Abstract
Pancreatic ductal adenocarcinoma (PDAC) is among the leading causes of cancer deaths and is unresponsive to existing therapy. Smoking and alcohol-induced pancreatitis are among the risk factors for PDAC. We have previously reported that beta-adrenergic receptors (β-ARs) stimulate the proliferation and migration of human PDAC cells in vitro via cAMP-dependent signaling and that the nicotine-derived nitrosamine NNK activates this pathway directly in vitro while additionally stimulating the release of noradrenaline/adrenaline by binding to α7 nicotinic acetylcholine receptors in hamsters. In the current study, we have tested the hypothesis that the β-AR antagonist propranolol prevents the development of PDAC induced in hamsters with ethanol-induced pancreatitis by NNK. We found that propranolol had strong cancer preventive effects in this animal model. Western blots of pancreatic duct cells and PDAC cells harvested by laser capture microscopy showed significant upregulation of the α7nicotinic acetylcholine receptor (α7nAChR) associated with significant inductions of p-CREB, p-ERK1/2 and increases in EGF and VEGF in PDAC cells of hamsters not treated with propranolol. These effects were reversed by treatment with propranolol. Our data suggest that propranolol may prevent the development of PDAC by blocking cAMP-dependent intracellular signaling, cAMP-dependent release of EGF and PKA-dependent release of VEGF while additionally downregulating the α7nAChR via inhibition of cAMP-mediated subunit assembly. We conclude that increased cAMP signaling is an important factor that drives the development and progression of PDAC and that the inhibition of cAMP formation is a promising new target for the prevention and adjuvant therapy of PDAC.
Introduction
Pancreatic cancer is the fourth leading cause of cancer mortality in both men and women in Western countries [1]. Smoking, diabetes mellitus and pancreatitis from any etiology, including alcohol abuse, are risk factors for this malignancy [2]. Although pancreatic cancer can arise from endocrine or exocrine pancreatic cells, more than 95% of all pancreatic cancers are pancreatic ductal adenocarcinomas (PDACs). PDAC is one of the most aggressive human cancers, with extensive invasiveness and metastasis precluding surgical resection in the majority of patients at the time of diagnosis. In addition, PDAC is generally unresponsive to conventional radio-and chemotherapy, resulting in a mortality rate near 100% within six months of diagnosis.
The growth regulation of PDAC and its putative cells of origin, pancreatic duct epithelia, is poorly understood. The majority of PDACs (about 75%) harbor activating point mutations in K-ras while also over-expressing the epidermal growth factor receptor (EGFR), leading to the generally accepted view that EGFR signaling via ras and its downstream effector, the extracellular signal regulated protein kinases (ERK1/2) play important roles in the regulation of this cancer. We have shown that human PDAC and pancreatic duct epithelial cells in vitro are stimulated in their growth by β-AR agonists that activate signaling via adenylyl cyclase and its downstream effectors cAMP, PKA and p-CREB as well asPKA-dependent transactivation of the EGFR pathway [3, 4]. Interestingly, our studies also identified the powerful nicotine-derived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) as a β-AR agonist that stimulated the same signaling cascade in PDAC and pancreatic duct epithelial cells [3, 4]. Beta-adrenergic activity of NNK resulting in mitogenic and/or anti-apoptotic signaling has additionally been reported by us and others in cell lines derived from human small airway-derived adenocarcinomas, immortalized human small airway epithelia [5, 6] and in colon cancer cells [7]. In addition, it has been shown that the migration and invasiveness of adenocarcinomas of the colon, prostate, breast and ovaries are under beta-adrenergic control [8-11].
In analogy to the increased risk of smokers and individuals with alcohol-induced panreatitis, studies in our laboratory have shown that pregnant hamsters given ethanol in the drinking water and treated with NNK give birth to offspring that developed a high incidence of pancreatitis-associated PDAC while the offspring of females given ethanol alone developed pancreatitis [12, 13]. Molecular analysis of the hamster PDACs showed upregulated expression levels of p-CREB and p-ERK1/2, suggestive of a hyperactive cAMP-dependent regulatory pathway [14]. Using this animal model, the current study has tested the hypothesis that the general antagonist for β-ARs (synonym: beta-blocker), propranolol prevents the development of PDAC by blocking this signaling pathway.
Materials and Methods
The animal experiment was approved by the Institutional Animal Care and Use Committee. Outbred male and female Syrian golden hamsters were purchased from Charles River to establish a breeding colony at our Laboratory Animal Facility. The animals were mated during evening hours under supervision by an experienced technician. Successful copulation was determined the following morning by the presence of a vaginal plug. Treatment of the pregnant hamsters with ethanol (10% in the drinking water from day 5 to end of pregnancy) alone or in combination with NNK (50mg/kg by subcutaneous injection on day 15 of pregnancy) was done as in previous experiments that reproducibly yielded a 65-75% incidence of pancreatitis-associated PDAC in the offspring [12, 13]. The offspring remained with their mothers until they were four weeks old and could feed themselves. One group (n=12) treated prenatally with ethanol and NNK was subcutaneously injected five times a week with the beta-blocker propranolol (0.3 mg/100g bodyweight) until the end of the observation period (8 months) whereas a second group (n=12) not treated with propranolol served as positive controls. A group of offspring exposed prenatally to ethanol alone (n=12) served as negative control. All animals were euthanized 8 months after start of the cancer preventive treatments and all major organs harvested.
Pancreatic tissues were fixed in 70% ethanol and embedded in paraffin for histopathology evaluation of tissue sections stained with hematoxylin/eosin. Survival curves were established and assessed for significant differences among groups using Prism Graphpad software and Chi-square log-rank test. A contingency table was established with the columns representing the alternate outcome of pancreatic cancer or no pancreatic cancer and the rows representing the treatment groups. Association of the variable “pancreatic cancer” with treatment group was ascertained by Chi-square test.
Pancreatic duct epithelial cells and PDAC cells were harvested from tissue sections by laser capture microscopy as previously described [14, 15], using a PixCell II Laser Capture Microdissection system (Arcturus, Mountain View, CA). From each cell type in the treatment and control groups, 30,000 cells randomly allocated to three equal samples were analyzed. The proteins were extracted from the CapSure caps using protein extract buffer (Pierce, Rockford, IL).
It has been shown in the nervous system and in colon cancer cells that the α7nicotinic acetylcholine receptor (α7nAChR) stimulates the synthesis and release of adrenaline and noradrenaline which activate cAMP signaling downstream of β-ARs [16-18]. In accord with these findings, we have shown that the α7nAChR is upregulated in the hamster PDACs and that these animals have significantly higher levels of systemic noradrenaline and adrenaline than untreated controls or animals prenatally exposed to ethanol alone [14]. We therefore analyzed the protein expression of nAChRs expressing the α7 subunits, as well as p-CREB and p-ERK1/2 in the harvested pancreatic cells. In addition, we assessed the protein levels of EGF and vascular endothelial growth factor (VEGF) that support the development and progression of pancreatic cancer. Following lysis of the thawed tissues, protein was determined using the BCA Protein Assay (Pierce, Rockford, IL, USA). Equal amounts of protein were treated with loading buffer at 100°C for five minutes and applied to each lane for electrophoresis in 12% polyacrylamide gel. The electrophoresed proteins were transferred onto nitrocellulose membranes in transfer buffer at 100 mV for one hour. After transfer, the membranes were treated with blocking buffer (5% nonfat dry milk in tris buffered saline with Tween 20, TBST) for one hour, and incubated with primary antibody overnight at 4°C. Primary antibodies against the following signaling proteins were used: total CREB (Upstate Biotechnology, Lake Placid, NY, USA), p-CREB, p-ERK1/2 and ERK1/2 (Cell Signaling, Danvers, MA, USA), α7nAChR (Millipore, Billerica, MA, USA), EGF (Santa Cruz Biotechology, Santa Cruz, CA, USA ) and VEGF (Abcam, Cambridge, MA, USA) and beta-actin (Sigma). After being washed with TBST, the membranes were incubated with horseradish peroxidase-labeled secondary antibody (goat anti-mouse, or goat anti-rabbit, Cell Signaling) for one hour. Immunoreactive bands were detected using a chemiluminescent reaction (ECL, Amersham Biosciences, Piscataway, NJ, USA) via autoradiography on Kodak Bio-Max XAR film. Three separate Western blots were conducted for each antibody per sample and yielded similar data. Relative densities of the bands were determined by image analysis using NIH SCION image analysis software. Mean values and standard errors from five densitometric readings per band were analyzed by one way ANOVA and Tukey-Kramer multiple comparison test.
Results
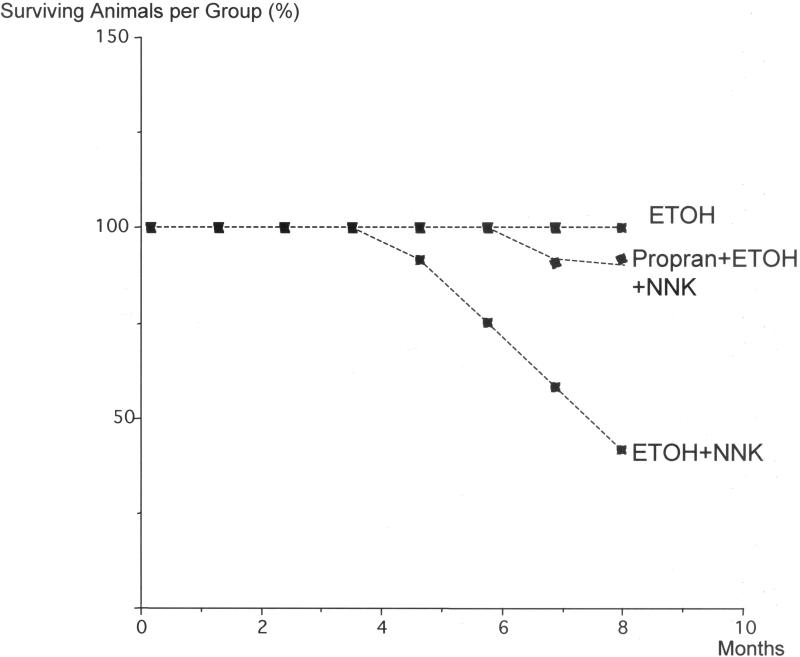
As shown in Figure 1, all offspring treated with the beta-blocker propranolol survived until the end of the observation period. By contrast, numerous animals initiated for PDAC development by transplacental treatment with ethanol and NNK and maintained without further treatment showed clinical symptoms of pancreatic cancer (loss of appetite and bodyweight, diarrhea) and were therefore euthanized prior to the end of the experiment. The survival curve of this treatment group was significantly different from the propranolol treated group (p<0.0001 by Logrank test) with only 42% of the animals surviving until the end of the study (Figure 1).
Figure 1.
Survival curves of hamsters expressed as percent surviving animals per treatment group (n=12 in each group) over time. Cancer preventive treatment with propranolol significantly (p<0.001 by Logrank test) increased the survival time of hamsters induced for the development of pancreatic cancer by prenatal treatment with ethanol and NNK.
Histopathology evaluation showed pancreatitis in all of the animals treated transplacentally with ethanol and NNK and maintained postnatally without further treatment. In accordance with the classification of pancreatitis suggested by Klöppel [19], this fibro-inflammatory disease was classified as alcoholic chronic pancreatitis, advanced stage (intensive perilobular and intralobular fibrosis affecting the entire pancreas and replacing most exocrine and endocrine pancreatic cells). In addition, 66.6% of the hamsters in this group (n=12) had developed PDAC. In accordance with the validated 6th edition [20] of the American Joint Committee on Cancer (AJCC), the induced PDACs were classified as T1 (tumor limited to pancreas, 2 cm or less in greatest diameter) or T2 (tumor limited to pancreas, greater than 2 cm in greatest diameter) and graded by histopathology as grade 2 (moderately differentiated duct-like structures and tubular glands) as suggested by Hruban and Fukushima [21]. By contrast, only one of the animals (8.3%, n+12) treated with propranol developed PDAC while all of these hamsters had pancreatitis. The hamsters exposed prenatally to ethanol alone all developed pancreatitis but no pancreatic cancer.
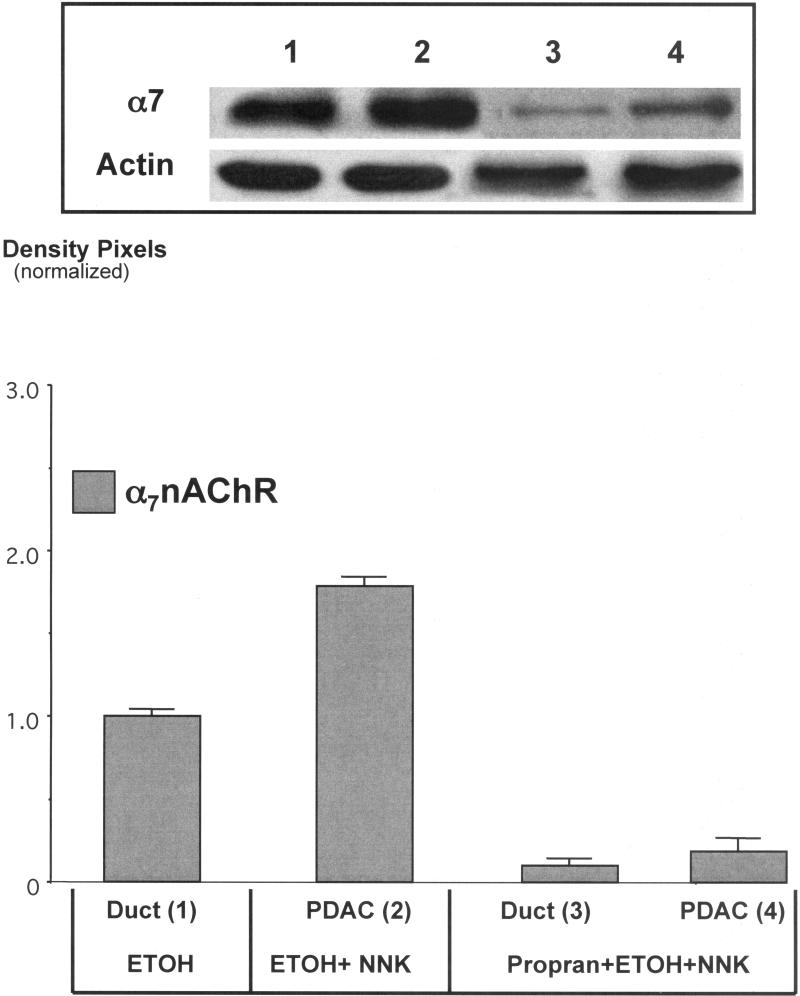
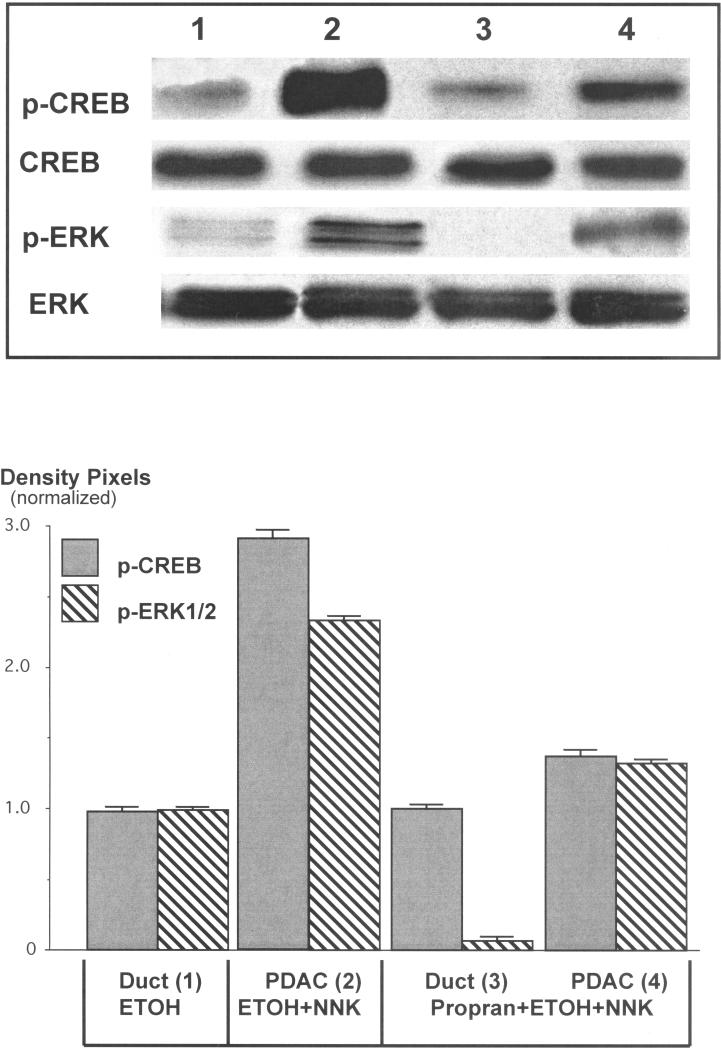
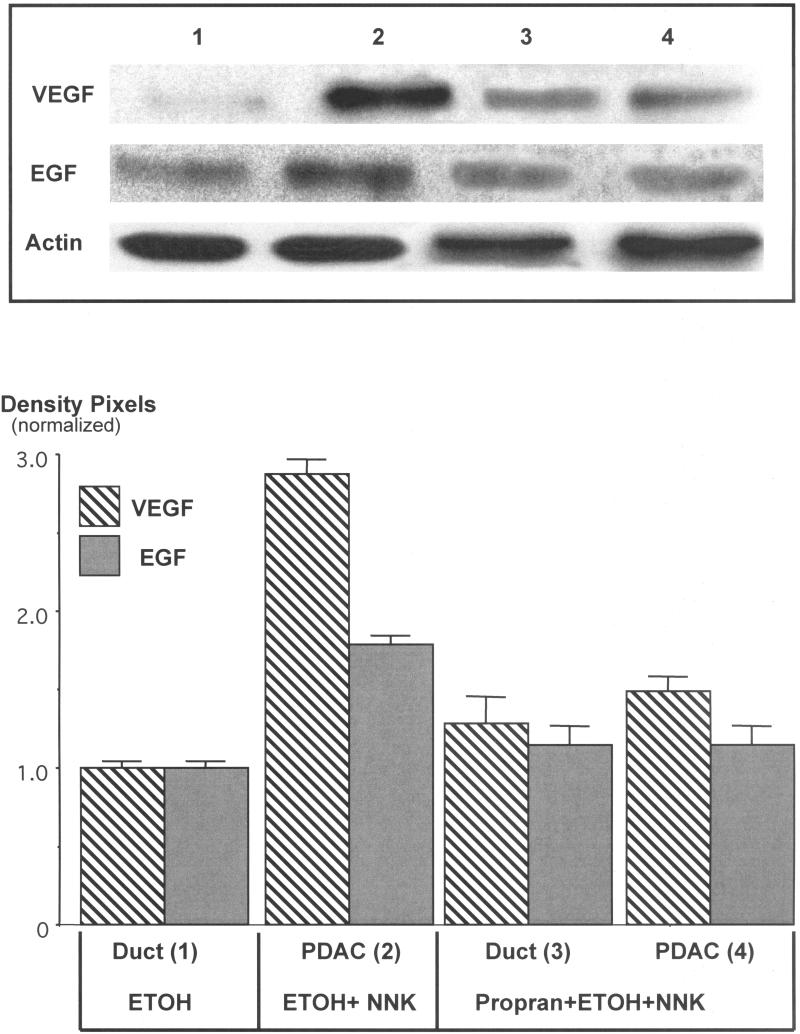
Protein analysis by Western blotting of pancreatic cells harvested by laser capture microscopy showed a 1.9-fold increase (p<0.001) in α7nAChR protein in PDAC cells (Figure 2). By contrast, the protein expression of this receptor in pancreatic duct epithelia and in cells from the single PDAC that developed in this group was reduced below the levels of the ethanol control group (Figure 2). The upregulation of the α7nAChR was accompanied by 2.9-fold induction of p-CREB (p<0.001) and a 2.2-fold increase in p-ERK1/2 (p<0.001) in the ETOH/NNK-induced PDACs (Figure 3). Both responses were reduced (p<0.001) to levels not significantly different from the controls by treatment with propranolol (Figure 3), suggesting activation of these signaling protein as effectors of β-ARs . Interestingly, the expression of VEGF (2.9-fold, p<0.001) and EGF (1.8-fold, p<0.001) was also significantly (p<0.001) increased in the ETOH/NNK-induced PDAC cells (Figure 4). Both of these responses were significantly (p<0.001) reduced by propranolol treatment (p<0.001), suggesting that they were largely under β-AR control.
Figure 2.
Western blot showing upregulation (1.9-fold; p<0.001) of the α7nAChR in cells harvested from ETOH+NNK-induced PDACs and dowregulation of this receptor below the levels of the ETOH controls by propranolol (p<0.001). Columns in the graph represent mean values and standard errors of five densitometric readings per band expressed as ratio of α7nAChR over actin. This Western was conducted three times with lysates from three separate samples per treatment group and yielded similar data.
Figure 3.
Western blots showing induction of p-CREB (2.9-fold, p<0.001) and p-ERK1/2 (2.2-fold, p<0.001) in cells harvested from ETOH/NNK-induced PDACs and inhibition of these responses (p<0.001) by treatment with propranolol. Columns in the graph represent mean values and standard errors of five densitometric readings per band expressed as ratio of p-CREB over CREB or p-ERK1/2 over ERK1/2. Each Western was conducted three times with lysates from three separate samples per treatment group and yielded similar data.
Figure 4.
Western blots exemplifying the induction of VEGF (2.9-fold, p<0.001) and EGF (1.8-fold, p<0.001) in cells from ETOH/NNK-induced PDACs and inhibition of these responses (p<0.001) by propranolol. Columns in the graph represent mean values and standard errors of five densitometric readings per band expressed as ratio of VEGF or EGF over actin. Each Western was conducted three times with lysates from three separate samples per treatment group and yielded similar data.
Discussion
Our data show that the beta-blocker propranolol has strong cancer preventive effects on PDAC induced by prenatal exposure to ETOH/NNK in hamsters, an effect that involved the reversal of increases in EGF/VEGF and of CREB/ERK phosphorylation. In addition, a pronounced upregulation of the α7nAChR in PDAC cells was not only reversed by propranolol but even downregulated below control levels. These findings are in accord with in vitro studies that have shown the activation of CREB and PKA-dependent transactivation of the EGFR downstream of β-ARs following ligand-binding of NNK to β-ARs in cell lines derived from human PDACs or lung adenocarcinomas and their respective cells of origin [3, 4, 6, 22]. More recently, it has been shown that NNK additionally increases the systemic levels of the stress neurotransmitters noradrenaline and adrenaline in hamsters [14, 23]. Since noradrenaline and adrenaline are the physiological agonists for β-ARs, this effect of NNK further intensifies beta-adrenergic signaling.
The observed upregulation of α7nAChR protein in PDAC cells of the group treated with ETOH/NNK alone is in accord with the documented function of NNK as an nAChR agonist [24-26] and mirror images the paradoxical upregulation of this receptor reported upon chronic exposure to nicotine in the nervous system [27]. However, in the current animal model a single dose of NNK injected to pregnant hamsters one day before the delivery of the pups upregulated this receptor in the offspring. This may be the reflection of the higher affinity of NNK to the α7nAChR [24-26] or a greater sensitivity of the receptor to agonist-induced upregulation in fetal tissues. It has also been reported that cAMP positively regulates the subunit assembly of nAChRs, thereby increasing their protein expression [28]. The NNK-induced direct and indirect stimulation of cAMP signaling downstream of β-ARs may thus have contributed to the observed upregulation of the α7nAChR. Conversely, inhibition of cAMP formation by propranolol may have triggered the observed reduction in expression levels of this receptor below the levels in controls. In light of the fact that the α7nAChR stimulates the synthesis and release of noradrenaline and adrenaline in the nervous system [29, 30] and in colon cancer cells [18], the observed induction of p-CREB and p-ERK1/2 in PDAC cells were in part caused by stress neurotransmitter-induced stimulation of beta-adrenergic signaling. This interpretation is supported by our recent finding that NNK-treated hamsters have significantly increased systemic levels of noradrenaline and adrenaline [14, 23]. The observed simultaneous increases in VEGF and EGF in PDAC cells and their reduction by propranolol are in accord with the concept that both are stimulated by β-adrenergic signaling [31-34].
In summary, the beta-blocker propranolol had strong cancer preventive effects on PDAC by inhibiting several important targets that drive the development and progression of this cancer (Figure 5). These findings further emphasize the importance of cAMP signaling downstream of β-ARs in the regulation of PDAC. They suggest that interference with this signaling cascade is a promising target for PDAC prevention, a strategy that may also be applicable to other cancers that are stimulated by stress neurotransmitters and β-AR signaling, including adenocarcinoma of the colon [7, 8], prostate [9], stomach [35] and ovarian cancer [11].
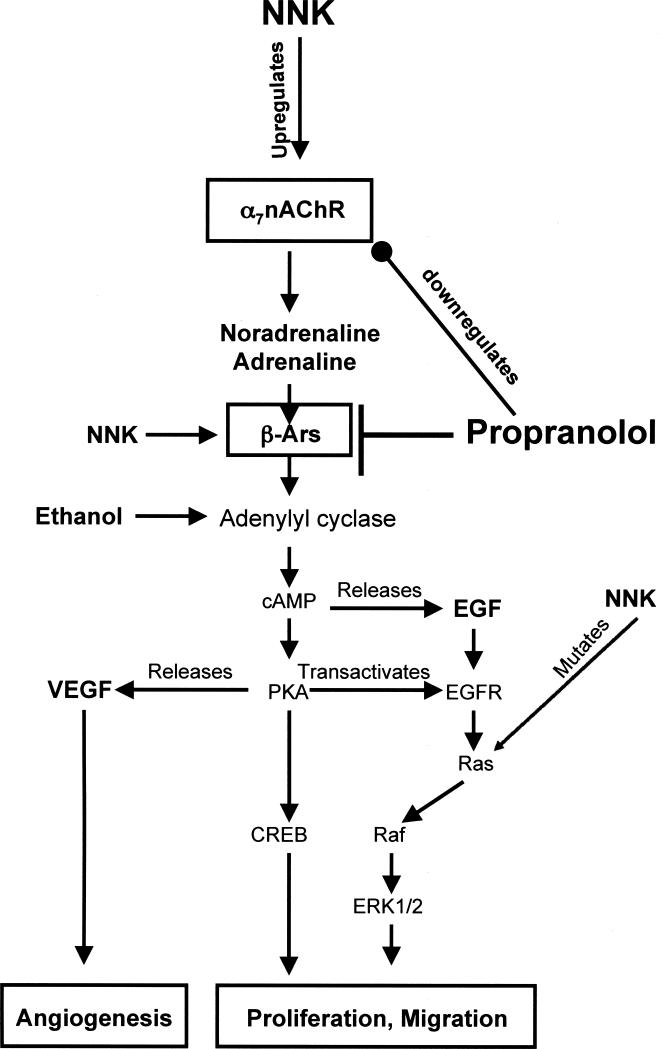
Figure 5.
Working model of the proposed mechanisms how propranolol prevented the development of PDAC induced in hamsters by ethanol+NNK. NNK-induced upregulation of the α7nAChR increased the production and release of the stress neurotransmitter noradrenaline from which adrenaline is formed enzymatically. Both neurotransmitters bind as agonists to β-ARs, activating its effector adenylyl cyclase, the rate-limiting step for the formation of intracellular cAMP. In turn, cAMP causes the release of EGF [34] and activates PKA that phosphorylates the transcription factor CREB while additionally stimulating the release of VEGF [32] and transactivating the EGFR. This signaling cascade is further intensified by direct agonist binding of NNK to β-ARs and activation of adenylyl cyclase by ethanol [36]. Propranolol blocks all signaling events downstream of β-ARs by binding as an antagonist to these receptors. In addition, propranolol downregulates the α7nAChR by inhibiting cAMP-mediated assembly of its subunits [28], thus indirectly reducing the synthesis and release of noradrenaline and adrenaline. Collectively, these multiple actions of propranolol have strong inhibiting effects on the proliferation, migration and angiogenesis of PDAC.
Acknowledgement
The authors thank Kindra Walker (University of Tennessee) for her technical assistance with the animal experiment.
Supported by grant RO1CA042829 with the National Cancer Institute and the State of Tennessee Center of Excellence in Livestock diseases and Human Health.
Footnotes
None of the authors has any conflict of interest to report.
References
- 1.Mancuso A, Calabro F, Sternberg CN. Current therapies and advances in the treatment of pancreatic cancer. Crit Rev Oncol Hematol. 2006;58:231–41. doi: 10.1016/j.critrevonc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Lowenfels AB, Maisonneuve P. Risk factors for pancreatic cancer. J Cell Biochem. 2005;95:649–56. doi: 10.1002/jcb.20461. [DOI] [PubMed] [Google Scholar]
- 3.Weddle DL, Tithoff P, Williams M, Schuller HM. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22:473–9. doi: 10.1093/carcin/22.3.473. [DOI] [PubMed] [Google Scholar]
- 4.Askari MD, Tsao MS, Schuller HM. The tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone stimulates proliferation of immortalized human pancreatic duct epithelia through beta-adrenergic transactivation of EGF receptors. J Cancer Res Clin Oncol. 2005;131:639–48. doi: 10.1007/s00432-005-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin Z, Gao F, Flagg T, Deng X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone promotes functional cooperation of Bcl2 and c-Myc through phosphorylation in regulating cell survival and proliferation. J Biol Chem. 2004;279:40209–19. doi: 10.1074/jbc.M404056200. [DOI] [PubMed] [Google Scholar]
- 6.Laag E, Majidi M, Cekanova C, Masi T, Takahashi T, Schuller HM. NNK activates ERK1/2 and CREB/ATF-1 via beta-1-AR and EGFR signaling in human lung adenocarcinoma and small airway epithelial cells. Int J Cancer. 2006;119:1547–52. doi: 10.1002/ijc.21987. [DOI] [PubMed] [Google Scholar]
- 7.Wu WK, Wong HP, Luo SW, Chan K, Huang FY, Hui MK, et al. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone from cigarette smoke stimulates colon cancer growth via beta-adrenoceptors. Cancer Res. 2005;65:5272–7. doi: 10.1158/0008-5472.CAN-05-0205. [DOI] [PubMed] [Google Scholar]
- 8.Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61:2866–9. [PubMed] [Google Scholar]
- 9.Palm D, Lang K, Niggemann B, Drell TLt, Masur K, Zaenker KS, et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer. 2006;118:2744–9. doi: 10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]
- 10.Drell TLt, Joseph J, Lang K, Niggemann B, Zaenker KS, Entschladen F. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res Treat. 2003;80:63–70. doi: 10.1023/A:1024491219366. [DOI] [PubMed] [Google Scholar]
- 11.Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–75. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuller HM, Jorquera R, Reichert A, Castonguay A. Transplacental induction of pancreas tumors in hamsters by ethanol and the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1993;53:2498–501. [PubMed] [Google Scholar]
- 13.Schuller HM, Zhang L, Weddle DL, Castonguay A, Walker K, Miller MS. The cyclooxygenase inhibitor ibuprofen and the FLAP inhibitor MK886 inhibit pancreatic carcinogenesis induced in hamsters by transplacental exposure to ethanol and the tobacco carcinogen NNK. J Cancer Res Clin Oncol. 2002;128:525–32. doi: 10.1007/s00432-002-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Wadei HAN, Schuller HM. Upregulation of nicotinic receptors is associated with systemic increase in stress neurotransmitters and cAMP, induction of p-CREB and p-ERK and suppression of GABA in NNK-induced adenocarcinoma of the lungs and pancreas. J Pathol. (in press) [Google Scholar]
- 15.Cekanova M, Majidy M, Masi T, Al-Wadei HA, Schuller HM. Overexpressed Raf-1 and phosphorylated cyclic adenosine 3′-5′-monophosphatate response element-binding protein are early markers for lung adenocarcinoma. Cancer. 2007;109:1164–73. doi: 10.1002/cncr.22520. [DOI] [PubMed] [Google Scholar]
- 16.Barik J, Wonnacott S. Indirect modulation by alpha7 nicotinic acetylcholine receptors of noradrenaline release in rat hippocampal slices: interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol Pharmacol. 2006;69:618–28. doi: 10.1124/mol.105.018184. [DOI] [PubMed] [Google Scholar]
- 17.Haass M, Kubler W. Nicotine and sympathetic neurotransmission. Cardiovasc Drugs Ther. 1997;19:657–665. doi: 10.1007/BF00053022. [DOI] [PubMed] [Google Scholar]
- 18.Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH. Nicotine promotes cell proliferation via alpha7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol Appl Pharmacol. 2007;221:261–7. doi: 10.1016/j.taap.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Klöppel G. Chronic pancreatitis, pseudotumors and other tumor-like lesions. Modern Pathology. 2007;20:S113–S131. doi: 10.1038/modpathol.3800690. [DOI] [PubMed] [Google Scholar]
- 20.Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110:738–44. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 21.Hruban RH, Fukushima N. Pancreatic adenocarcinoma: update on the surgical pathology of carcinomas of ductal origin and PanINs. Modern Pathology. 2007;20:S61–S70. doi: 10.1038/modpathol.3800685. [DOI] [PubMed] [Google Scholar]
- 22.Schuller HM, Tithof PK, Williams M, Plummer H., 3rd The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a beta-adrenergic agonist and stimulates DNA synthesis in lung adenocarcinoma via beta-adrenergic receptor-mediated release of arachidonic acid. Cancer Res. 1999;59:4510–5. [PubMed] [Google Scholar]
- 23.Al-Wadei HAN, Schuller HM. Beta-carotene promotes the development of NNK-induced small airway-derived lung adenocarcinoma. Eur J Cancer. doi: 10.1016/j.ejca.2008.10.035. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuller HM, Orloff M. Tobacco-specific carcinogenic nitrosamines. Ligands for nicotinic acetylcholine receptors in human lung cancer cells. Biochem Pharmacol. 1998;55:1377–84. doi: 10.1016/s0006-2952(97)00651-5. [DOI] [PubMed] [Google Scholar]
- 25.Schuller HM. Nitrosamines as nicotinic receptor ligands. Life Sci. 2007;80:2274–80. doi: 10.1016/j.lfs.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arredondo J, Chernyavsky AI, Grando SA. Nicotinic receptors mediate tumorigenic action of tobacco-derived nitrosamines on immortalized oral epithelial cells. Cancer Biol Ther. 2006;5:511–7. doi: 10.4161/cbt.5.5.2601. [DOI] [PubMed] [Google Scholar]
- 27.Lindstrom J, Anand R, Gerzanich V, Peng X, Wang F, Wells G. Structure and function of neuronal nicotinic acetylcholine receptors. Prog Brain Res. 1996;109:125–37. doi: 10.1016/s0079-6123(08)62094-4. [DOI] [PubMed] [Google Scholar]
- 28.Wanamaker CP, Christianson JC, Green WN. Regulation of nicotinic acetylcholine receptor assembly. Ann N Y Acad Sci. 2003;998:66–80. doi: 10.1196/annals.1254.009. [DOI] [PubMed] [Google Scholar]
- 29.Schuller HM. Neurotransmission and cancer: implications for prevention and therapy. Anticancer Drugs. 2008;19:655–71. doi: 10.1097/CAD.0b013e3283025b58. [DOI] [PubMed] [Google Scholar]
- 30.Mozayan M, Lee TJ. Statins prevent cholinesterase inhibitor blockade of sympathetic alpha7nAChR-mediated currents in rat superior cervical ganglion neurons. Am J Physiol Heart Circ Physiol. 2007;293:H1737–H1744. doi: 10.1152/ajpheart.00269.2007. [DOI] [PubMed] [Google Scholar]
- 31.Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH. Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activatriton. Toxicol Sci. 2007;97:279–287. doi: 10.1093/toxsci/kfm060. [DOI] [PubMed] [Google Scholar]
- 32.Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66:10357–64. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 33.Grau M, Soley M, Ramirez I. Interaction between adrenaline and epidermal growth factor in the control of liver glycogenolysis in mouse. Endocrinology. 1997;138:2601–2609. doi: 10.1210/endo.138.6.5183. [DOI] [PubMed] [Google Scholar]
- 34.Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer. 2009;9:195–205. doi: 10.1038/nrc2590. [DOI] [PubMed] [Google Scholar]
- 35.Shin VY, Wu WK, Chu KM, Koo MW, Wong HP, Lam EK, et al. Functional role of beta-adrenergic receptors in the mitogenic action of nicotine on gastric cancer cells. Toxicol Sci. 2007;96:21–9. doi: 10.1093/toxsci/kfl118. [DOI] [PubMed] [Google Scholar]
- 36.Kou J, Yoshimura M. Isoform-specific enhancement of adenylyl cyclase activity by n-alkanols. Alcohol Clin Exp Res. 2007;31:1467–72. doi: 10.1111/j.1530-0277.2007.00455.x. [DOI] [PubMed] [Google Scholar]