Abstract
Two different and generally noncomplimentary disruptions of timing by pharmacological agents have been found. One is a lateral shift of the psychophysical curve for time, indicating a subjective shortening or lengthening of time, whereas the other is a flattening of the curve and decrease in temporal accuracy. This study assessed the role of a methodological variation in producing this discrepancy. The procedure used required pigeons to classify stimulus duration intervals as short or long, using response alternatives that were defined either by the location of response keys (spatial), or by their color (nonspatial). D-amphetamine was used to replicate earlier findings, whereas nicotine and haloperidol extended the research to different drug classes. Dose-dependent decreases in the accuracy of classifying temporal intervals and a flattening of the psychophysical curve were found across drug classes for both spatial and nonspatial procedural variations. Procedural variations, under these conditions, could not explain the discrepancy. However, the results from this study added to the mounting body of literature showing decrements in temporal accuracy and a flattening of the psychophysical curve because of a number of diverse pharmacological and nonpharmacological disruptors.
Keywords: attention, d-amphetamine, haloperidol, nicotine, pigeon, response latency, stimulus control, temporal bisection, temporal discrimination, timing
Introduction
The symbolic matching to sample of durations procedure (Stubbs, 1968; Church and Deluty, 1977) is used commonly in the laboratory to assess temporal discrimination. It consists of the presentation of stimuli of varying durations followed by two choice alternatives. The subject is trained to classify these durations as short or long by responding on different alternatives. These response alternatives may be in different locations (e.g. Church and Deluty, 1977), making this version spatial (Location procedure). Response alternatives may also be illuminated with different colors with location randomized (e.g. Stubbs, 1968), making this version nonspatial (Color procedure). The resulting data are psychophysical curves for time (proportion of long alternative responses as a function of stimulus duration; e.g. Stubbs, 1968; Church and Deluty, 1977; Blough, 1996).
Pharmacological agents produce two distinct forms of disruption of the psychophysical curve. Dopaminergic agonists (i.e. amphetamines) and antagonists (i.e. haloperidol) have been shown to produce leftward or rightward shifts of the psychophysical curve, respectively (Maricq et al., 1981; Maricq and Church, 1983; Meck, 1983, 1986, 1996; Chiang et al. 2000, exp. 1; Cevik, 2003, exp. 2; Cheng et al., 2006). Nicotine, which acts on nicotinic acetylcholine receptors, has also been shown to produce leftward shifts of the curve (Bizot, 1997). There is a growing body of literature, however, that does not report lateral shifts in the psychophysical curve due to the administration of pharmacological disruptors. Rather, these studies have shown a decrease in the accuracy for classifying short and long intervals in the form of a flattening of the psychophysical curve and reduction in slope (Stubbs and Thomas, 1974; Rapp and Robbins, 1976; Stanford and Santi, 1998; Chiang et al. 2000, exp. 2; Santi et al., 2001; Odum et al., 2002; Cevik, 2003, exp. 1; McClure et al., 2005, 2009a, 2009b; Ward and Odum, 2005; Harper et al., 2006; Odum and Ward, 2007; Sanchez-Castillo et al., 2007). This decrease in accuracy has also been found after the administration of nicotine (Popke et al., 2000; Ward et al., 2009).
The use of spatial or nonspatial variants of the matching to sample of durations procedure (Location or Color) has been examined as a potential variable in explaining differential disruption of temporal behavior. These studies have yielded mixed results when various disruptors and dosing regimens have been used (Odum and Ward, 2007; McClure et al., 2009a, 2009b, 2010), although examination of earlier temporal discrimination studies shows a trend in terms of the procedure used and disruption produced. Most studies that have used the Location procedure found lateral shifts in the psychophysical curve due to the presentation of pharmacological and nonpharmacological disruptors (Maricq et al., 1981; Maricq and Church, 1983; Meck, 1983, 1986, 1996; Bizot, 1997; Kraemer et al., 1995; Chiang et al., 2000, exp. 1; Cevik, 2003, exp. 2; Cheng et al., 2006; McClure et al., 2009b, 2009c), whereas other studies found a flattening of the curve because of the disruptors with the use of both Color and Location procedures (Stubbs and Thomas, 1974; Rapp and Robbins, 1976; Wilkie et al., 1988; Morgan et al., 1993; Bizo and White, 1994; Kraemer et al., 1995; Stanford and Santi, 1998; Killeen et al., 1999; Chiang et al., 2000, exp 2; Santi et al., 2001; Odum et al., 2002; Cevik, 2003, exp 1; McClure et al., 2005, 2009a, 2009b, 2010; Ward and Odum, 2005, 2007; Harper et al., 2006; Odum and Ward, 2007; Sanchez-Castillo et al., 2007; Ward et al., 2009). This experiment tested whether lateral shifts occurred during administration of various doses of d-amphetamine, nicotine, and haloperidol in the Location, but not the Color procedural variant.
Intermittent d-amphetamine administration served as a replication of previous results, in which no difference was found in temporal disruption between procedural variations (Odum and Ward, 2007; McClure et al., 2009a). This study also extended procedural comparisons to nicotine and haloperidol. Haloperidol (a dopamine antagonist) is used frequently in the timing literature, mostly because of the implication of dopamine as vital in modifying the clock speed, which determines temporal accuracy and the form of disruption (Maricq et al., 1981; Maricq and Church, 1983; Meck, 1983, 1986, 1996; Buhusi and Meck, 2002, 2005; Cheng et al., 2006; Meck et al., 2008). Effects of nicotine on timing have only been shown in a handful of reports (Bizot, 1997; Popke et al., 2000; Ward et al., 2009), the results of which were not in agreement, making a case for further inquiry. In addition, certain doses of nicotine have been shown to increase measures of attention (Levin and Simon, 1998; Levin et al., 2006). As attention has been suggested as the mediating factor by which temporal disruption occurs (Heinemann et al., 1969; Santi et al., 1995; Blough, 1996; Ward et al., 2009), nicotine was used in this study to determine whether its administration altered measures of attention (e.g. latency to classify temporal intervals) and discriminability of durations.
Methods
Subjects
Twelve white Carneau pigeons (Columba livia), with an earlier behavioral testing history, served as subjects. All birds had some drug history either with d-amphetamine or cocaine, but at the time of this experiment they had not experienced any drug in at least 3 months. The birds were individually housed in a humidity-controlled and temperature-controlled colony room with a 16:8 h light–dark cycle. Water and grit were continuously available in the home cages. Postsession feedings were given when necessary to maintain body weights at 83% of free-feeding levels.
Apparatus
Twelve standard operant test chambers (Model ENV-007, Med Associates Inc., St Albans, Vermont, USA) were used. The chambers had internal dimensions of 30.5 × 24.1 × 29.2 cm. The doors and opposite side panels consisted of clear polycarbonate. The intelligence panels and back walls were constructed with aluminum. The intelligence panel contained three circular response keys with a diameter of 2.5 cm (Model ENV-123AM) that could be transilluminated with red, green, and white light. The three keys were 6.5 cm from the top of the chamber. Each side key was located 2.25 cm from the sidewalls and the center response key was 6 cm from each side key. The force required to depress a response key was between 0.12 and 0.15 N. Grain could be accessed from a hopper (Model ENV-205M) through a 5.5 by 6.5 cm rectangular opening that was positioned 13 cm below the center response key and 8.5 cm from both sides of the chamber. A light in the hopper was activated whenever grain was available, while all other lights in the chamber were extinguished. On the opposite aluminum wall a house light (28 V; Model ENV-215M) was placed 1 cm from the top of the chamber and 11.5 cm from the sides. The equipment was contained in a sound-attenuating chamber (Model ENV-018M) and controlled with Med-PC IV software (Med Associates Inc.).
Procedure
Behavioral training
No key peck training was required. Subjects were randomly distributed into Color and Location procedural groups. Sessions started with a 5-min blackout for both groups. Each trial began with white illumination of the center key; a single response to this key initiated the trial. This initiating response terminated the center-key illumination and was followed immediately by the house light presentation for one of the two training durations, either 2 or 8 s. The training durations were chosen randomly, with the limitation that a duration could not appear more than twice in succession, and were presented an equal number of times for each session. Directly following the termination of the house light sample, two side response keys were illuminated simultaneously. For the Color group, one alternative was red, the other green. Location of the colors was randomized, with the constraint that no color could appear on the same side more than twice in succession. The location of the reinforced response choice was also randomized across sides and could not be the same for more than two trials. For three of six subjects in the Color group, a single peck on the red key after a 2-s stimulus duration was reinforced with 2s of access to grain, whereas a single peck on the green was reinforced after an 8-s stimulus duration. For the other three subjects in this group, the Color response alternatives were counterbalanced (response on the red key was reinforced after an 8-s duration, and a response on the green key was reinforced after a 2-s duration).
For the Location group, both choice alternatives were illuminated red. For three subjects in the Location group, a response on the left key was reinforced after a 2-s duration, and a response on the right key was reinforced after an 8-s duration. Key locations were counterbalanced for the other three subjects in the Location group. Reinforcement was followed by an average 10-s intertrial interval, with a Range of 1–20 s. Incorrect responses led directly to the intertrial interval. Training sessions consisted of 96 trials for 50min whichever came first.
Once an accuracy of the subject in discriminating the training durations was above 80% for five consecutive sessions, intermediate stimulus durations were introduced. The values of the intermediate duration stimuli were 2.6, 3.48, 4.6, and 6.1 s. Sessions consisted of 96 trials for 80 min whichever came first. A time constraint was placed on sessions to be sure that we were capturing the effects of drug during peak levels, rather than after significant metabolism had occurred. Training durations (2 and 8 s) were presented for 48 of the 96 trials, and the four intermediate durations were presented 12 times each. Durations could not appear more than twice in the succession in each block of the 24 trials. For both procedural groups, correct responses to training durations were reinforced at all times and responses to intermediate durations were never reinforced.
Drug administration
When each subject’s psychophysical curve and all derived parameters [Range, Standard deviation (SD), Minimum (Min), and Point of Subjective Equality (PSE), see below] for the last 10 days of baseline were deemed stable by visual inspection, the acute dosing regimen began. Three different drugs were given to each of the subjects in a counterbalanced order. The drugs and doses used were d-amphetamine (vehicle, 0.3, 1.0, 1.7, 2.25, and 3.0 mg/kg), nicotine (vehicle, 0.03, 0.1, 0.3, 1.0, and 1.7 mg/kg), and haloperidol (vehicle, 0.01, 0.03, 0.1, 0.3, 0.5, and 0.7 mg/kg). D-amphetamine was mixed in 0.9% of saline solution. All doses were determined by the weight of the base, and the injection volume was 1 ml/kg. Nicotine hydrogen tartrate was dissolved in a potassium phosphate solution (1.13 g/l monobasic KPO4, 7.33 g/l dibasic KPO4, 9 g/l NaCl in distilled H2O; Fisher Scientific, Pittsburgh, Pennsylvania, USA) to decrease the irritation caused by nicotine, and increases the pH to approximately 7.4. Haloperidol was mixed in a 0.03% solution of acetic acid and distilled water.
Doses of drug and vehicle were given twice per week, with each dose being separated by two-to-three experimental sessions with no injections. Drug and vehicle administration involved two-to-three cycles of all drug doses so that any systematic changes in effects across successive administrations would be evident. All drug doses, along with vehicle, yielded a dose–response curve. After all doses for one particular drug had been administered two-to-three times, the next drug administration began after 30 experimental sessions with no injections. All injections were given intramuscularly in the breast muscle 5 min before the beginning of the experimental session. During haloperidol dosing, subjects experienced a 15-min blackout in the experimental chamber before the start of the session rather than the normal 5-min blackout experienced for other drug determinations. This was because of a longer latency for haloperidol to exert its peak effects.
All 12 subjects experienced three drugs, therefore making a total of 36 dose–response curve determinations. For 18 of these 36 determinations, injections occurred in a descending (d-amphetamine) or ascending (nicotine and haloperidol) order. The other 18 of 36 determinations were given in a random order. It should be noted that, some of the higher doses of drug were not administered to all subjects because of a complete suppression of responding during sessions. When this occurred, we continued to test that dose for two-to-three more sessions before excluding it. The 0.7 mg/kg dose of haloperidol was added later in the experiment, and only eight of 12 subjects were tested with that dose. Preliminary results showed that the lowest doses did not always have an effect on behavior, and so not all subjects received the lowest dose because of time constraints.
Data analyses
The data analyzed came from each presentation of each drug dose and vehicle administration. Subjects had to complete at least 48 of 96 trials during vehicle and drug dosing sessions for data to be included in analyses. For each session, the proportion of responses to the long alternative was plotted as a function of the duration of the stimulus on that trial. The resultant psychophysical curves were analyzed by fitting a cumulative Gaussian function with four free parameters, following Blough (1996). The equation fit was the integral of:
in which f(t) is the proportion of long responses at a given duration t of a stimulus, a is the Min of the function, b is the Range, which is the difference between the point at the upper asymptote of the curve compared with the point at the lower asymptote of the curve, μ is the mean (PSE), and σ is the SD. All curve fitting was done using the normal distribution command in the Microsoft Excel (see McClure et al., 2005).
Derived parameters for each drug were analyzed separately using mixed-model analyses of variance (ANOVAs). For d-amphetamine and nicotine, all doses were included in analyses, and subjects that did not experience all doses were excluded. Three of 12 subjects were excluded for the d-amphetamine analysis, whereas two were excluded for the nicotine analysis. For haloperidol, the lowest and highest doses of drug were excluded from analysis, which allowed 11 of 12 subjects to be included. The within-subjects factor for all analyses was Dose (d-amphetamine: vehicle, 0.3, 1.0, 1.7, 2.25, and 3.0 mg/kg; nicotine: vehicle, 0.03, 0.1, 0.3, 1.0, and 1.7 mg/kg; and haloperidol: vehicle, 0.03, 0.1, 0.3, and 0.5 mg/kg). The between-subjects factor was Group (Color and Location). If a significant main effect was found, the Holm–Sidak method was used for multiple post-hoc comparisons.
Three separate mixed-model ANOVAs were also used to analyze response latency (RL) for each drug. Dose was used as the within-subjects factor (see above for doses) and the between-subjects factor was Group (Color and Location). These analyses assessed main effects of Dose and Group on (i) RL at a 2-s duration, (ii) RL at an 8-s duration, and (iii) differences between the longest and shortest RL for all six durations. The Holm–Sidak method was used for post-hoc comparisons. Subjects included in each AVOVA for RL were the same as for the derived parameters analyses, with the only exception being that seven of 12 subjects were included in the d-amphetamine analyses due to missing data.
Results
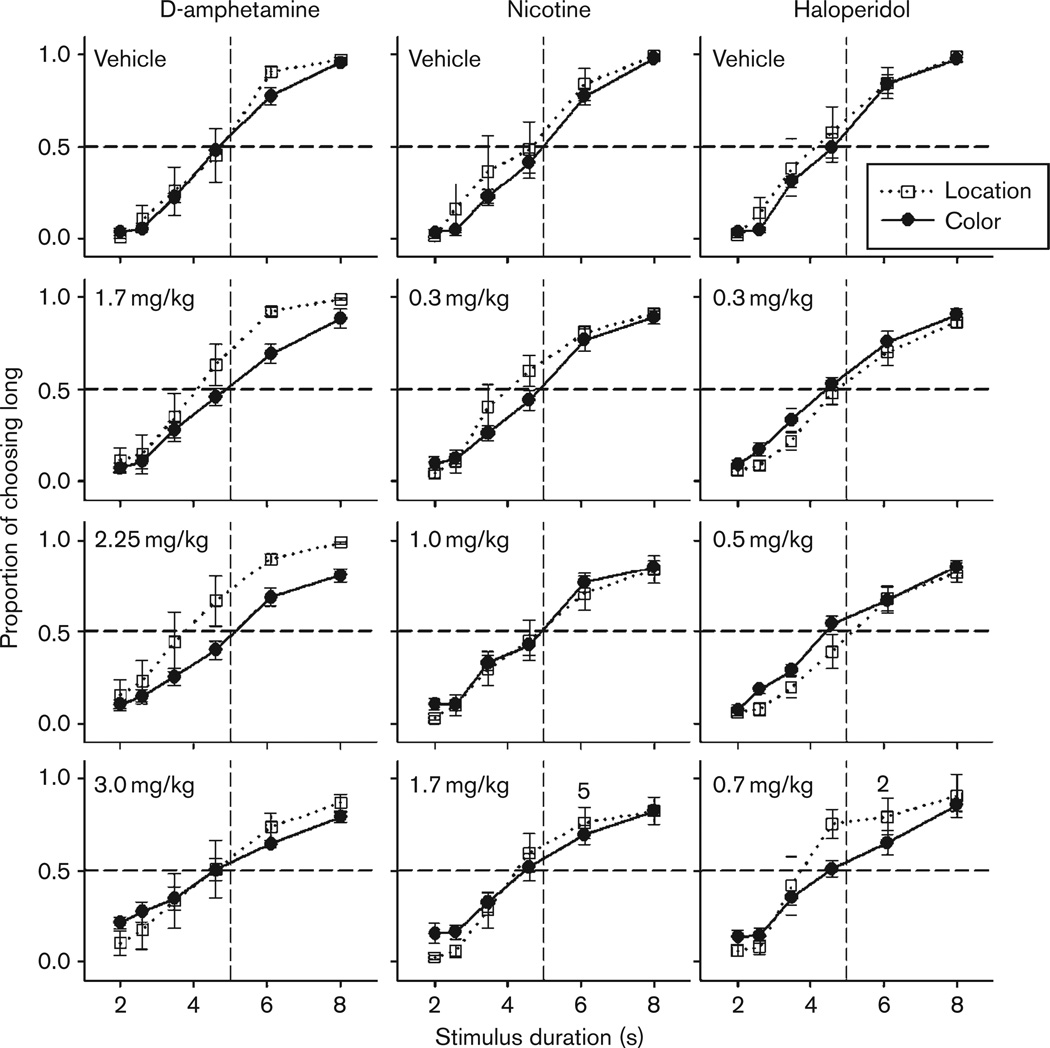
Psychophysical curves for d-amphetamine, nicotine, and haloperidol for both Color and Location groups are shown in Fig. 1. Low doses of all drugs had little effect on temporal behavior and are not shown here. The most pronounced effect across drugs was the flattening of the psychophysical curves, and decrease in accuracy at extreme durations with the administration of increased doses. The Color group showed slightly larger decrements in accuracy compared with the Location group during d-amphetamine and nicotine administration, whereas decrements in accuracy were more comparable during haloperidol administration. It should be noted that, some subjects did not always experience the high doses of nicotine and haloperidol and the number of subjects contributing to the average is noted in the figure legend.
Fig. 1.
Averaged psychophysical curves across procedural groups for vehicle and the three highest drug doses. Location and Color groups are compared within the same panels. Data from the Location group are represented by open squares, whereas closed circles represent data from the Color group. The number of subjects being averaged for each dose is six, except for the following doses and groups: nicotine, Location, 1.7 mg/kg (5 s); haloperidol, Location, 0.7 mg/kg (2 s). Those numbers are shown within each panel. Error bars represent standard error of the mean.
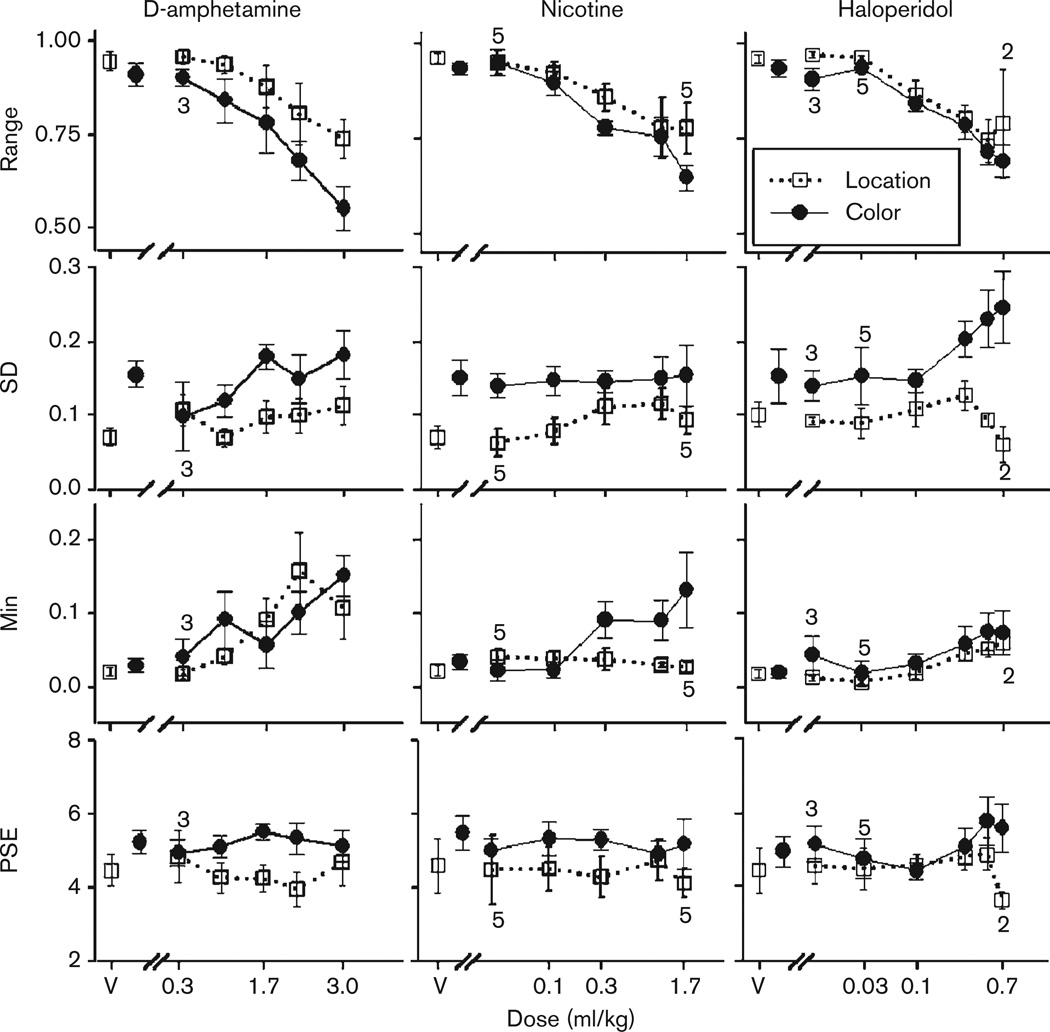
The four parameters derived from equation 1 (Range, SD, Min, and PSE) are shown in Fig. 2. All significant main effects, interactions, and post-hoc comparisons are shown in Table 1. Decreased Range values indicate decrements in accuracy at one or both of the extreme durations, which can also be seen as a flattening of the psychophysical curves. During d-amphetamine administration, dose-dependent decreases in the Range occurred for both procedural groups. This was confirmed by a main effect of Dose on Range and significant differences between vehicle and the three highest doses of drug. In addition, during d-amphetamine administration, the Color group had lower Range values compared with the Location group for all doses, and the difference between groups at the 3.0 mg/kg dose was significant (t = 2.7). Dose-dependent decreases in the Range were also found during higher doses of nicotine and haloperidol administration, which was confirmed by a significant main effect of Dose for both of these drugs. The only significant group difference was at the 1.7 mg/kg dose of nicotine (t = 2.0), in which the Color group had lower Range values compared with the Location group. For Range, no other significant main effects or interactions were found (largest F = 3.7). Range values for individual subjects at each drug dose are found in Appendix 1
Fig. 2.
Derived parameter values [Range, Standard deviation (SD), Minimum of the function (Min), and Point of Subjective Equality (PSE)] for all drugs and doses within a procedural group. Location group values are represented with open squares, whereas Color group values are represented with closed circles. Derived parameters from top to bottom are Range, SD, Min, and PSE. Note the differences in scale for each parameter. Error bars represent standard error of the mean. Small numbers above and below data paths indicate how many subjects contributed to the average. The number of subjects being averaged for each dose is six, except for the following doses and groups d-amphetamine, Color, 0.3 mg/kg (3 s); nicotine, Location, 0.03 mg/kg (5 s); 1.7 mg/kg (5 s); haloperidol, Location, 0.7 mg/kg (2 s); haloperidol, Color, 0.01 mg/kg (3 s); 0.03 mg/kg (5 s).
Table 1.
F values of mixed-model analysis of variance, separated by drug, with repeated measures during acute dosing for all derived parameters
| Parameter | Main effect/interaction | D-amphetamine d.f. = (5,47) | Nicotine d.f. = (5,48) | Haloperidol d.f. = (4,39) |
|---|---|---|---|---|
| Range | Dose | 19.5** | 24.0** | 29.5** |
| Holm–Sidak | V − 1.7: 2.8* | V − 0.3: 4.5** | V − 0.1: 3.8** | |
| V − 2.25: 5.2** | V − 1.0: 6.3** | V − 0.3: 6.3** | ||
| V − 3.0: 7.9** | V − 1.7: 8.2** | V − 0.5: 8.9** | ||
| SD | Group | 21.9** (d.f. = 1,7) | 16.1* (d.f. = 1,8) | 20.9** (d.f. = 1,9) |
| Holm–Sidak | V: 2.8* | V: 2.7* | 0.3: 2.3* | |
| 1.7: 2.7* | 0.03: 2.4* | 0.5: 4.2** | ||
| 3.0: 2.3* | 0.1: 2.3* | |||
| 1.7: 2.2* | ||||
| Min | Dose | 4.5* | 4.9* | 6.4** |
| Holm–Sidak | V − 2.25: 3.5* | V − 1.7: 3.8** | V − 0.3: 2.9* | |
| V − 3.0: 3.5* | V − 0.5: 3.8** | |||
| Dose by Group | — | 4.4* | — |
Significant main effects, interactions, and post-hoc comparisons using the Holm–Sidak method are shown. Degrees of freedom are listed below each drug, and are different for the between-subject factor main effect. Values from post-hoc comparisons represent significant differences between vehicle (V) and the listed dose.
d.f. degree of freedom; Min, Minimum of the function; SD, Standard deviation.
P<0.05.
P<0.001.
SD values are shown in Fig. 2 and represent the slope of the psychophysical curve. Individual subject values are shown in Appendix 2. SD values for Color and Location groups were different, which is confirmed by a significant main effect of Group for all drugs. Procedural groups had significantly different SD values at vehicle during d-amphetamine and nicotine dosing cycles, which persisted throughout drug administration. Differences were also found between groups at certain doses of haloperidol (0.3 and 0.5 mg/kg), but not during vehicle administration. There were no significant main effects of Dose for any drug for either group (largest F = 1.7).
Min values are shown in Fig. 2, and represent the lower asymptote of the psychophysical curve, which increases signifying decrements in accuracy for a 2-s duration. Individual subject’s Min values are shown in Appendix 3. Slight-to-moderate increase in the Min values occurred during high-dose administrations of d-amphetamine and haloperidol for both procedural groups, and was confirmed by a significant main effect of Dose for Min for those drugs. A main effect of Dose on Min was also found during nicotine administration, as was a Dose–Group interaction, which is evident by the increase in Min for only the Color group. During nicotine administration, significant differences were also found between groups at the dose of 1.0 mg/kg (t = 2.1) and at the Dose of 1.7 mg/kg (t = 3.2). No other significant main effects or interactions were significant (largest F = 2.8).
PSE values are shown in Fig. 2, and individual subject values are show in Appendix 4. Increases in PSE values indicate a shift in the psychophysical curve to the right, whereas decreases indicate a shift in the curve to the left. Very little effect on PSE was found for all drugs, and any slight changes were inconsistent and not significant (largest F = 2.8).
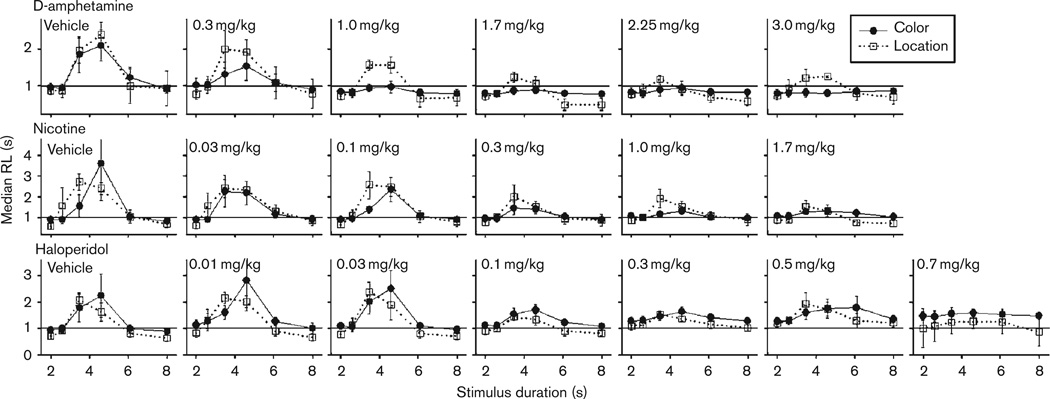
Median RL to choice alternatives after presentation of the temporal stimulus is shown in Fig. 3 for each drug dose. During vehicle administration for all drug cycles, RL was higher for intermediate durations, specifically for the 3.48 and 4.6-s sample durations compared with training durations (2 and 8 s). As drug dose increased, RL for all durations became more uniform and approached latency measures found during training duration trials. For analyses of changes in RL across doses, the difference between the longest latency and the shortest latency for all of the six durations was calculated for each subject and was averaged within procedural group (RL difference). All significant main effects, interactions, and results from post-hoc comparisons for RL are shown in Table 2. As dose increased, differences in RL between intermediate and training durations decreased (lower RL difference values), which was confirmed by a significant main effect of Dose for RL difference for all drugs. Post-hoc comparisons also confirmed that as drug dose increased, RL difference values were becoming much different from RL difference of vehicle values. A significant main effect of Group was also found for the RL difference during d-amphetamine administration, and post-hoc comparisons showed differences between groups at the 0.3 and 1.0 mg/kg doses.
Fig. 3.
Median response latency in seconds as a function of stimulus duration for each drug and dose across Color and Location groups. D-amphetamine dosing is shown in the top panel, nicotine in the middle panel, and haloperidol in the bottom panel. A solid line is set at 1 s as a point of reference across doses. Note the differences in scale across drugs. Error bars represent standard error of the mean. RL, response latency.
Table 2.
F values of mixed-model analysis of variance, separated by drug, with repeated measures during acute dosing for response latency
| D-amphetamine | Nicotine | Haloperidol | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Main effect | RL difference | 2-s RL | 8-s RL | RL difference | 2-s RL | 8-s RL | RL difference | 2-s RL | 8-s RL |
| Dose | 13.8** (5,37) | 5.9** | — | 4.5* (5,48) | 18.7** | 4.1* | 5.1* (4,39) | 43.6** | 14.8** |
| Holm–Sidak | V − 0.3: 3.3* | V − 1.0: 3.8** | — | V − 0.3: 3.2* | V − 0.3: 3.2* | V − 0.3: 2.5* | V − 0.3: 2.9* | V − 0.03: 2.3* | V − 0.3: 4.8** |
| V − 1.0: 5.1** | V − 1.7: 4.7** | V − 1.0: 3.5** | V − 1.0: 6.3** | V − 1.0: 3.4* | V − 0.1: 5.3** | V − 0.5: 6.5** | |||
| V − 1.7: 6.5** | V − 2.25: 3.5* | V − 1.7: 4.1** | V − 1.7: 7.1** | V − 1.7: 3.9** | V − 0.3: 9.8** | ||||
| V − 2.25: 6.9** | V − 3.0: 4.4** | V − 0.5: 11.0** | |||||||
| V − 3.0: 6.6** | |||||||||
| Group | 12.4* (1,5) | — | — | — | — | — | — | — | — |
| Holm–Sidak | 0.3: 2.9* | — | — | — | — | — | — | — | — |
| 1.0: 2.7* | |||||||||
For each drug, separate analyses were conducted for (i) the difference between the maximum and minimum response latency for all durations (RL difference), (ii) the difference in RL for 2-s stimulus duration trials, and (iii) the difference in RL for 8-s stimulus duration trials. Significant main effects, interactions, and post-hoc comparisons are shown. Degrees of freedom are shown in parentheses after F values for dose and group. Values from post-hoc comparisons represent significant differences between vehicle (V) and the listed dose.
P<0.05.
P<0.001.
Analyses were also carried out separately for changes in RL for training duration trials across drug doses (2 and 8-s RL). All significant main effects, interactions, and results from post-hoc comparisons for RL during 2 and 8-s duration trials are shown in Table 2. Changes in RL during drug administration were generally different for training duration trials compared with trials with intermediate (nonreinforced) durations. During d-amphetamine administration, RL decreased for a 2-s duration dose dependently, but RL for an 8-s duration trial was unaffected by drug. RL for 2 and 8-s duration trials increased dose dependently for both nicotine and haloperidol. No significant group differences were found for either nicotine or haloperidol.
Discussion
D-amphetamine, nicotine, and haloperidol, in both the Color and Location procedural groups, dose dependently decreased Range values and produced decrements in temporal accuracy. Procedural variation and class of drug were not sufficient to produce differential effects on temporal behavior, and did not show consistent lateral shifts of the psychophysical curve. Our results add to the wealth of evidence that show a flattening of the psychophysical curve due to diverse pharmacological agents under both procedural variations (Stubbs and Thomas, 1974; Rapp and Robbins, 1976; Stanford and Santi, 1998; Chiang et al., 2000, exp. 2; Popke et al., 2000; Santi et al., 2001; Odum et al., 2002; Cevik, 2003, exp. 1; McClure et al., 2005, 2009b, 2009c; Ward and Odum, 2005; Harper et al., 2006; Odum and Ward, 2007; Sanchez-Castillo et al., 2007; Ward et al., 2009).
Procedural variations
These results, in part, replicated the findings of two earlier studies that showed no difference in the acute effects of d-amphetamine administration on performance in Color and Location procedural variations (Odum and Ward, 2007; McClure et al., 2009a), and extended this effect to nicotine and haloperidol. Despite the similarities shown across procedural variations, some notable differences were found: a slightly greater decrease in the Range for the Color group compared with the Location group during d-amphetamine and nicotine administration, greater increases in Min during nicotine administration for the Color group, and shorter RL under low doses of d-amphetamine (0.3 and 1.0 mg/kg) for the Color group. In addition, the Location group had significantly lower SD values compared with the Color group, which represents a steeper slope of the psychophysical curve. All these differences, taken together, indicate a higher level of discriminability of temporal intervals and superior stimulus control for the Location group over the Color group. The disruptive effects of drug were somewhat attenuated for the Location group, specifically for the Range and Min parameters, and RL during d-amphetamine administration, which may suggest that the Location group is in some way more resistant to drug effects. This could be due to greater stimulus control and could lead to differential disruption under other experimental configurations, which would be problematic when comparisons across studies and procedures are made within the literature.
Other reports have shown differences in the performance on these procedural variations with and without the use of disruptive agents. For example, the Location version of the task was acquired more quickly compared with the Color version (Chatlosh and Wasserman, 1987; Odum and Ward, 2007). In addition, mediating behavior, which has been suggested to aid in accurate timing, was shown to develop with the Location version and not the Color version (Fetterman et al., 1998; Machado and Keen, 2003). Our laboratory has shown more substantial disruption in the Color group during a chronic regimen of d-amphetamine (McClure et al., 2009b) and with the use of an increased stimulus intensity of the temporal sample (McClure et al., 2010). As noted above, many studies that found lateral shifts were using the Location procedure, whereas studies using both Color and Location procedural variations have found a flattening of the psychophysical curve by drugs.
It may be the case that lateral shifts can only be found when the Location variation is being used, and only some of the time. This is a curious trend that requires further inquiry.
Attention
Attention to the temporal stimulus has been suggested as the process that is disrupted and leads to temporal inaccuracies (Heinemann et al., 1969; Santi et al., 1995; Blough, 1996; Lejeune et al., 1999; Sutton and Roberts, 2002; Ward et al., 2009). In this experiment, attention to the temporal stimulus was quantified through measures of latency to respond to choice alternatives, as reported earlier (Ward and Odum, 2007; Ward et al., 2009). It seems likely that if the animal were attending to the preceding temporal stimulus, it would respond quickly to the correct choice alternative, but not so quickly as to indicate premature responding. We expected that if attention to the stimulus were disrupted, response latencies to choice alternatives would increase. This was not the case for responding under d-amphetamine administration, which showed decreased or unchanged RL at all stimulus durations. During nicotine and haloperidol administration, RL changes were similar to each other, yet varied in magnitude. For nicotine and haloperidol, RL decreased during intermediate duration trials, but to a much smaller degree for haloperidol, and increased for training duration trials, but to a much larger degree for haloperidol. It should also be noted that, nicotine did not show any differential effects on attention compared with d-amphetamine and haloperidol.
Some of the drug-induced changes in RL observed in this study are consistent with the effects of these drugs shown elsewhere in the literature. For example, studies using the five-choice serial reaction time task showed that d-amphetamine led to an increase in the number of premature responses and decrease in RL, with decrements in accuracy at higher doses (Cole and Robbins, 1987, 1989). Our results agree with these earlier findings, as d-amphetamine decreased RL for most stimulus durations. Haloperidol has been shown to increase RL (Carli and Samanin, 1992). Our results showed an increase in RL at training durations, and only a slight decrease at intermediate durations. Finally, nicotine has been shown to produce an increase in premature responses and decrease in RL, much like d-amphetamine (Blondel et al., 1998). The effects of nicotine on RL in this study, specifically decreased RL on intermediate durations and increased latencies during training durations, were more similar to haloperidol than d-amphetamine. For all drugs, RL decreased for intermediate duration trials. It should be noted that, responses during intermediate duration trials were never reinforced, whereas correct responses during training duration trials were always reinforced. Thus, the differential drug effects found between reinforced and nonreinforced responding are of interest and should be explored in future temporal discrimination studies.
Our results show that rapid responding does not necessarily equate to temporal accuracy. For example, decrements in temporal accuracy occurred during d-amphetamine administration though RL was rapid. Haloperidol and nicotine had the opposite effect, with increased RL at training durations, which was also accompanied by decrements in accuracy. In this case, however, RL did not increase to a point that would suggest that the subject could no longer accurately discriminate the interval. These findings are particularly interesting in light of the fact that a number of temporal discrimination studies have excluded trials from analyses in which RL was greater than 3 s (Maricq et al., 1981; Maricq and Church, 1983; Cheng et al., 2006). The argument for exclusion was that choices made after this cutoff were not controlled by the temporal sample and thus not an accurate representation of temporal disruption. On the basis of our results, responding to choice keys within 3 s does not necessarily translate into accurate temporal discrimination.
Concluding remarks
The results of this study are not easily incorporated into existing neurobiological theories of timing. Theoretical predictions dictate that d-amphetamine and haloperidol should produce lateral shifts, in opposing directions, because of their actions as dopamine agonist and antagonist, respectively (Meck, 1983, 1986, 1996; Buhusi and Meck, 2005; Meck et al., 2008). Nicotine exerts its effects through nicotinic acetylcholine receptors, and yet our results showed that all drugs produced nearly identical disruptions of the psychophysical curve. It seems from our results that responding after drug administration was no longer controlled by the preceding duration, suggesting that it was controlled by other variables that may very well be nontemporal. If the drug effects are thought of as a breakdown in stimulus control and disruption of attention, rather than as effects on a specific timing mechanism, that conceptualization may be able to account for the similar effects seen across drug classes and with nonpharmacological disruptors. It is well accepted in the literature that a wide range of drugs impair nontemporal discrimination responding in a number of species (Berryman et al., 1962; Eckerman et al., 1978; Dykstra, 1979; Grilly et al., 1980; Ridley et al., 1980; Koek and Slangen, 1983, 1984; Andrews and Holtzman, 1988). Future experiments may determine whether similar mechanisms control temporal and nontemporal behavior, and how those mechanisms are disrupted by drugs.
In a retrospective temporal discrimination procedure, such as the one used in this experiment, it is assumed that what is being disrupted by drug is temporal accuracy. However, there are a number of other processes occurring simultaneously that could also be disrupted, such as attention to the temporal sample, reinforcer efficacy, motoric activity, color or spatial discrimination, etc. Any of these processes could be affected by drug, which would undermine conclusions regarding temporal mechanisms specifically. It is possible, in this study and others that the temporal stimulus was discriminated accurately, but some aspect of choice behavior was disrupted. We believe that this possibility provides more reasons to suggest that temporal discrimination and its seeming disruption should be thought of and tested as a breakdown in stimulus control, paying specific attention to the separation of different processes occurring during temporal discrimination procedures.
Two recent studies have attempted to compare the effects of pharmacological agents on temporal and nontemporal discrimination. Ward and Odum (2005) found that morphine disrupted temporal discrimination, but did not disrupt performance on a simple red–green match-to-sample discrimination when the tasks were presented within session. These tasks varied greatly in difficulty though, which could have been the reason for differential disruption. A study by Hampson et al. (2010), however, showed that d-amphetamine produced similar disruption of temporal and light intensity discriminations that were of comparable difficulty. This same study also showed that 2,5-dimethoxy-4-iodoamphetamine, a 5-HT2 receptor agonist, had selective effects on temporal discrimination, while leaving the nontemporal discrimination unaffected. These types of experimental designs should be replicated and expanded to determine the differences between temporal discrimination disruption and a more general breakdown of stimulus control.
Future research should aim to provide explanations of the empirical discrepancy, paying close attention to the Location procedure as necessary, but not sufficient in producing lateral shifts. Our results do not serve as a justification to ignore and discount the role of procedural variations in the assessment of temporal discrimination, but help to isolate the conditions under which procedural variations produce differential disruption. The variables leading to this discrepancy have yet to be shown, and future studies should aim to uncover these variables, as they will surely lead to reconciliation of divergent findings in the literature. Methodological concerns should be carefully examined, as they may provide clarification in the best way to understand and conceptualize timing behavior.
Acknowledgements
The authors thank Marc Branch, Timothy Hackenberg, and Jesse Dallery for their assistance and comments on the experimental design and interpretation of results, and Paul Soto for statistical assistance. They also thank two anonymous reviewers for useful suggestions on previous versions of this manuscript.
Appendix
Appendix 1.
Averaged Range values for individual subjects during all vehicle and drug doses of d-amphetamine, nicotine, and haloperidol
| D-amphetamine | Nicotine | Haloperidol | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | V | 0.3 | 1.0 | 1.7 | 2.25 | 3.0 | V | 0.03 | 0.1 | 0.3 | 1.0 | 1.7 | V | 0.01 | 0.03 | 0.1 | 0.3 | 0.5 | 0.7 | |
| L | 673 | 0.87 | 0.88 | 0.91 | 0.88 | 0.86 | 0.67 | 0.94 | 1.00 | 0.96 | 0.86 | 0.74 | 0.72 | 0.93 | 0.95 | 0.96 | 0.89 | 0.88 | 0.88 | 0.89 |
| 648 | 0.94 | 0.95 | 0.91 | 0.77 | 0.56 | 0.80 | 0.97 | 1.02 | 0.94 | 0.89 | 0.88 | 0.92 | 0.92 | 0.98 | 0.96 | 0.86 | 0.75 | 0.70 | — | |
| 622 | 0.94 | 0.98 | 1.00 | 0.95 | 0.96 | 0.94 | 0.97 | 0.92 | 0.96 | 0.94 | 0.90 | 0.86 | 0.96 | 0.99 | 0.96 | 0.69 | 0.82 | 0.62 | — | |
| 4423 | 0.94 | 0.98 | 0.86 | 0.69 | 0.57 | 0.60 | 0.92 | 0.86 | 0.82 | 0.72 | 0.69 | — | 0.98 | 0.99 | 0.95 | 0.94 | 0.79 | 0.61 | — | |
| 883 | 1.00 | 0.98 | 0.99 | 0.98 | 0.91 | 0.70 | 1.00 | — | 0.98 | 0.92 | 0.97 | 0.81 | 0.98 | 0.97 | 0.96 | 0.88 | 0.68 | 0.76 | — | |
| 774 | 1.01 | 0.99 | 0.96 | 1.00 | 0.97 | 0.72 | 0.97 | 0.96 | 0.90 | 0.83 | 0.48 | 0.58 | 0.98 | 0.95 | 0.98 | 0.91 | 0.90 | 0.90 | 0.69 | |
| C | 811 | 0.96 | 0.92 | 0.92 | 0.98 | 0.86 | 0.76 | 0.96 | 1.00 | 0.95 | 0.81 | 0.92 | 0.58 | 0.94 | — | 0.94 | 0.84 | 0.75 | 0.71 | 0.66 |
| 965 | 0.91 | 0.92 | 0.68 | 0.61 | 0.67 | 0.42 | 0.87 | 1.03 | 0.93 | 0.80 | 0.86 | 0.72 | 0.95 | 0.86 | 0.93 | 0.82 | 0.67 | 0.61 | 0.59 | |
| 642 | 0.79 | — | 0.85 | 0.81 | 0.72 | 0.63 | 0.92 | 0.87 | 0.88 | 0.82 | 0.67 | 0.54 | 0.90 | — | — | 0.79 | 0.79 | 0.76 | 0.68 | |
| 657 | 1.00 | — | 0.99 | 0.93 | 0.74 | 0.58 | 0.95 | 0.99 | 0.81 | 0.71 | 0.72 | 0.68 | 0.99 | — | 0.91 | 0.88 | 0.92 | 0.83 | 0.87 | |
| 63 | 0.88 | 0.87 | 0.70 | 0.53 | 0.58 | 0.47 | 0.94 | 0.96 | 0.98 | 0.78 | 0.70 | 0.72 | 0.86 | 0.91 | 0.92 | 0.90 | 0.77 | 0.68 | 0.67 | |
| 750 | 0.92 | — | 0.92 | 0.84 | 0.54 | 0.46 | 0.96 | 0.85 | 0.82 | 0.75 | 0.65 | 0.64 | 0.97 | 0.95 | 0.98 | 0.82 | 0.81 | 0.69 | 0.68 | |
Location subjects (L) are in the top six rows, whereas Color subjects (C) are in the bottom six rows. Missing values indicate that the subject (S) did not receive that particular dose of drug. V, vehicle.
Appendix 2.
Averaged Standard deviation values for individual subjects
| D-amphetamine | Nicotine | Haloperidol | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | V | 0.3 | 1.0 | 1.7 | 2.25 | 3.0 | V | 0.03 | 0.1 | 0.3 | 1.0 | 1.7 | V | 0.01 | 0.03 | 0.1 | 0.3 | 0.5 | 0.7 | |
| L | 673 | 0.07 | 0.15 | 0.10 | 0.06 | 0.07 | 0.18 | 0.03 | 0.03 | 0.06 | 0.06 | 0.18 | 0.07 | 0.10 | 0.11 | 0.07 | 0.10 | 0.16 | 0.11 | 0.08 |
| 648 | 0.05 | 0.18 | 0.04 | 0.07 | 0.08 | 0.05 | 0.09 | 0.10 | 0.12 | 0.14 | 0.08 | 0.08 | 0.15 | 0.08 | 0.15 | 0.19 | 0.15 | 0.09 | — | |
| 622 | 0.11 | 0.12 | 0.09 | 0.10 | 0.11 | 0.13 | 0.10 | 0.08 | 0.07 | 0.18 | 0.15 | 0.09 | 0.09 | 0.09 | 0.03 | 0.03 | 0.08 | 0.12 | — | |
| 4423 | 0.10 | 0.07 | 0.06 | 0.19 | 0.20 | 0.05 | 0.04 | 0.02 | 0.03 | 0.04 | 0.13 | — | 0.04 | 0.08 | 0.11 | 0.15 | 0.10 | 0.10 | — | |
| 883 | 0.05 | 0.06 | 0.10 | 0.08 | 0.07 | 0.09 | 0.11 | — | 0.13 | 0.16 | 0.12 | 0.07 | 0.09 | 0.09 | 0.12 | 0.10 | 0.19 | 0.06 | — | |
| 774 | 0.05 | 0.07 | 0.05 | 0.10 | 0.07 | 0.17 | 0.07 | 0.10 | 0.08 | 0.09 | 0.05 | 0.16 | 0.13 | 0.11 | 0.06 | 0.09 | 0.08 | 0.08 | 0.04 | |
| C | 811 | 0.15 | 0.05 | 0.10 | 0.17 | 0.09 | 0.09 | 0.19 | 0.16 | 0.09 | 0.11 | 0.17 | 0.05 | 0.29 | — | 0.14 | 0.10 | 0.17 | 0.37 | 0.46 |
| 965 | 0.17 | 0.18 | 0.11 | 0.12 | 0.13 | 0.15 | 0.06 | 0.14 | 0.16 | 0.11 | 0.16 | 0.08 | 0.14 | 0.11 | 0.12 | 0.20 | 0.28 | 0.29 | 0.22 | |
| 642 | 0.22 | — | 0.08 | 0.19 | 0.20 | 0.22 | 0.13 | 0.20 | 0.16 | 0.15 | 0.11 | 0.11 | 0.21 | — | — | 0.18 | 0.24 | 0.17 | 0.18 | |
| 657 | 0.11 | — | 0.19 | 0.18 | 0.07 | 0.28 | 0.20 | 0.12 | 0.23 | 0.17 | 0.26 | 0.20 | 0.13 | — | 0.29 | 0.13 | 0.19 | 0.12 | 0.19 | |
| 63 | 0.15 | 0.07 | 0.07 | 0.20 | 0.27 | 0.23 | 0.14 | 0.13 | 0.14 | 0.17 | 0.07 | 0.24 | 0.07 | 0.16 | 0.14 | 0.16 | 0.13 | 0.21 | 0.27 | |
| 750 | 0.13 | — | 0.18 | 0.23 | 0.15 | 0.12 | 0.18 | 0.10 | 0.13 | 0.18 | 0.14 | 0.27 | 0.09 | 0.16 | 0.09 | 0.13 | 0.22 | 0.23 | 0.17 | |
Location subjects (L) are in the top six rows, whereas Color subjects (C) are in the bottom six rows. Missing values indicate that the subject (S) did not receive that particular dose of drug. V, vehicle.
Appendix 3.
Averaged Minimum of the function values for individual subjects
| D-amphetamine | Nicotine | Haloperidol | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | V | 0.3 | 1.0 | 1.7 | 2.25 | 3.0 | V | 0.03 | 0.1 | 0.3 | 1.0 | 1.7 | V | 0.01 | 0.03 | 0.1 | 0.3 | 0.5 | 0.7 | |
| L | 673 | 0.05 | 0.01 | 0.04 | 0.08 | 0.09 | 0.08 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.03 |
| 648 | 0.03 | 0.00 | 0.05 | 0.23 | 0.36 | 0.06 | 0.03 | 0.01 | 0.03 | 0.04 | 0.03 | 0.05 | 0.03 | 0.03 | 0.00 | 0.08 | 0.08 | 0.07 | — | |
| 622 | 0.01 | 0.02 | 0.01 | 0.00 | 0.02 | 0.00 | 0.02 | 0.06 | 0.05 | 0.03 | 0.03 | 0.05 | 0.02 | 0.02 | 0.00 | 0.01 | 0.07 | 0.11 | — | |
| 4423 | 0.03 | 0.01 | 0.10 | 0.20 | 0.39 | 0.39 | 0.06 | 0.08 | 0.08 | 0.14 | 0.05 | — | 0.01 | 0.00 | 0.00 | 0.00 | 0.03 | 0.02 | — | |
| 883 | 0.00 | 0.05 | 0.00 | 0.04 | 0.07 | 0.05 | 0.00 | — | 0.01 | 0.02 | 0.02 | 0.00 | 0.00 | 0.03 | 0.01 | 0.02 | 0.07 | 0.09 | — | |
| 774 | 0.01 | 0.02 | 0.06 | 0.00 | 0.02 | 0.06 | 0.03 | 0.06 | 0.07 | 0.00 | 0.04 | 0.01 | 0.05 | 0.00 | 0.03 | 0.00 | 0.03 | 0.02 | 0.09 | |
| C | 811 | 0.02 | 0.06 | 0.05 | 0.02 | 0.08 | 0.09 | 0.04 | 0.00 | 0.02 | 0.12 | 0.11 | 0.17 | 0.00 | — | 0.00 | 0.02 | 0.08 | 0.00 | 0.00 |
| 965 | 0.02 | 0.00 | 0.19 | 0.15 | 0.14 | 0.21 | 0.07 | 0.00 | 0.01 | 0.14 | 0.02 | 0.09 | 0.04 | 0.09 | 0.08 | 0.00 | 0.05 | 0.11 | 0.13 | |
| 642 | 0.06 | — | 0.12 | 0.02 | 0.07 | 0.18 | 0.04 | 0.08 | 0.07 | 0.16 | 0.19 | 0.35 | 0.03 | — | — | 0.09 | 0.08 | 0.12 | 0.15 | |
| 657 | 0.01 | — | 0.01 | 0.00 | 0.13 | 0.10 | 0.02 | 0.00 | 0.00 | 0.01 | 0.03 | 0.05 | 0.01 | — | 0.00 | 0.02 | 0.01 | 0.10 | 0.00 | |
| 63 | 0.05 | 0.06 | 0.19 | 0.15 | 0.00 | 0.10 | 0.04 | 0.01 | 0.02 | 0.06 | 0.08 | 0.04 | 0.03 | 0.03 | 0.02 | 0.04 | 0.14 | 0.12 | 0.12 | |
| 750 | 0.03 | — | 0.00 | 0.01 | 0.18 | 0.23 | 0.00 | 0.04 | 0.02 | 0.06 | 0.11 | 0.10 | 0.02 | 0.02 | 0.00 | 0.02 | 0.00 | 0.01 | 0.04 | |
Location subjects (L) are in the top six rows, whereas Color subjects (C) are in the bottom six rows. Missing values indicate that the subject (S) did not receive that particular dose of drug. V, vehicle.
Appendix 4.
Averaged Point of Subjective Equality values for individual subjects
| D-amphetamine | Nicotine | Haloperidol | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | V | 0.3 | 1.0 | 1.7 | 2.25 | 3.0 | V | 0.03 | 0.1 | 0.3 | 1.0 | 1.7 | V | 0.01 | 0.03 | 0.1 | 0.3 | 0.5 | 0.7 | |
| L | 673 | 3.54 | 3.06 | 3.14 | 3.68 | 3.33 | 3.74 | 2.48 | 2.51 | 2.64 | 2.66 | 3.85 | 3.04 | 2.97 | 2.97 | 2.74 | 3.53 | 3.99 | 3.69 | 3.50 |
| 648 | 4.90 | 6.26 | 4.30 | 4.25 | 3.30 | 3.67 | 5.07 | 4.84 | 5.20 | 4.15 | 3.71 | 3.76 | 6.21 | 5.27 | 5.78 | 4.95 | 4.64 | 4.34 | — | |
| 622 | 5.07 | 6.85 | 5.25 | 4.69 | 4.52 | 5.99 | 6.98 | 6.92 | 6.31 | 6.31 | 6.78 | 4.82 | 5.84 | 5.89 | 5.96 | 5.43 | 5.72 | 6.62 | — | |
| 4423 | 3.02 | 3.10 | 3.16 | 2.89 | 2.57 | 2.87 | 2.89 | 2.72 | 3.28 | 3.57 | 3.92 | - | 2.92 | 3.50 | 3.48 | 4.65 | 4.39 | 4.90 | — | |
| 883 | 4.90 | 4.84 | 4.71 | 4.84 | 4.84 | 5.42 | 5.08 | — | 4.76 | 4.73 | 4.63 | 4.07 | 4.24 | 4.73 | 4.58 | 4.05 | 6.01 | 4.50 | — | |
| 774 | 5.40 | 4.90 | 5.07 | 5.14 | 5.13 | 6.40 | 5.02 | 5.38 | 4.87 | 4.27 | 5.58 | 4.85 | 4.68 | 5.15 | 4.58 | 4.98 | 4.29 | 5.39 | 3.83 | |
| C | 811 | 4.91 | 5.13 | 5.13 | 5.59 | 5.19 | 5.26 | 6.78 | 5.32 | 4.93 | 4.47 | 5.13 | 3.73 | 6.39 | — | 4.72 | 3.55 | 6.54 | 7.85 | 7.43 |
| 965 | 5.59 | 5.33 | 5.19 | 5.09 | 6.37 | 4.90 | 5.08 | 4.37 | 6.54 | 4.68 | 4.72 | 4.27 | 5.37 | 4.87 | 4.61 | 4.47 | 6.31 | 7.17 | 5.01 | |
| 642 | 6.51 | — | 6.36 | 5.25 | 6.26 | 6.89 | 5.18 | 6.36 | 6.48 | 5.77 | 4.06 | 4.13 | 5.46 | — | — | 4.73 | 4.32 | 5.87 | 3.87 | |
| 657 | 4.73 | — | 4.91 | 5.05 | 4.32 | 4.16 | 6.79 | 4.68 | 5.38 | 5.25 | 6.33 | 5.04 | 4.48 | — | 6.60 | 5.01 | 5.29 | 4.24 | 5.12 | |
| 63 | 5.15 | 4.37 | 4.57 | 6.36 | 4.07 | 4.62 | 4.42 | 4.86 | 4.58 | 5.56 | 4.81 | 6.36 | 4.30 | 5.97 | 4.32 | 4.23 | 4.17 | 4.49 | 7.27 | |
| 750 | 4.54 | — | 4.50 | 5.75 | 5.81 | 4.92 | 4.65 | 4.47 | 4.04 | 6.06 | 4.44 | 7.55 | 3.90 | 4.73 | 3.68 | 4.72 | 4.05 | 5.20 | 4.97 | |
Location subjects (L) are in the top six rows, whereas Color subjects (C) are in the bottom six rows. Missing values indicate that the subject (S) did not receive that particular dose of drug. V, vehicle.
Footnotes
This experiment was conducted at the University of Florida, and served in part to fulfill the requirements of a Doctorate of Philosophy from the University of Florida for Erin A. McClure.
References
- Andrews JS, Holtzman SG. Effects of d-amphetamine, morphine, naloxone, and drug combinations on visual discrimination in rats. Psychopharmacology. 1988;94:172–177. doi: 10.1007/BF00176840. [DOI] [PubMed] [Google Scholar]
- Berryman R, Jarvik ME, Nevin JA. Effects of pentobarbital, lysergic acid diethylamide and chlorpromazine on matching behavior in the pigeon. Psychopharmacologia. 1962;3:60–65. doi: 10.1007/BF00413108. [DOI] [PubMed] [Google Scholar]
- Bizo LA, White GW. The behavioral theory of timing: reinforcer rate determines pacemaker rate. J Exp Anal Behav. 1994;61:19–33. doi: 10.1901/jeab.1994.61-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizot JC. Effects of psychoactive drugs on temporal discrimination in rats. Behav Pharmacol. 1997;8:293–308. doi: 10.1097/00008877-199708000-00003. [DOI] [PubMed] [Google Scholar]
- Blondel A, Simon H, Sanger DJ, Moser PC. The effects of repeated nicotine administration on performance of drug-naive rats in a 5-choice serial reaction time task. Behav Pharmacol. 1998;10:665–673. doi: 10.1097/00008877-199911000-00013. [DOI] [PubMed] [Google Scholar]
- Blough DS. Error factors in pigeon discrimination and delayed matching. J Exp Psychol Anim Behav Process. 1996;22:118–131. [Google Scholar]
- Buhusi CV, Meck WH. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behav Neurosci. 2002;116:291–297. doi: 10.1037//0735-7044.116.2.291. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Carli M, Samanin R. Serotonin 2 receptor agonists and serotonergic anorectic drugs affect rats’ performance differently in a five-choice serial reaction time task. Psychopharmacology. 1992;106:228–234. doi: 10.1007/BF02801977. [DOI] [PubMed] [Google Scholar]
- Cevik M. Effects of methamphetamine on duration discrimination. Behav Neurosci. 2003;117:774–784. doi: 10.1037/0735-7044.117.4.774. [DOI] [PubMed] [Google Scholar]
- Chatlosh DL, Wasserman EA. Delayed temporal discrimination in pigeons: a comparison of two procedures. J Exp Anal Behav. 1987;47:299–309. doi: 10.1901/jeab.1987.47-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RK, MacDonald CJ, Meck W. Differential effects of cocaine and ketamine on time estimation: implications for neurobiological models of interval timing. Pharmacol Biochem Behav. 2006;85:114–122. doi: 10.1016/j.pbb.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Chiang TJ, Al-Ruwaitea ASA, Mobini S, Ho MY, Bradshaw CM, Szabadi E. The effect of d-amphetamine on performance on two operant timing schedules. Psychopharmacology. 2000;150:170–184. doi: 10.1007/s002130000422. [DOI] [PubMed] [Google Scholar]
- Church RM, Deluty MZ. Bisection of temporal intervals. J Exp Psychol Anim Behav Process. 1977;3:216–228. doi: 10.1037//0097-7403.3.3.216. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Amphetamine impairs the discrimination performance of rats with dorsal bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic–noradrenergic interactions. Psychopharmacology. 1987;91:458–466. doi: 10.1007/BF00216011. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res. 1989;33:165–179. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- Dykstra LA. Effects of morphine, diazepam, and chlorpromazine on discrimination of electric shock. J Pharmacol Exp Ther. 1979;209:297–303. [PubMed] [Google Scholar]
- Eckerman DA, Lanson RN, Berryman R. Effects of sodium pentobarbital on symbolic matching and symbolic oddity performance. Psychon Bull Rev. 1978;11:171–174. [Google Scholar]
- Fetterman JG, Killeen PR, Hall S. Watching the clock. Behav Process. 1998;44:211–224. doi: 10.1016/S0376-6357(98)00050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilly DM, Genovese RF, Nowak MJ. Effects of morphine, d-amphetamine, and pentobarbital on shock and light discrimination performance in rats. Psychopharmacology. 1980;70:213–217. doi: 10.1007/BF00435317. [DOI] [PubMed] [Google Scholar]
- Hampson CL, Body S, den Boon FS, Cheung THC, Bezzina G, Langley RW, et al. Comparison of the effects of 2,5-dimethoxy-4-iodoamphetamine and d-amphetamine on the ability of rats to discriminate durations and intensities of light stimuli. Behav Pharmacol. 2010;21:11–20. doi: 10.1097/FBP.0b013e328334707a. [DOI] [PubMed] [Google Scholar]
- Harper DN, Bizo LA, Peters H. Dopamine agonists and antagonists can produce an attenuation of response bias in a temporal discrimination task depending on discriminability of target duration. Behav Process. 2006;71:286–296. doi: 10.1016/j.beproc.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Heinemann EG, Avin E, Sullivan MA, Chase S. Analysis of stimulus generalization with a psychophysical method. J Exp Psychol. 1969;80:215–224. [Google Scholar]
- Killeen PR, Hall S, Bizo LA. A clock not wound runs down. Behav Process. 1999;45:129–139. doi: 10.1016/s0376-6357(99)00014-5. [DOI] [PubMed] [Google Scholar]
- Koek W, Slangen JL. Effects of d-amphetamine and morphine on discrimination: signal detection analysis and assessment of response repetition in the performance deficits. Psychopharmacology. 1983;80:125–128. doi: 10.1007/BF00427954. [DOI] [PubMed] [Google Scholar]
- Koek W, Slangen JL. Effects of d-amphetamine and morphine on delayed discrimination: signal detection analysis and assessment of response repetition in the performance deficits. Psychopharmacology. 1984;83:346–350. doi: 10.1007/BF00428543. [DOI] [PubMed] [Google Scholar]
- Kraemer PJ, Brown RW, Randall CK. Signal intensity and duration estimation in rats. Behav Process. 1995;34:265–268. doi: 10.1016/0376-6357(95)00003-d. [DOI] [PubMed] [Google Scholar]
- Lejeune H, Macar F, Zakay D. Attention and timing: dual task performance in pigeons. Behav Process. 1999;45:141–157. doi: 10.1016/s0376-6357(99)00015-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Simon BB. Nicotinic acetylcholine receptor involvement in cognitive function in animals. Psychopharmacology. 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Machado A, Keen R. Temporal discrimination in a long operant chamber. Behav Process. 2003;62:157–182. doi: 10.1016/s0376-6357(03)00023-8. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Church RM. The differential effects of haloperidol and methamphetamine on time estimation in the rat. Psychopharmacology (Berl) 1983;79:10–15. doi: 10.1007/BF00433008. [DOI] [PubMed] [Google Scholar]
- Maricq A, Roberts S, Church R. Methamphetamine and time estimation. J Exp Psychol Anim Behav Process. 1981;7:18–30. doi: 10.1037//0097-7403.7.1.18. [DOI] [PubMed] [Google Scholar]
- McClure EA, Saulsgiver KA, Wynne CDL. Effects of d-amphetamine on temporal discrimination in pigeons. Behav Pharmacol. 2005;16:193–208. doi: 10.1097/01.fbp.0000171773.69292.bd. [DOI] [PubMed] [Google Scholar]
- McClure EA, Saulsgiver KA, Wynne CDL. Effects of acute and chronic d-amphetamine on two variations of a temporal discrimination procedure. Behav Pharmacol. 2009a;20:668–672. doi: 10.1097/FBP.0b013e328331ba08. [DOI] [PubMed] [Google Scholar]
- McClure EA, Saulsgiver KA, Wynne CDL. ABA chronic dosing of d-amphetamine produces differential drug effects in two variants of a temporal discrimination procedure in pigeons. Behav Pharmacol. 2009b;20:705–719. doi: 10.1097/FBP.0b013e328333b251. [DOI] [PubMed] [Google Scholar]
- McClure EA, Saulsgiver KA, Wynne CDL. Manipulating pre-feed, density of reinforcement, and extinction produces disruption in the Location variation of a temporal discrimination task in pigeons. Behav Process. 2009c;82:85–89. doi: 10.1016/j.beproc.2009.03.009. [DOI] [PubMed] [Google Scholar]
- McClure EA, Saulsgiver KA, Wynne CDL. Disruptive effects of stimulus intensity on two variations of a temporal discrimination procedure. J Exp Anal Behav. 2010;94:57–68. doi: 10.1901/jeab.2010.94-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH. Selective adjustment of the speed of internal clock and memory processes. J Exp Psychol Anim Behav Process. 1983;9:171–201. [PubMed] [Google Scholar]
- Meck WH. Affinity for the dopamine D2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharmacol Biochem Behav. 1986;25:1185–1189. doi: 10.1016/0091-3057(86)90109-7. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Cognit Brain Res. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck WH, Penney TB, Pouthas V. Cortico-striatal representation of time in animals and humans. Curr Opin Neurobiol. 2008;18:145–152. doi: 10.1016/j.conb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Morgan L, Killeen PR, Fetterman JG. Changing rates of reinforcement perturbs the flow of time. Behav Process. 1993;30:259–272. doi: 10.1016/0376-6357(93)90138-H. [DOI] [PubMed] [Google Scholar]
- Odum AL, Ward RD. Characterizing the effects of d-amphetamine on temporal discrimination. Behav Process. 2007;175:156–166. doi: 10.1016/j.beproc.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Odum AL, Lieving LM, Schaal DW. Effects of d-amphetamine in a temporal discrimination procedure: selective changes in timing or rate dependency? J Exp Anal Behav. 2002;78:195–214. doi: 10.1901/jeab.2002.78-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popke EJ, Mayorga AJ, Folge CM, Paule MG. Effects of acute nicotine on several operant behaviors in rats. Pharmacol Biochem Behav. 2000;65:247–254. doi: 10.1016/s0091-3057(99)00205-1. [DOI] [PubMed] [Google Scholar]
- Rapp DL, Robbins TW. The effects of d-amphetamine on temporal discrimination in the rat. Psychopharmacology (Berl) 1976;51:91–100. doi: 10.1007/BF00426328. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Baker HF, Weight ML. Amphetamine disrupts successive but not simultaneous visual discrimination in the monkey. Psychopharmacology. 1980;67:241–244. doi: 10.1007/BF00431263. [DOI] [PubMed] [Google Scholar]
- Sanchez-Castillo H, Chavez A, Miranda F, Velazquez-Martinez DN. Effect of d-amphetamine on a retrospective timing task. Revista Mexicana de Psicologia. 2007;24:65–75. [Google Scholar]
- Santi A, Weise L, Kuiper D. Amphetamine and memory for event duration in rats and pigeons: disruption of attention to temporal samples rather than changes in the speed of the internal clock. Psychobiology. 1995;23:224–232. [Google Scholar]
- Santi A, Coppa R, Ross L. Effects of the dopamine D2 agonist, quinpirole, on time and number processing in rats. Pharmacol Biochem Behav. 2001;68:147–155. doi: 10.1016/s0091-3057(00)00452-4. [DOI] [PubMed] [Google Scholar]
- Stanford L, Santi A. The dopamine D2 agonist quinpirole disrupts attention to temporal signals without selectively altering the speed of the internal clock. Psychobiology. 1998;26:258–266. [Google Scholar]
- Stubbs DA. The discrimination of stimulus duration by pigeons. J Exp Anal Behav. 1968;11:223–238. doi: 10.1901/jeab.1968.11-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs DA, Thomas JR. Discrimination of stimulus duration and d-amphetamine in pigeons: a psychophysical analysis. Psychopharmacologia. 1974;36:313–322. doi: 10.1007/BF00422563. [DOI] [PubMed] [Google Scholar]
- Sutton JE, Roberts WA. The effect of nontemporal information processing on time estimation in pigeons. Learn Motiv. 2002;33:123–140. [Google Scholar]
- Ward RD, Odum AL. Effects of morphine on temporal discrimination and color matching: general disruption of stimulus control or selective effects on timing? J Exp Anal Behav. 2005;84:401–415. doi: 10.1901/jeab.2005.94-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Odum AL. Disruption of temporal discrimination and the choose short effect. Learn Behav. 2007;35:60–70. doi: 10.3758/bf03196075. [DOI] [PubMed] [Google Scholar]
- Ward RD, Barrett ST, Johnson RN, Odum AL. Nicotine dose not enhance discrimination performance in a temporal bisection procedure. Behav Pharmacol. 2009;20:99–108. doi: 10.1097/FBP.0b013e3283242fc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie DM, Symons LA, Tees RC. Effects of intertrial reinforcers on rats timing behavior. Behav Process. 1988;17:229–238. doi: 10.1016/0376-6357(88)90006-X. [DOI] [PubMed] [Google Scholar]