Abstract
Objective: To compare the first two weeks of tolerability of clindamycin/benzoyl peroxide gel versus adapalene/benzoyl peroxide gel followed by six weeks of open-label clindamycin/benzoyl peroxide gel therapy in subjects with mild-to-moderate acne who participated in two eight-week, identically designed, clinical studies. Methods: Using a split-face method, patients received both clindamycin/benzoyl peroxide gel and adapalene/benzoyl peroxide gel once daily for two weeks (allocation to the right or left side of the face was randomized) in an investigator-blinded fashion. Patients then went on to receive a further six weeks of open-label, full-face clindamycin/benzoyl peroxide gel. The primary outcome was to compare signs and symptoms of tolerability during the first two weeks of treatment using an investigator-assessed 4-point rating scale. Secondary endpoints included assessment of acne severity (Investigator Static Global Assessment and lesion counts), quality of life, product acceptability/preference, and patient assessments of tolerability and safety. Results: Of the 76 subjects enrolled in the two studies, 72 completed them. Overall both products were well tolerated, but mean scores for erythema, dryness, and peeling were significantly higher with adapalene/benzoyl peroxide gel than with clindamycin/benzoyl peroxide gel at both Weeks 1 and 2 (p<0.03). Patients also rated clindamycin/benzoyl peroxide gel significantly more tolerable than adapalene/benzoyl peroxide gel for redness, dryness, burning, itching, and scaling at Weeks 1 and 2 (p 0.0073). Mean Investigator Static Global Assessment score improved with both products during the first two weeks of treatment and continued to show significant improvement versus baseline when treatment with clindamycin/benzoyl peroxide gel was continued for a further six weeks (p<0.001 at Week 8). Lesion counts improved throughout the study with significant reductions from baseline occurring at Weeks 5 and 8 (p<0.0001 for both time points for total lesion counts). Clindamycin/benzoyl peroxide gel and adapalene/benzoyl peroxide gel were well tolerated, with most adverse events of mild-to-moderate severity. Conclusion: Clindamycin/benzoyl peroxide gel had better tolerability with regard to erythema, dryness, and peeling than adapalene/benzoyl peroxide gel during the first two weeks of treatment.
Acne is a multifactorial disease with the following four primary pathogenic features: sebum production Propionibacterium acnes colonization, altered keratinization, and release of inflammatory mediators.1 Topical combination therapy can target multiple pathogenic mechanisms and therefore is currently recommended as the standard of care in the treatment of mild-to-moderate acne, particularly in patients with an inflammatory component.1 The Global Alliance to Improve Outcomes in Acne recommends the combination of a retinoid with an antimicrobial, preferably the nonantibiotic benzoyl peroxide (BPO), as first-line therapy for mild-to-moderate acne.1 Topical antibiotics also have a role in acne management, but they should be used in combination with BPO to limit the development of P. acnes resistance.1 Fixed-combination products are reported to be effective, well tolerated, and more convenient for patients than multiple individual agents,2 and by reducing the number of medications and applications, fixed-combination products may improve patient adherence and treatment outcomes.2
A number of fixed-combination topical products are available for the treatment of acne, including clindamycin-BPO combinations and adapalene-BPO combinations. The fixed combination of adapalene and BPO (A/BPO) is a retinoid-antimicrobial combination that has proven to be more effective than monotherapy with either component or placebo.3 Local irritation, including erythema, peeling, dryness, burning, and itching, is the most common adverse effect of topical retinoids, although the potential for irritation appears to be lower with adapalene than with other retinoids such as tretinoin.4–6 BPO can also cause local irritation,7 but combining adapalene and BPO has a comparable safety and tolerability profile relative to adapalene alone.3,8 The combination of clindamycin and BPO (C/BPO) has been shown to more rapidly reduce the number of total and inflammatory lesions compared with adapalene monotherapy,9 erythromycin and zinc combination,10 and A/BPO.11 C/BPO has a good tolerability profile, minimizes irritation, and does not have the early flare effect characteristic of topical retinoids.12 Levels of hydrating excipients have been increased in a combination formulation of C/BPO to improve tolerability.13 Both C/BPO and A/BPO are once-daily formulations, making them convenient for patients to use. In a 12-week comparative study, A/BPO and C/BPO proved to be similarly effective in reducing inflammatory and noninflammatory acne lesions, but C/BPO had a more rapid effect on lesion counts, particularly inflammatory lesions, and was better tolerated.11
The authors present pooled data from two similarly designed studies using C/BPO and A/BPO in subjects with acne. A randomized, investigator-blind, split-face design was used to compare the agents during the first two weeks of treatment, followed by six weeks of open-label treatment with C/BPO over the entire face. The primary objective of the study was to compare the tolerability of C/BPO and A/BPO during the first two weeks of treatment in subjects with acne, using a study design that minimized the potential for variation by having patients act as their own control.
Patients and Methods
Study design. Two multicenter, eight-week studies were conducted, one in the United States (study 410) and one in Argentina (study 401). The study designs were identical and therefore suitable for pooling, but there were some slight differences in patient inclusion criteria and endpoint analyses. For example, study 401 enrolled subjects aged Ž18 years and included investigator assessments of tolerability while study 410 enrolled subjects aged Ž21 years and included both investigator- and subject-rated assessments of tolerability.
For the first two weeks of the study, a randomized, single-blind, split-face study design was conducted. Subjects applied C/BPO (Duac® or Clindoxyl®, Stiefel, a GSK Company, Research Triangle Park, North Carolina) and A/BPO (Epiduo®, Galderma Laboratories, Fort Worth, Texas) in a bilateral split-face fashion (allocation to the left or right side of the face was randomized). Investigators were blinded during the first two weeks of treatments. For the remaining six weeks, subjects applied C/BPO to the entire face, in an open-label, full-face fashion.
Both studies were approved by their local Institutional Review Boards and Ethics Committees and conducted in accordance with the guidelines of the International Conference on Harmonisation Good Clinical Practice (ICH GCP).
Patients. Subjects were eligible for study entry if they were Ž18 years of age (study 401) or Ž21 years of age (study 410), were in good health, had documented acne vulgaris (15–60 inflammatory and noninflammatory facial lesions excluding nose, nasolabial fold, and upper and lower eyelids), and were willing to avoid all other topical or systemic acne therapies for the duration of the studies. Female subjects who were pregnant, planning to become pregnant, or breastfeeding were excluded, and sexually active female subjects had to be using a medically acceptable form of contraception (oral contraception, injectable or implantable methods, or intrauterine devices); barrier methods were considered acceptable in study 410 but not in study 401.
Hormonal treatments, initiated before entry to the trial, including contraceptives (those containing estrogen, androgens, or anti-androgens), were allowed as long as there was no expected change to the dose or drug or discontinuation during the study. Other exclusion criteria were severe systemic disease or diseases of the facial skin other than acne; presence of facial hair that could interfere with the accurate assessment of acne severity; history or presence of regional enteritis, inflammatory bowel disease or photosensitivity; recent use of topical antibiotics (in the preceding 2 weeks) or systemic antibiotics (in the preceding 4 weeks), topical corticosteroids (in the preceding 4 weeks), systemic retinoids (preceding 6 months), or other topical anti-acne medications (preceding 2 weeks); concomitant use of photosensitizing or neuromuscular blocking agents or medications known to exacerbate acne, including vitamins; current use of facial products that could potentially affect results (e.g., astringents, toners, peels, hair removal wax, cleansers, washes or soaps containing BPO, sulfacetamide sodium or salicylic acid, or moisturizers containing retinol, salicylic, or hydroxyl acids); facial procedure (peel, dermabrasion, or ultraviolet light therapy) within the past four weeks; use of an investigational drug or treatment within the previous four weeks; and/or sharing a household with another study participant. All subjects provided written informed consent before entering the study.
Procedures and study endpoints. Data collected during the baseline study visit included information about patient demographics, medical/medication histories, and lesion counts. A number of assessment procedures were also performed including an Investigator Static Global Assessment (ISGA; face only), SKINDEX-29, local tolerability assessments, and a pregnancy test. Patients were then dispensed one 45g tube of C/BPO and one 45g tube of A/BPO. Subjects were instructed to wash their face in the evening with soap-free cleanser (Physiogel®, Stiefel, a GSK Company, in study 401), rinse thoroughly, and pat dry with soft towel before applying a thin film of each study product to either side of the face (as per the randomization schedule). Each gram of C/BPO gel contained 10mg (1%) clindamycin as clindamcyin phosphate and 50mg (5%) BPO and each gram of A/BPO gel contained 1mg (0.1%) adapalene and 25mg (2.5%) BPO in an aqueous gel.
Subjects were instructed not to wash their skin for at least four hours, and preferably to leave the study product on for eight hours. In the morning, subjects washed their face with the same cleanser and applied moisturizer/ sunscreen. This was undertaken daily for two weeks. At the end of Week 2, subjects applied C/BPO to the entire face each evening for the next six weeks and undertook the same procedures for cleansing and moisturizer/sunscreen application as used in the first two weeks.
Following the Baseline visit, subsequent study visits were performed at Weeks 1, 2, 5, and 8. At each visit, subjects returned used product tubes for weighing and provided updated information about concomitant medication, and investigators undertook ISGAs, lesion counts after Week 5 and 8, and tolerability assessments. Adverse events (AEs) were also monitored at each visit.
Diary cards were collected at Weeks 1 and 2 and SKINDEX-29 quality-of-life (QOL) assessments were undertaken at Baseline, Week 2, and Week 8 in study 401 and at Baseline plus Week 8 in study 410. Product acceptability and preference questionnaires were also completed by subjects at Weeks 1, 2, and 8 in both studies.
The primary endpoint for both studies was the investigator assessment of the signs and symptoms of local tolerability (erythema, peeling, and dryness) during the first two weeks of treatment. Investigators measured erythema, peeling, and dryness using a 4-point scale for each where 0=no signs/symptoms and 3=intense signs/symptoms. Secondary endpoints were signs of local tolerability (erythema, peeling, and dryness) at Weeks 5 and 8, ISGA assessments of acne severity using a 6-point scale from 0 (clear) to 5 (very severe), SKINDEX-29 QOL assessments, product acceptability, and preference.
As part of the Product Acceptability and Preference questionnaire, subjects in both studies assessed local tolerability for each product individually as a secondary endpoint. Assessments were undertaken for each side of the face separately at Weeks 1 and 2, using a 6-point scale from 0 (none) to 5 (very severe) to describe any redness, dryness, burning, itching, or scaling.
Safety was determined by recording all AEs that were observed or spontaneously reported throughout the study by subjects, investigators, or designees. The main safety outcomes investigated were the frequency of treatment-emergent events, treatment-related events (all AE reports were reviewed by the investigator to determine causality), events leading to discontinuation, and serious events.
Data analysis and statistical methods. Assuming a standard deviation (SD) of 2 in tolerability scores, it was estimated that 45 subjects per treatment arm (sides of face) would detect a 1.2 difference with 80 percent power using a 2-sided type I error rate of 0.05. Once subjects gave informed consent and were found to have met the inclusion criteria, their treatment was randomly allocated to either side of their face by a computer-generated randomization schedule (generated by the sponsor). To maintain the single blind during the initial two weeks, subjects and study-center staff were instructed not to reveal the treatment allocation to the investigator and subjects were instructed not to apply the product in their presence. Subjects were enrolled and assigned their interventions by a study coordinator, nurse, or pharmacist.
Analysis was undertaken on pooled endpoint data from the intent-to-treat (ITT) populations in the two studies (i.e., all patients who received Ž1 application of study medication). At Weeks 1 and 2, the individual differences between both sides of the face in terms of investigator and subject tolerability scores, ISGA, and each question of the Product Acceptability and Preference questionnaire were analyzed using the Wilcoxon signed-rank test at an alpha level of 0.05. No adjustments were made for multiplicity. The assumption of the normality was tested using a Shapiro-Wilk test at an alpha of 0.01, and if not verified, a nonparametric method (Wilcoxon signed-rank test) was used. All endpoint data at Weeks 5 and 8 were presented in a descriptive fashion and AE data were analyzed in terms of frequencies and percentages.
Results
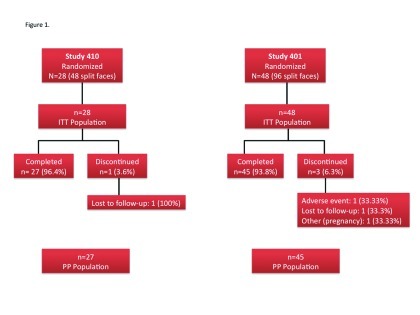
Subjects. Seventy-six subjects were enrolled in the two studies: 28 in study 410 and 48 in study 401. Enrollment for the 401 study began in February 2009 and the last subject completed the trial in April 2009. For the 410 study, enrollment began in July 2009 and the study was completed in December 2009. A total of 72 subjects completed the studies and four discontinued (Figure 1). Demographic characteristics were generally similar at Baseline (Table 1). Most subjects (82%) were female and the median age was 26 to 27 years. There was a clear difference between the studies in the ethnic/racial mix. In study 401, all subjects were white and of these, 69 percent were of Hispanic or Latino ethnicity, whereas in study 410, 54 percent of subjects were white (the rest were African American or Asian) with only 11 percent Hispanic or Latino. Subjects in study 410 also tended to have more severe disease compared with subjects in study 401. Approximately 93 percent of subjects in study 410 had moderate-to-severe scores on ISGA compared with 71 percent in study 401. Likewise, mean baseline lesion counts (inflammatory, noninflammatory, and total) were higher in the 410 than the 401 population. The mean (SD) number of days subjects were exposed to treatment was 52.3 (9.2) days in study 401 and 58.4 (4.2) days in study 410.
Figure 1.
Flow chart of subject disposition in each of the two studies
TABLE 1.
Baseline demographics and disease characteristics
| STUDY 401 (n=48) | STUDY 410 (n=28) | |
|---|---|---|
| Age, years | ||
| Mean (SD) | 27.6 (5.5) | 29.6 (9.5) |
| Median | 26 | 27.6 |
| Range | 21.6-45.6 | 18.6-48.4 |
| Sex, n (%) | ||
| Male | 10 (20.8) | 4 (14.3) |
| Female | 38 (79.2) | 24 (85.7) |
| Race, n (%) | ||
| White | 48 (100) | 15 (53.6) |
| African American | 0 | 11 (39.3) |
| Asian | 0 | 2 (7.1) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 33 (68.8) | 3 (10.7) |
| No Hispanic or Latino | 15 (31.3) | 25 (89.3) |
| ISGA score, n (%) | ||
| 2 - Mild | 14 (29.2) | 2 (7.1) |
| 3 - Moderate | 31 (64.6) | 20 (71.4) |
| 4 - Severe | 3 (6.3) | 6 (21.4) |
| Lesion count, mean (SD) | ||
| Inflammatory | 14.2 (9.1) | 21.5 (9.3) |
| Noninflammatory | 24.8 (12.8) | 33.0 (24.7) |
| Total | 39.1 (13.0) | 54.5 (27.1) |
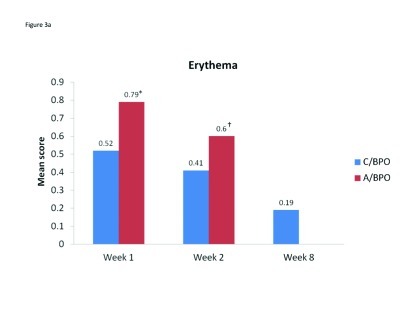
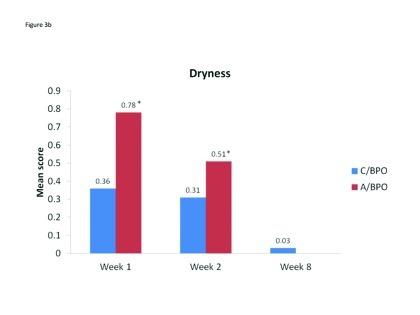
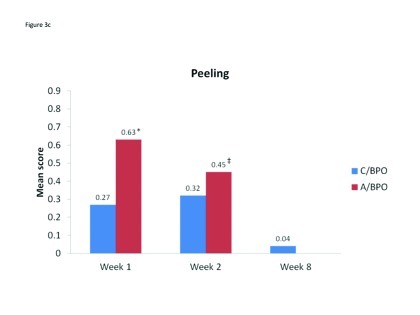
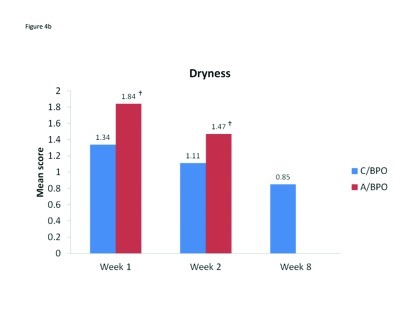
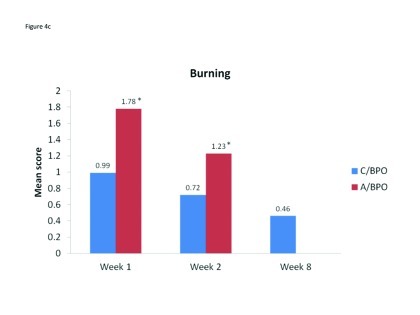
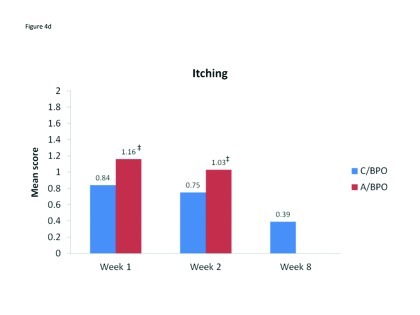
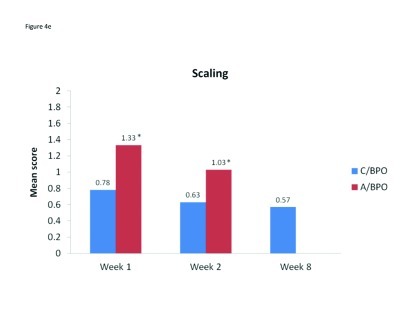
Local tolerability. During the split-face study, both C/BPO and A/BPO were well tolerated, with low investigator-rated scores for erythema, dryness, and peeling (primary endpoint; Figure 2). However, mean scores for these parameters were significantly higher after application of A/BPO than C/BPO at Weeks 1 and 2 (p<0.03 vs. C/BPO; Figure 2). Mean subject ratings for signs and symptoms of local tolerability (redness, dryness, burning, itching, and scaling) were also significantly lower with C/BPO than with A/BPO at Weeks 1 and 2 (p 0.0073; Figure 3).
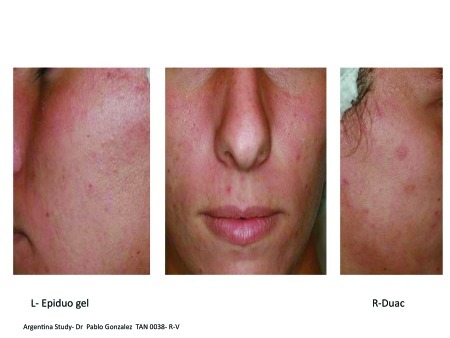
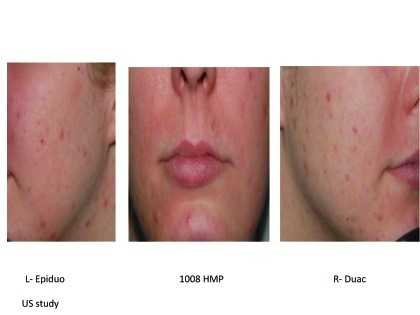
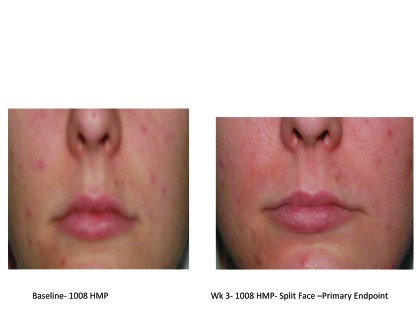
Figures 2A–2E.
Visual examples of outcomes following 2 weeks of split-face application.
Figure 2A. Left = Epiduo; Right = Duac Argentina study, Dr. Pablo Gonzalez; TAN 0038ȓR-V
Figure 2B. Left = Epiduo; Right = Duac United States study
Figure 2C. Left = Baseline—1008 HMP; Right = Week 3; 1008 HMP; split-face—primary endpoint
Figure 2D. Left = Baseline—1008 HMP; Right = Week 3; 1008 HMP; split-face—primary endpoint
Figure 2E. Left = Baseline—1008 HMP; Right = Week 3; 1008 HMP; split-face—primary endpoint
Figure 3A–3C.
Mean scores for (A) erythema, (B) dryness, and (C) peeling, as rated by investigators using a 4-point scale at Weeks 1, 2, and 8. *p<0.0001 vs. C/BPO, †p=0.002 vs. C/BPO and ‡p<0.03 vs. C/BPO
The incidence and ratings as assessed by investigators for erythema, dryness, and peeling continued to decline from Week 2 when C/BPO therapy only began, such that at Week 8 mean scores for each of these signs were negligible and, in each case, nearly two thirds or more of patients had no signs present (Table 2). Subject ratings for tolerability parameters also continued to decrease during full-face treatment with C/BPO, such that at Week 8, the mean (SD) score for each parameter was <1, very minimal (Table 3).
TABLE 2.
Investigator assessments of C/BPO local tolerability at Weeks 2 and 8
| INVESTIGATOR ASSESSMENTS (n=76) WEEK 2 | INVESTIGATOR ASSESSMENTS (n=76) WEEK 8 | |||
|---|---|---|---|---|
| NO. (%) WITH NO SIGN/SYMPTOM PRESENT | MEAN (SD) SCORE ON 4-POINT SCALE* | NO. (%) WITH NO SIGN/SYMPTOM PRESENT | MEAN (SD) SCORE ON 4-POINT SCALE* | |
| Redness | 47 (62.7) | 0.41 (0.57) | 62 (83.8) | 0.19 (0.46) |
| Dryness | 54 (72.0) | 0.31 (0.52) | 73 (98.6) | 0.03 (0.23) |
| Peeling | 54 (72.0) | 0.32 (0.55) | 71 (95.9) | 0.04 (0.20) |
| Irritant/allergic contact dermatitis | 73 (97.3) | 0.03 (0.16) | 74 (100.0) | 0.00 (0.00) |
0=none, 1=slight, 2=moderate, and 3=intense
TABLE 3.
Subject assessments of C/BPO local tolerability at Weeks 2 and 8
| SUBJECT ASSESSMENTS (n=76) WEEK 2 | SUBJECT ASSESSMENTS (n=76) WEEK 8 | |||
|---|---|---|---|---|
| NO. (%) WITH NO SIGN/SYMPTOM PRESENT | MEAN (SD) SCORE ON 6-POINT SCALE* | NO. (%) WITH NO SIGN/SYMPTOM PRESENT | MEAN (SD) SCORE ON 6-POINT SCALE* | |
| Redness | 34 (46.6) | 0.74 (0.83) | 39 (52.7) | 0.85 (1.11) |
| Dryness | 24 (33.3) | 1.11 (1.01) | 37 (50.0) | 0.85 (1.06) |
| Burning | 37 (51.4) | 0.72 (0.89) | 52 (70.3) | 0.46 (0.83) |
| Itching | 39 (54.2) | 0.75 (0.98) | 52 (70.3) | 0.39 (0.74) |
| Scaling | 39 (54.2) | 0.63 (0.78) | 51 (68.9) | 0.57 (1.02) |
0=none, 1=very minimal, 2=mild, 3=moderate, 4=severe, and 5=very severe
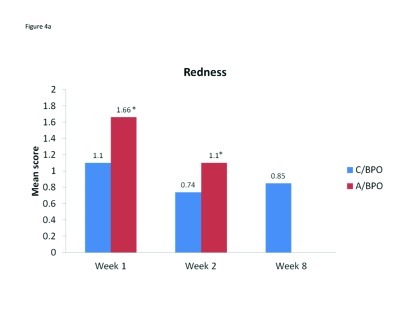
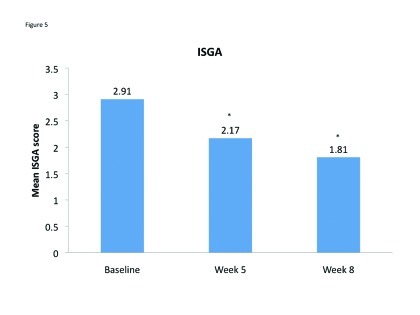
Acne severity. Mean ISGA improved for both sides of the face and there was no significant difference between the scores for C/BPO and A/BPO during the split-face portion of the study. Specifically, mean (SD) ISGA scores were 2.42 (0.83) and 2.48 (0.78) for C/BPO and A/BPO, respectively, at Week 1 (p=0.4850), and 2.16 (0.87) and 2.17 (0.86), respectively, at Week 2 (p=1.0). Over the course of the entire study, there was a significant improvement in full-face ISGA ratings (p<0.001) (Figure 4).
Figure 4A–4E.
Mean scores for (A) redness, (B) dryness, (C) burning, (D) itching, and (E) scaling as rated by subjects using a 6-point scale at Weeks 1, 2, and 8. *p<0.0001 vs. C/BPO, †p<0.0006 vs. C/BPO; ‡p<0.0073 vs. C/BPO
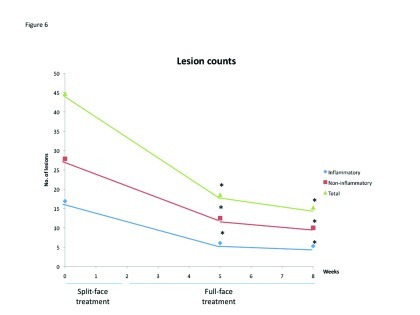
In terms of lesion counts, pooled data showed a significant reduction in the number of inflammatory, noninflammatory, and total lesions at Weeks 5 and 8 compared with baseline (p<0.0001; Figure 5). No comparative analysis was undertaken for lesion counts during the split-face phase of the study because baseline lesion counts were undertaken on the full face (not separately for each side) in study 401.
Figure 5.
Mean Investigator Static Global Assessment scores at baseline, Week 5 and Week 8. *p<0.0001 vs. baseline
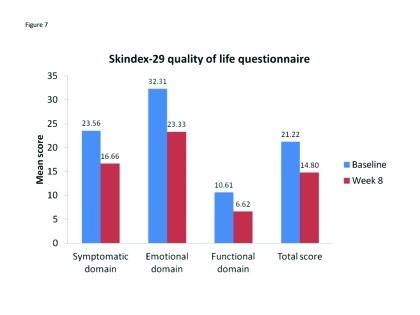
Patient preference and QOL. Patient QOL improved over the course of the study, with reductions in scores for all domains of the Skindex-29 quality-of-life questionnaire, as well as the total score (Figure 6). During the split-face portion of the study, almost all subjects (95–98%) rated C/BPO and A/BPO as “easy” or “very easy” to use, even with make-up, and there were no between-group differences. Similarly, both treatments were rated equally effective at reducing acne breakouts. However, A/BPO had significantly worse scores for skin comfort compared with C/BPO at Week 1 (p<0.02) and Week 2 (p=0.0036), and more subjects reported being more satisfied with C/BPO than with A/BPO at Week 1 (65.3% vs. 31.9% of patients; 2.8% of patients were equally satisfied with both treatments) and at Week 2 (56.2% vs. 42.5% of patients; 1.4% of patients were equally satisfied with both treatments).
Figure 6.
Lesion counts over the course of the 8-week studies. *p<0.0001 vs. baseline
Figure 7.
Mean Skindex-29 scores for all patients (n=76) at Baseline and Week 8. A reduction in score reflects improvement in quality of life.
Neither product rated well in terms of leaving the skin moisturized or hydrated with fewer than 50 percent in each group reporting a sensation of hydration at Week 1 or Week 2 (45–46% with C/BPO and 38–40% with A/BPO). At the end of Week 1, 63/76 subjects (88.7%) said they would choose to use C/BPO again and 41/76 (56.9%) said they would use A/BPO again. The corresponding number of subjects responding in this way at the end of Week 2 was 55/76 (76.4%) for C/BPO and 50/76 (68.5%) for A/BPO. At the end of Week 8 (after 6 weeks of full-face treatment with C/BPO), 61/76 subjects (83.6%) said they would choose to use this product again. Overall treatment satisfaction was high; 54/73 subjects (74%) rated being “satisfied” or “very satisfied” with C/BPO and 48/76 (66%) with A/BPO at Week 1. The corresponding rates at Week 2 were 61/74 (82.4%) with C/BPO and 56/74 (76%) with A/BPO. The between-group differences were not significant. After an additional six weeks of full-face C/BPO treatment, 55/73 (75%) of subjects were “satisfied” or “very satisfied.”
Compliance with both agents was reported to be high; 93 percent of patients in each group reported they were 80 to 100 percent compliant with treatment during the first week and 89 percent in each group reported the same at Week 2. During the full-face portion of the study, 92 percent of subjects reported that they used C/BPO every day.
Adverse events. Three subjects in study 410 developed an AE (10.7%). One had diarrhea, one dizziness, and one erythema. None of these was considered treatment related or serious and no subject discontinued treatment because of AEs. In contrast, 41/48 subjects in study 401 (85.4%) developed a treatment-related AE. Almost all of these events (in 40/41 subjects with an AE) occurred during the split-face portion of the study and involved application-site conditions (Table 4). A post-hoc analysis indicated that irritation, dryness, and erythema were significantly more common with A/BPO than with C/BPO (p<0.015; Table 4). Eleven subjects (22.9%) reported an AE during full-face treatment with C/BPO. Most events were of mild or moderate severity, but three subjects developed serious severe cutaneous AEs and one of these withdrew from the study.
TABLE 4.
Adverse events occurring during the course of the split-face portion (Weeks 1 and 2) of the 401 study
| SUBJECTS WITH AEs, n (%) | |||
|---|---|---|---|
| C/BPO (N=48) | A/BPO (N=48) | P-VALUE | |
| Any AE | |||
| 31 (64.6) | 40 (83.3) | 0.0067 | |
| Application site conditions | |||
| Irritation | 23 (47.9) | 33 (68.8) | 0.0124 |
| Erythema | 13 (27.1) | 19 (39.6) | 0.0143 |
| Dryness | 10 (20.8) | 18 (37.5) | 0.0114 |
| Exfoliation | 8 (16.7) | 10 (20.8) | 0.1573 |
| Pruritus | 8 (16.7) | 10 (20.8) | 0.3173 |
| Dermatitis | 2 (4.2) | 1 (2.1) | 0.3173 |
Discussion
These studies have demonstrated that topical C/BPO is better tolerated than A/BPO during the initial two weeks of treatment for acne, with significantly lower overall scores for all investigator- and subject-rated tolerability parameters (p<0.05). These data are consistent with a previous randomized study comparing these two agents.11 Zouboulis et al11 reported a significantly greater incidence of local reactions with A/BPO than with C/BPO from Weeks 1 through 12 and that, among patients who experienced tolerability reactions, C/BPO was significantly better tolerated than A/BPO at all grades from Week 1 onward.11 This was true for both investigator-rated (erythema, dryness, peeling) and participant-rated (pruritus, burning/stinging) outcomes. The study by Zouboulis et al11 also showed that both treatments effectively reduced inflammatory, noninflammatory, and total lesion counts over the 12-week treatment period. A similarly effective reduction was observed in these three parameters at both five and eight weeks in the current study, although subjects in the current study received two weeks of split-face C/BPO and A/BPO followed by full-face C/BPO, whereas subjects in the study by Zouboulis et al11 received 12 weeks’ treatment with each therapy. It should be noted that the use of A/BPO for just two weeks during the comparative phase of the current study is insufficient to assess this agent’s efficacy in treating acne; rather, the study was designed primarily to assess short-term tolerability differences.
Although there was no difference in the overall incidence of AEs occurring with C/BPO or A/BPO use in one of the studies (410), the other (401) showed a significantly higher rate of local AEs with A/BPO than C/BPO, albeit in a post-hoc analysis.
In addition to the improvement of local irritation and reduction in acne lesions, the authors’ study also demonstrated that continued use of C/BPO was associated with improvements in QOL. Moreover, QOL parameters also improved throughout the studies with subjects reporting improvements in emotional distress and ability to function as well as symptomatic improvement in physical signs and symptoms.
As with most clinical trials, this study is not without limitations. The authors pooled data from two almost identical studies, allowing for a larger study population and greater statistical power. However, this meant there were some slight differences in the study populations and in the way that endpoint data were collected. Nevertheless, the authors believe that these factors are unlikely to have demonstrably impacted the results. Another limitation is that the authors’ study was a single-blind analysis, and the fact that patients were not blinded to treatment allocation may have introduced some bias. However, the primary endpoint was the investigator rating of local tolerability, and investigators were blinded to treatment allocation, minimizing the impact of any bias on the primary results. The last limitation is that this study was of eight weeks’ duration with only two weeks of direct comparison and therefore no conclusions should be drawn about the comparative efficacy of the two products at 12 weeks where maximal benefit of acne treatment is achieved. The results from this study do not allow statements about therapeutic equivalence or noninferiority of A/BPO and C/BPO to be made as the study was not powered to address such issues. However, the focus of this study was the evaluation of acute tolerability, and since irritation potential is highest during the first two weeks of treatment, the study duration was deemed appropriate.
Conclusion
In conclusion, C/BPO gel has demonstrated a better tolerability profile than A/BPO during the first two weeks of treatment. Both agents are effective in reducing overall acne severity and achieving high levels of patient satisfaction, and continued use of C/BPO for a further six weeks may be associated with better adherence to therapy, clinical improvement in acne, and QOL.
Acknowledgment
Medical writing and editorial support were provided by Catherine Rees and Natalie Avenell-Mills, medical writers, and Medisys Health Communications, and funded by Stiefel, a GSK company. These studies were sponsored by Stiefel, a GSK company. The authors gratefully acknowledge the 401 and 410 study investigators Steve Grekin, MD, C000-401: Lawrence Green, MD, FAAD; Pablo J. Gonzalez, MD; Eduardo Adolfo Rodriguez, MD, and Tania Zarowsky, MD.
Footnotes
DISCLOSURE:Dr. Green was paid by Stiefel as an investigator for this study. Dr. Cirigliano and Ms. Gwazdauskas are employees of Stiefel.
Dr. Gonzalez serves as a researcher and/or speaker for GSK. These studies were sponsored by Stiefel, a GSK company.
References
- 1.Thiboutot D, Gollnick H, Bettoli V, et al. Global Alliance to Improve Outcomes in Acne. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5Suppl):S1–S50. doi: 10.1016/j.jaad.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Ghali F, Kang S, Leyden J, et al. Changing the face of acne therapy. Cutis. 2009;83(2 Suppl):4–15. [PubMed] [Google Scholar]
- 3.Thiboutot DM, Weiss J, Bucko A, et al. Adapalene-benzoyl peroxide, a fixed-dose combination for the treatment of acne vulgaris: results of a multicenter, randomized double-blind, controlled study. J Am Acad Dermatol. 2007;57:791–799. doi: 10.1016/j.jaad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Thielitz A, Gollnick H. Topical retinoids in acne vulgaris: update on efficacy and safety. Am J Clin Dermatol. 2008;9:369–381. doi: 10.2165/0128071-200809060-00003. [DOI] [PubMed] [Google Scholar]
- 5.Campbell JL., Jr A comparative review of the efficacy and tolerability of retinoid-containing combination regimens for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:625–629. [PubMed] [Google Scholar]
- 6.Zaenglein Al. Topical retinoids in the treatment of acne vulgaris. Semin Cutan Med Surg. 2008;27:177–182. doi: 10.1016/j.sder.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Exp Opin Pharmacother. 2009;10:2555–2562. doi: 10.1517/14656560903277228. [DOI] [PubMed] [Google Scholar]
- 8.Tan JKL. Adapalene 0.1% and benzoyl peroxide 2.5%: a novel combination for treatment of acne vulgaris. Skin Ther Lett. 2009;14:4–5. [PubMed] [Google Scholar]
- 9.Langner A, Chu A, Goulden V, et al. A randomized, single-blind comparison of topical clindamycin + benzoyl peroxide and adapalene in the treatment of mild to moderate facial acne vulgaris. Br J Dermatol. 2008;158:122–129. doi: 10.1111/j.1365-2133.2007.08308.x. [DOI] [PubMed] [Google Scholar]
- 10.Langner A, Sheehan-Dare R, Layton A. A randomized, single-blind comparison of topical clindamycin + benzoyl peroxide (Duac®) and erythromycin + zinc acetate (Zineryt®) in the treatment of mild to moderate facial acne vulgaris. J Eur Acad Dermatol Venereol. 2007;21:311–319. doi: 10.1111/j.1468-3083.2006.01884.x. [DOI] [PubMed] [Google Scholar]
- 11.Zouboulis CC, Fischer TC, Wohlrab J, et al. Study of the efficacy, tolerability, and safety of 2 fixed-dose combination gels in the management of acne vulgaris. Cutis. 2009;84:223–229. [PubMed] [Google Scholar]
- 12.Berson DS, Shalita AR. The treatment of acne: the role of combination therapies. J Am Acad Dermatol. 1995;32:S31–S41. doi: 10.1016/0190-9622(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 13.Dhawan SS. Comparison of 2 clindamycin 1%-benzoyl peroxide 5% topical gels used once daily in the management of acne vulgaris. Cutis. 2009;83:265–272. [PubMed] [Google Scholar]