling osteocytes are discrete populations. While dying osteocytes did not produce pro-osteoclastogenic signals, a population of non-apoptotic osteocytes surrounding the apoptotic cells near the microdamage zone displayed altered expression of RANKL and OPG in a spatially and temporally coherent fashion.
These studies show that the RANKL/OPG ratio is shifted to create a pro-osteoclastogenic environment surrounding the microdamage area, indicated by upregulation of RANKL and corresponding downregulation of OPG. RANKL is typically formed as a membrane-bound molecule (40–45 kDa), which under the appropriate conditions can be cleaved by metalloproteinases into a smaller soluble form (30 kDa) [53]. It seems reasonable to hypothesize that osteocyte signaling involves both the membrane-bound and soluble forms. The molecular size order for soluble RANKL would allow it to move readily through the osteocyte lacunar-canalicular system [54••]. It is noteworthy that these studies found upregulation of RANKL staining in osteocytes to be highest some 150 to 200 µm from the microdamage-osteocyte apoptotic core, which would place this signaling in direct proximity to the nearest bone surface (periosteal or endocortical) at which osteoclast precursors can be recruited. Other studies have used different approaches to characterize the expression of RANKL by osteocytes. It has recently been shown that selective ablation of RANKL in an osteocyte population in mice results in reduced resorption and its phenotypic consequences during development as well as an impaired ability to respond to mechanical loading [55••].
The fatigue microdamage repair model in rats is particularly well suited to the examination of pro-osteoclastogenic signaling by osteocytes for two reasons. First, baseline intracortical remodeling in this model is zero [47, 56]. Thus, any remodeling-related activities or signals that are observed can be attributed to the experimental intervention. This is in contrast to constitutive genetic mouse model where osteocyte-derived RANKL was eliminated. In such studies, the loss of osteocyte-derived RANKL during the rapid resorption phases in bone growth and development led to osteopetrosis [54••]. Together these studies indicate that osteocytes are a major source of RANKL production, with different functional contributions at different skeletal ages. Second, unlike development and hindlimb unloading, induction of bone remodeling in response to microdamage is highly localized, and allows the relationship between pro-osteoclastogenic signaling to be assessed with respect to both the inducing stimuli (damage and the requisite osteocyte apoptosis) and the tissue response to it (subsequent remodeling). The finding that a dramatic induction of an increased RANKL/OPG ratio occurs in bone after damage, was limited to a finite region where resorption consistently occurs in this model, and yet extended far enough to reach the periosteal or endocortical surface where remodeling initiates, strongly support a direct role for osteocytes—specifically non-apoptotic osteocytes—in RANKL-based signaling to osteoclast progenitors.
Conclusions
Our knowledge of the contribution of osteocytes to distinct aspects of skeletal metabolism is expanding at an unprecedented rate. The early belief that osteocytes were merely passive “placeholders” in the mineralized matrix has been displaced by exciting, and sometimes unexpected, discoveries that reveal this population of cells to be a highly sensitive, active and responsive network. Moreover, the signaling abilities of this cell type are capable of influencing local and systemic mineral metabolism as well as bone formation and resorption via direct or indirect action on osteoblasts and osteoclasts, respectively. The recent discovery that apoptosis in osteocytes is essential for osteoclastogenesis, but that it is not those dying osteocytes but rather nearby neighbors that carry out RANKL-based pro-osteoclastogenic signaling indicates a division of labor among osteocytes in response to damage paralleling that seen in ischemic injury [57]. Such similarities suggest that common pathways and mechanisms probably exist in localized remodeling of many tissue types.
Footnotes
Disclosure
Conflicts of interest: M.B. Schaffler: has received grant support from the National Institutes of Health; and receives royalties from Carolina Biological; O.D. Kennedy: none.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Bonewald L. Osteocytes. In: Marcus R, editor. Osteoporosis. 3rd. Elsevier; 2008. pp. 170–189. [Google Scholar]
- 2.Gu G, Nars M, Hentunen TA, et al. Isolated primary osteocytes express functional gap junctions in vitro. Cell Tissue Res. 2006;323(2):263–271. doi: 10.1007/s00441-005-0066-3. [DOI] [PubMed] [Google Scholar]
- 3.Feng JQ, Ward LM, Liu S, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38(11):1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He G, George A. Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J Biol Chem. 2004;279(12):11649–11656. doi: 10.1074/jbc.M309296200. [DOI] [PubMed] [Google Scholar]
- 5.Ruchon AF, Tenenhouse HS, Marcinkiewicz M, et al. Developmental expression and tissue distribution of Phex protein: effect of the Hyp mutation and relationship to bone markers. J Bone Miner Res. 2000;15(8):1440–1450. doi: 10.1359/jbmr.2000.15.8.1440. [DOI] [PubMed] [Google Scholar]
- 6.A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995;11(2):130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 7.Fisher LW, Fedarko NS. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 2003;44(Suppl 1):33–40. [PubMed] [Google Scholar]
- 8.Rowe PS, Garrett IR, Schwarz PM, et al. Surface plasmon resonance (SPR) confirms that MEPE binds to PHEX via the MEPE-ASARM motif: a model for impaired mineralization in X-linked rickets (HYP) Bone. 2005;36(1):33–46. doi: 10.1016/j.bone.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gowen LC, Petersen DN, Mansolf AL, et al. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem. 2003;278(3):1998–2007. doi: 10.1074/jbc.M203250200. [DOI] [PubMed] [Google Scholar]
- 10.Liu SG, Quarles LD. How fibroblast growth factor 23 works. Journal of the American Society of Nephrology. 2007;18(6):1637–1647. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Zhou J, Tang W, et al. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291(1):E38–E49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 12.Liu S, Guo R, Simpson LG, et al. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278(39):37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 13.Poole KE, van Bezooijen RL, Loveridge N, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. Faseb J. 2005;19(13):1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 14.Beighton P. Sclerosteosis. J Med Genet. 1988;25(3):200–203. doi: 10.1136/jmg.25.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10(5):537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23(6):860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 17.Bodine PV, Vernon SK, Komm BS. Establishment and hormonal regulation of a conditionally transformed preosteocytic cell line from adult human bone. Endocrinology. 1996;137(11):4592–4604. doi: 10.1210/endo.137.11.8895322. [DOI] [PubMed] [Google Scholar]
- 18.Kato Y, Boskey A, Spevak L, et al. Establishment of an osteoid preosteocyte-like cell MLO-A5 that spontaneously mineralizes in culture. J Bone Miner Res. 2001;16(9):1622–1633. doi: 10.1359/jbmr.2001.16.9.1622. [DOI] [PubMed] [Google Scholar]
- 19.Kato Y, Windle JJ, Koop BA, et al. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res. 1997;12(12):2014–2023. doi: 10.1359/jbmr.1997.12.12.2014. [DOI] [PubMed] [Google Scholar]
- 20.Rosser J, Bonewald LF. Studying osteocyte function using the cell lines MLO-Y4 and MLO-A5. Methods Mol Biol. 2012;816:67–81. doi: 10.1007/978-1-61779-415-5_6. [DOI] [PubMed] [Google Scholar]
- 21. Al-Dujaili SA, Lau E, Al-Dujaili H, et al. Apoptotic osteocytes regulate osteoclast precursor recruitment and differentiation in vitro. J Cell Biochem. 2011;112(9):2412–2423. doi: 10.1002/jcb.23164. Describes an in vitro model used to study gene expression of MLO-Y4 cells undergoing apoptosis, and the subsequent effect on osteoclastogenesis.
- 22.Zahm AM, Bucaro MA, Srinivas V, et al. Oxygen tension regulates preosteocyte maturation and mineralization. Bone. 2008;43(1):25–31. doi: 10.1016/j.bone.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genetos DC, Kephart CJ, Zhang Y, et al. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212(1):207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon RY, Temiyasathit S, Tummala P, et al. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. Faseb J. 2010;24(8):2859–2868. doi: 10.1096/fj.09-148007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xia X, Kar R, Gluhak-Heinrich J, et al. Glucocorticoid-induced autophagy in osteocytes. J Bone Miner Res. 2010;25(11):2479–2488. doi: 10.1002/jbmr.160. Demonstrates the effects of glucocorticoids on osteocytes in terms of autophagy and apoptosis. Proposes new mechanisms responsible for bone loss in patients receiving glucocorticoid therapy.
- 26.Plotkin LI, Aguirre JI, Kousteni S, et al. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. J Biol Chem. 2005;280(8):7317–7325. doi: 10.1074/jbc.M412817200. [DOI] [PubMed] [Google Scholar]
- 27. Batra N, Burra S, Siller-Jackson AJ, et al. Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci U S A. 2012;109(9):3359–3364. doi: 10.1073/pnas.1115967109. Shows that mechanical perturbation or conformational activation of integrin á5â1 leads to the opening of the connexin 43 hemichannel, a potentially important pathway for cell-cell communication.
- 28.Heino TJ, Hentunen TA, Vaananen HK. Conditioned medium from osteocytes stimulates the proliferation of bone marrow mesenchymal stem cells and their differentiation into osteoblasts. Exp Cell Res. 2004;294(2):458–468. doi: 10.1016/j.yexcr.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka-Kamioka K, Kamioka H, Ris H, et al. Osteocyte shape is dependent on actin filaments and osteocyte processes are unique actin-rich projections. J Bone Miner Res. 1998;13(10):1555–1568. doi: 10.1359/jbmr.1998.13.10.1555. [DOI] [PubMed] [Google Scholar]
- 31.Cowin SC, Moss-Salentijn L, Moss M L. Candidates for the mechanosensory system in bone. Journal of Biomechanical Engineering. 1991;113(2):191–197. doi: 10.1115/1.2891234. [DOI] [PubMed] [Google Scholar]
- 32.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. Journal of Biomechanics. 1994;27(3):339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 33.Skerry TM, Bitensky L, Chayen J, et al. Early strain-related changes in enzyme activity in osteocytes following bone loading in vivo. Journal of Bone and Mineral Research. 1989;4(5):783–788. doi: 10.1002/jbmr.5650040519. [DOI] [PubMed] [Google Scholar]
- 34.Dallas SL, Zaman G, Pead MJ, et al. Early strain-related changes in cultured embryonic chick tibiotarsi parallel those associated with adaptive modeling in vivo. J Bone Miner Res. 1993;8(3):251–259. doi: 10.1002/jbmr.5650080302. [DOI] [PubMed] [Google Scholar]
- 35.Dodds RA, Ali N, Pead MJ, et al. Early loading-related changes in the activity of glucose 6-phosphate dehydrogenase and alkaline phosphatase in osteocytes and periosteal osteoblasts in rat fibulae in vivo. J Bone Miner Res. 1993;8(3):261–267. doi: 10.1002/jbmr.5650080303. [DOI] [PubMed] [Google Scholar]
- 36.Gluhak-Heinrich J, Ye L, Bonewald LF, et al. Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J Bone Miner Res. 2003;18(5):807–817. doi: 10.1359/jbmr.2003.18.5.807. [DOI] [PubMed] [Google Scholar]
- 37.Verborgt O, Gibson GJ, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res. 2000;15(1):60–67. doi: 10.1359/jbmr.2000.15.1.60. [DOI] [PubMed] [Google Scholar]
- 38.Yang W, Lu Y, Kalajzic I, et al. Dentin matrix protein 1 gene cis-regulation: use in osteocytes to characterize local responses to mechanical loading in vitro and in vivo. J Biol Chem. 2005;280(21):20680–20690. doi: 10.1074/jbc.M500104200. [DOI] [PubMed] [Google Scholar]
- 39. Cardoso L, Herman BC, Verborgt O, et al. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res. 2009;24(4):597–605. doi: 10.1359/JBMR.081210. Demonstrates that osteocyte apoptosis is an obligatory step toward osteoclastogenesis in response to microdamage in cortical bone.
- 40.Zhang K, Barragan-Adjemian C, Ye L, et al. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26(12):4539–4552. doi: 10.1128/MCB.02120-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ajubi NE, Klein-Nulend J, Nijweide PJ, et al. Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes--a cytoskeleton-dependent process. Biochemical and Biophysical Research Communications. 1996;225(1):62–68. doi: 10.1006/bbrc.1996.1131. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Cowin SC, Weinbaum S, et al. Modeling tracer transport in an osteon under cyclic loading. Ann Biomed Eng. 2000;28(10):1200–1209. doi: 10.1114/1.1317531. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Wang Y, Han Y, et al. In situ measurement of solute transport in the bone lacunar-canalicular system. Proc Natl Acad Sci U S A. 2005;102(33):11911–11916. doi: 10.1073/pnas.0505193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Squire JM, Chew M, Nneji G, et al. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J Struct Biol. 2001;136(3):239–255. doi: 10.1006/jsbi.2002.4441. [DOI] [PubMed] [Google Scholar]
- 45.Burr DB, Martin RB, Schaffler MB, et al. Bone remodeling in response to in vivo fatigue microdamage. Journal of Biomechanics. 1985;18(3):189–200. doi: 10.1016/0021-9290(85)90204-0. [DOI] [PubMed] [Google Scholar]
- 46.Burr DB, Forwood MR, Fyhrie DP, et al. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. Journal of Bone and Mineral Research. 1997;12(1):6–15. doi: 10.1359/jbmr.1997.12.1.6. [DOI] [PubMed] [Google Scholar]
- 47.Bentolila V, Boyce TM, Fyhrie DP, et al. Intracortical remodeling in adult rat long bones after fatigue loading. Bone. 1998;23(3):275–281. doi: 10.1016/s8756-3282(98)00104-5. [DOI] [PubMed] [Google Scholar]
- 48.Verborgt O, Tatton NA, Majeska RJ, et al. Spatial distribution of Bax and Bcl-2 in osteocytes after bone fatigue: complementary roles in bone remodeling regulation? J Bone Miner Res. 2002;17(5):907–914. doi: 10.1359/jbmr.2002.17.5.907. [DOI] [PubMed] [Google Scholar]
- 49.Cheng Y, Deshmukh M, D'Costa A, et al. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest. 1998;101(9):1992–1999. doi: 10.1172/JCI2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicholson DW. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407(6805):810–816. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]
- 51.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 52. Kennedy OD, Herman BC, Laudier DM, et al. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone. 2012 doi: 10.1016/j.bone.2012.01.025. Demonstrates osteocyte expression of pro-osteoclastogenic factors by distinct cell populations in nearby sites of microdamage.
- 53.Nakashima T, Kobayashi Y, Yamasaki S, et al. Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokines. Biochem Biophys Res Commun. 2000;275(3):768–775. doi: 10.1006/bbrc.2000.3379. [DOI] [PubMed] [Google Scholar]
- 54. Nakashima T, Hayashi M, Fukunaga T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–1234. doi: 10.1038/nm.2452. Shows that purified osteocytes express RANKL and have the capacity to support osteoclastogenesis in vitro. Also shows osteocytes are a major source of RANKL in bone remodeling in vivo.
- 55. Xiong J, Onal M, Jilka RL, et al. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1124. doi: 10.1038/nm.2448. Uses a transgenic model with RANKL conditionally deleted to demonstrate that hypertrophic chondrocytes and osteocytes, both of which are embedded in matrix, are essential sources of the RANKL that controls mineralized cartilage resorption and bone remodeling, respectively
- 56.Baron R, Tross R, Vignery A. Evidence of Sequential Remodeling in Rat Trabecular Bone - Morphology, Dynamic Histomorphometry, and Changes during Skeletal Maturation. Anatomical Record. 1984;208(1):137–145. doi: 10.1002/ar.1092080114. [DOI] [PubMed] [Google Scholar]
- 57.Fisher M. The ischemic penumbra: identification, evolution and treatment concepts. Cerebrovasc Dis. 2004;17(Suppl 1):1–6. doi: 10.1159/000074790. [DOI] [PubMed] [Google Scholar]