Abstract
The sodium chloride cotransporter (NCC) is the principal salt absorptive pathway in the mammalian distal convoluted tubule (DCT) and is the site of action of thiazide diuretics. Utilizing a mammalian cell model system to assess NCC function we previously demonstrated that Ras Guanyl Releasing Protein 1 (RasGRP1) mediates phorbol ester induced suppression of function and surface expression of NCC in a PKC-independent and ERK1/2 dependent manner. Given that phorbol esters are functional analogues of DAG, this finding suggested a potential physiological regulation of NCC by DAG. The parathyroid hormone (PTH) receptor is a G protein-coupled receptor that is expressed in the DCT and activates PLC resulting in the generation of DAG. Here we demonstrate that PTH suppresses NCC function via a PLC/RasGRP1/ERK pathway. Functional assessment of NCC measuring thiazide-sensitive 22Na+ flux revealed that PTH suppresses NCC function. Inhibition of PLC prevented the suppression of NCC, indicating that PLC was necessary for this effect. Inhibitors of PKC and PKA had no effect on this suppression, but MAPK inhibitors completely prevented the PTH effect. RasGRP1 activates the MAPK pathway though activation of the small G protein Ras. Gene silencing of RasGRP1 prevented the PTH mediated suppression of NCC activity, the activation of the H-Ras isoform of Ras and the activation of ERK1/2 MAPK. This confirmed the critical role of RasGRP1 in mediating the PTH induced suppression of NCC activity through stimulation of the MAPK pathway.
Introduction
The thiazide-sensitive sodium-chloride cotransporter (NCC) is the salt reabsorptive pathway localized to the apical membrane of the mammalian distal convoluted tubule (DCT) that is responsible for reabsorbing 5–10% of the filtered load of sodium [1]. Pharmacological inhibition of NCC by thiazide diuretics decreases blood pressure and increases calcium reabsorption in the kidney [2–4]. NCC has also been shown to play a role in genetic disorders of calcium handling, hypotension, and hypertension [5–7]. Numerous studies have demonstrated that alterations of function of NCC result in changes in the handling of both calcium and sodium [3, 4, 7, 8]. Despite the importance of this cotransporter in human disease, the hormonal regulation of this cotransporter in the mammalian kidney has not been comprehensively studied. The relative difficulty in isolating the DCT for microperfusion studies and the lack of a mammalian DCT cell line that is amenable to physiological studies have been the primary hindrances to investigating the regulation of this cotransporter.
We recently established such a model and used it to examine the suppression of NCC function by diacylglycerol (DAG) analogues (phorbol esters) through activation of Ras Guanyl releasing protein 1 (RasGRP1) [9]. Interestingly, this effect of phorbol esters did not involve Protein Kinase C (PKC). Phorbol esters and DAG can bind and activate five different families of proteins, including the RasGRP family of proteins [10]. Phorbol esters appear to mediate the suppression of NCC through activation of RasGRP1 resulting in activation of Ras [9]. This triggers the Raf/MEK/ERK MAPK cascade of kinases ultimately resulting in decreased surface expression of NCC [9]. These studies were the first steps towards modeling the physiological regulation of NCC by DAG. However, although phorbol esters bind and activate the same five families of proteins to which DAG binds they are not metabolized like DAG, lacking appropriate negative feedback mechanisms. Additionally, they bypass the physiological pathway of an associated hormone, which would traditionally bind a G-protein coupled receptor (GPCR), activating Phospholipase C (PLC) and releasing DAG. Parathyroid Hormone (PTH) is a GPCR present in the DCT and known to stimulate the ERK 1/2 MAPK pathway [11–16]. PTH is known to trigger a proximal tubule acute diuretic effect, and most notably, it regulates calcium reabsorption in the DCT, a known site of uncoupled calcium and sodium transport [17–19]. We theorized that this uncoupling of sodium transport from calcium reabsorption seen in the DCT could be due in part to the action of PTH on NCC. Therefore, we examined the regulation of NCC by PTH. We now present evidence that PTH suppresses NCC function and surface expression through DAG activation of RasGRP1 and the ERK1/2 MAPK pathway.
Methods and Materials
Cell culture
Mouse distal convoluted tubule cells (mDCT cells, gift of Peter Friedman) were plated on 100mm cell culture plates and grown to the desired confluence in growth medium containing 50:50 mix of DMEM/F12, 5% (vol/vol) heat inactivated FBS and 1% PSN (50 units/mL penicillin, 50 µg/mL streptomycin, 100 µg/mL neomycin), at 37°C.
Assessment of NCC Function in mDCT Cells
mDCT cells were seeded in 12 well plates and grown to approximately 90% confluence in a medium containing a 50:50 mix of DMEM/F12, 5% heat-inactivated FBS, and 1% P/S/N. The cells were then incubated in a serum-free, preuptake medium (130 mM Na Gluconate, 2 mM K Gluconate, 1.0 mM Ca Gluconate, 1mM Mg Gluconate, 5 mM HEPES /Tris pH 7.4, 1 mM amiloride, 0.1 mM bumetanide) for 30 minutes. During this time period, the cells were treated with the indicated concentration of PTH. The medium was then changed to a 22Na+-containing medium (140 mM NaCl, 1 mM CaCl, 1 mM MgCl, 5 mM HEPES/Tris pH 7.4, 1 mM amiloride, 0.1 mM bumetanide, 0.1 mM benzamil, 1mM ouabain, and 1 microCi/ml of 22Na+) with or without thiazide (0.1 mM metolazone) and incubated for 20 minutes. Tracer uptake was then stopped via washes with ice-cold wash buffer. Cells were subsequently lysed with 0.1% SDS. Radioactivity was measured via liquid scintillation and protein concentrations of the lysates were determined (Bicinchoninic Acid (BCA) Protein Assay, Pierce). Uptakes were normalized to nmol/mg. Thiazide-sensitive uptake was given by the difference of the uptakes with and without thiazide.
Immunoblotting
SDS PAGE, blotting, and antibody incubations Eighty µg of kidney cortex lysate or mDCT cell lysates were loaded on polyacrylamide gels for SDS PAGE. For detection of RasGRP1and NCC detection a 7.5% polyacrylamide gel was used, while a 12.5% polyacrylamide gel was used for Ras and ERK1/2 detection. Proteins were transferred electrophoretically to PVDF membranes. After blocking with 5% (wt/vol) milk in TBST, the membranes were probed with corresponding primary antibodies (anti-RasGRP1, (dilution 1:200, Santa Cruz), anti-NCC (1:50,000, a gift from Mark Knepper), Ras (1:200, Pierce), H-Ras (1:1000, Santa Cruz), K-Ras (1:200, Santa Cruz), or N-Ras (1:200, Santa Cruz), Phospho-ERK1/2 (1:1000, Cell Signaling) and ERK1/2 (1:200, Santa Cruz)) overnight at 4°C. The blots were washed in TBST. Signal detection for RasGRP1 and Ras was done using IRDye800 goat anti-mouse IgG antibody, IRDye800 rabbit anti-goat IgG antibody, IRDye800 goat anti-rabbit, and IRDye680 goat anti-mouse (Rockland immunochemicals, dilution 1:10,000) and subsequent scanning of the membrane by the Odyssey Infrared Imager (Li-Cor Biosciences). Intensity of the protein bands was analyzed by using Odyssey Infrared Imaging Software (Li-Cor). The secondary antibody used for NCC detection was HRP conjugated goat anti rabbit antibody (Pierce, 1:500). Immunoblots were detected using enhanced chemiluminescence (GE Healthcare Amersham ECL plus Western Blotting Detection System) before exposure to X-ray film. The films were scanned and band densities were quantified using Image J. To facilitate comparisons, the densitometry values from control groups were normalized to 100%. p<0.05 was considered statistically significant
Activated Ras Assay
mDCT cells grown to 80% confluence after transfection with siRNA were incubated for 30 minutes in Opti-MEM medium. During this time period, the cells were treated with 10−7M PTH for 15 minutes. Cells were subsequently lysed and affinity-purified for activated Ras using the EZ-Detect Ras Activation Kit (Pierce). Immunoblotting was then using antibodies for Ras and its major isoforms.
ERK1/2 Phosphorylation Assay
mDCT cells grown to 80% confluence after transfection with siRNA were incubated for 30 minutes in Opti-Mem medium in the presence of various inhibitors as indicated. During this time period, the cells were treated with 10−7M PTH for 15 minutes. Cells were subsequently lysed in 1 ml of RIPA lysis buffer. The lysates were homogenized by sonication on ice. Samples were immunoblotted for phosphorylated ERK1/2 and total ERK1/2.
Immunoprecipitation
Cells for transfection were lysed in 1 ml of lysis buffer (50mM Tris pH 7.5, 150mM NaCl, 1% Nonidet P40, 0.5% SDS, and Complete protease inhibitors (Roche)) and homogenized by sonication on ice. 4 mg of total mDCT cell lysate protein was incubated with 4µg of anti RasGRP1 antibody (Santa Cruz Biotechnology) overnight at 4°C followed by incubation with protein G agarose for 3 hrs at 4°C. The immunocomplexes were centrifuged, washed twice with lysis buffer and resuspended in 30µl of SDS PAGE buffer and boiled to elute the immunoprecipitated proteins.
Gene Silencing
mDCT cells were grown to 80% confluence. siRNA specific for RasGRP1 (Dharmacon) was transfected into these cells using GeneSilencer siRNA Transfection Reagent per protocol (Genlantis). Non-targeting siRNA differing by a single base-pair from the siRNA for RasGRP1 (Dharmacon) was used as a control.
Statistical Analysis
Statistical analysis was performed using the SigmaStat software package (Systat, San Jose, CA). Data was analyzed for statistical significance using ANOVA (Holm-Sidak). A p-value of less than 0.05 was taken as statistically significant.
Results
PTH Decreases NCC Activity
To assess the impact of PTH on NCC function and its role as a potential partner for DAG/PE mediated reductions on NCC activity, mouse DCT cells were used to assess NCC function. These cells, which display the characteristics of the second portion of the distal convoluted tubule, have been used in the past by our laboratory to assess NCC function [9]. Assessment of NCC function was performed on mDCT cells grown in a monolayer by measuring 22Na+ flux as previously described [9]. Figure 1 outlines the effect on PTH on NCC activity. As shown, treatment of mDCT cells with 10−7M PTH reduces NCC activity (Fig. 1a). This effect is significant after 15 minutes (2.1±0.4 nmol/mg/min compared to control, n=6, p<0.01) and persists through 60 minutes. Based on this data, further radiotracer uptakes were conducted after 15 minutes of PTH stimulation. At this time point, PTH demonstrates a clear dose-response with a statistically significant reduction in NCC activity at 10−8M concentrations of PTH (Fig. 1b, n=6, p<0.01). This data demonstrates that PTH causes a significant and sustained decrease in NCC function.
Figure 1. Effect of PTH on NCC Activity.
A. mDCT cells were grown to confluence and then treated with 100nM PTH or vehicle (DMSO) for the indicated times. Cells were subsequently incubated in uptake medium with 22Na+ and either vehicle or 1 mM metolazone. Radioactive uptake was determined as in materials and methods. Thiazide-sensitive uptake was calculated as the difference in radiotracer uptake between the metolazone-containing and the metolazone-free groups. n=6, * p<0.01. B. mDCT cells grown as above were treated with the indicated concentrations of PTH for 15 minutes. Cells were subsequently incubated in uptake medium with 22Na+ and either vehicle or 1 mM metolazone. Radioactive uptake was determined as in materials and methods. Thiazide-sensitive uptake was calculated as the difference in radiotracer uptake between the metolazone-containing and the metolazone-free groups. n=6, * p<0.01.
PTH Actions on NCC are Phospholipase C-Dependent
If, as theorized, PTH acts to decrease NCC activity by activating RasGRP1 and ultimately ERK1/2, then PTH would likely act via its G-protein coupled receptor, activating phospholipase C (PLC) to trigger diacylglycerol (DAG) release. DAG would then bind to RasGRP1, allowing it to initiate the cascade featuring Ras, Raf, MEK1/2, and ultimately ERK1/2 activation. Therefore, to assess the PLC-dependence of the PTH effect, we measured NCC activity in the presence of U73122, a PLC inhibitor. As shown in Figure 2, inhibition of PLC prevented any significant reduction in NCC activity by PTH, providing evidence that PTH acts via PLC.
Figure 2. Effect of PLC Inhibition on the PTH Effect on NCC Activity.
mDCT cells were grown to confluence and then treated with 100nM PTH or vehicle (DMSO, Control) in the presence or absence of a PLC inhibitor, U73122 (PLCi), for 15 minutes. Cells were subsequently incubated in uptake medium with 22Na+ and either vehicle or 1 mM metolazone. Radioactive uptake was determined as in materials and methods. Thiazide-sensitive uptake was calculated as the difference in radiotracer uptake between the metolazone-containing and the metolazone-free groups. n=6, * p<0.01.
Activation of RasGRP1 and the ERK1/2 Pathway is Critical for PTH Effects on NCC
Having demonstrated the PLC dependence of this effect, we next assessed the role of RasGRP1 in PTH mediated decreases in NCC activity. For this, siRNA specific for RasGRP1 was used to silence RasGRP1 expression in mDCT cells. Knockdown efficiency was approximately 90% (Fig. 3a). Radiotracer uptake studies were then performed to measure NCC activity. mDCT cells for which RasGRP1 expression was silenced were unaffected by treatment with PTH, whereas PTH caused a 30% decrease in NCC function in the control group (mDCT cells transfected with non-targeting siRNA) from 2.6±0.2 nmol/mg/min to 1.9±0.3 nmol/mg/min (Fig. 3b, n=6, p<0.01).
Figure 3. Effect of RasGRP1 gene silencing on PTH-mediated effects on NCC Activity.
mDCT cells transfected as above were treated with 100nM PTH or vehicle (DMSO, Control) for 15 minutes. Cells were subsequently incubated in uptake medium with 22Na+ and either vehicle or 1 mM metolazone. Radioactive uptake was determined as in materials and methods. Thiazide-sensitive uptake was calculated as the difference in radiotracer uptake between the metolazone-containing and the metolazone-free groups. n=6, * p<0.01. Inset: mDCT cells were transfected with either non-targeting (NT) or siRNA specific to RasGRP1 (siRG). 48 hours after transfection, cells were lysed and immunoblotted for RasGRP1. Blot shown is representative of 4 experiments.
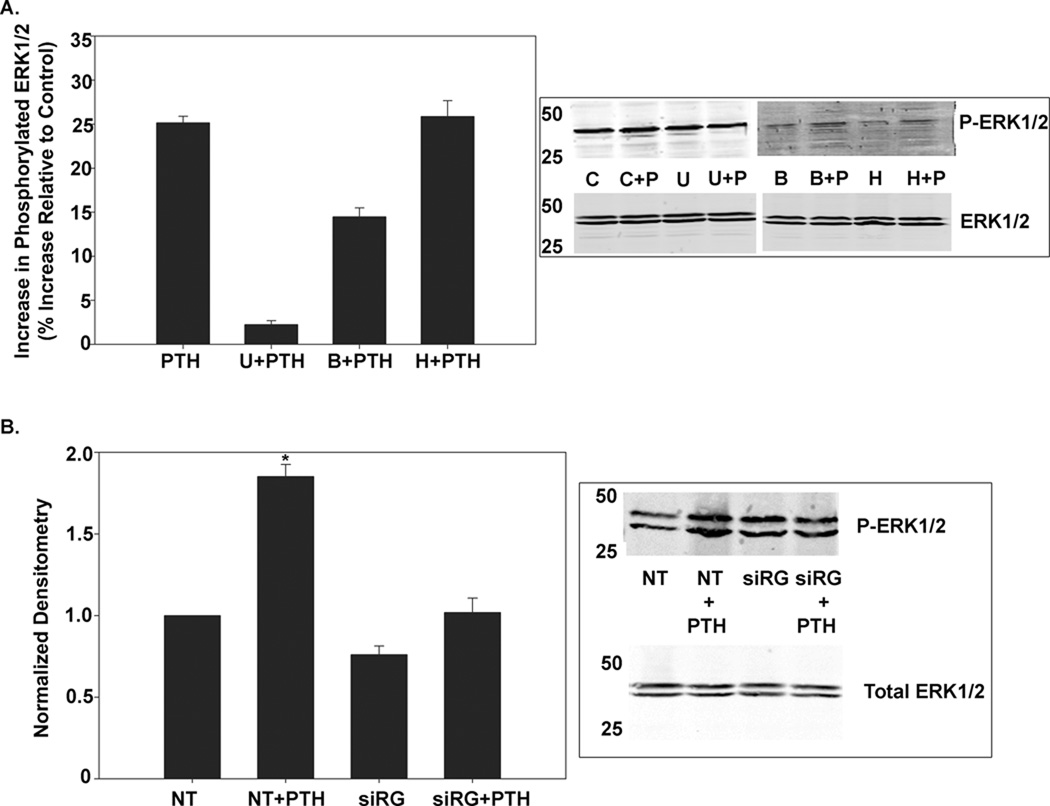
These findings demonstrated that RasGRP1 is essential in PTH modulation of NCC activity. To verify that RasGRP1 then proceeds to initiate a cascade that activates ERK1/2, we first assessed the dependence of the PTH effect upon ERK1/2 activity by treating our cells with the MEK1/2 inhibitor U0126. Measuring 22Na+ uptake in these cells showed that inhibiting the ERK1/2 pathway prevented any reduction in NCC activity with PTH treatment (Fig. 4). Inhibition of PKA or PKC did not change the PTH effect (Fig. 4).
Figure 4. Effect of ERK1/2, PKC, and PKA Inhibition on PTH-medaited effects on NCC Activity.
A. mDCT cells were grown to confluence and then treated with 100nM PTH or vehicle (DMSO, Control) in the presence or absence of an MEK1/2 inhibitor, U0126, for 15 minutes. Cells were subsequently incubated in uptake medium with 22Na+ and either vehicle or 1 mM metolazone. Radioactive uptake was determined as in materials and methods. Thiazide-sensitive uptake was calculated as the difference in radiotracer uptake between the metolazone-containing and the metolazone-free groups. n=6, * p<0.01. B. mDCT cells were grown to confluence and then treated with 100nM PTH or vehicle (DMSO, Control) in the presence or absence of either the PKC inhibitors BIM (B, B+T) and Gö6976 (G, G+T), the PKA inhibitor H89 (H, H+T) or control (DMSO vehicle, C) for 15 minutes. Cells were subsequently incubated in uptake medium with 22Na+ and either vehicle or 1 mM metolazone. Radioactive uptake was determined as in materials and methods. Thiazide-sensitive uptake was calculated as the difference in radiotracer uptake between the metolazone-containing and the metolazone-free groups. n=6, * p<0.01.
An examination of activation of Ras by PTH reinforces these findings. Using a pulldown assay for activated Ras-GTP followed by Western blotting for Ras, we found that PTH enhances activated Ras-GTP by 32.4±4.0% compared to control (Fig. 5, n=4, p<0.05). Consistent with our previous findings using phorbol esters, this increase was more prominent in the H-Ras isoform, with PTH enhancing H-Ras-GTP by 87.1±26% compared to control (n=4, p<0.05). Again, this effect was mediated by Ras-GRP1, as silencing RasGRP1 expression resulted in no significant effect on Ras or H-Ras activation with the addition of PTH.
Figure 5. Ras Activation in PTH-Treated mDCT Cells After Gene-Silencing of RasGRP1.
mDCT cells transfected with siRNA for RasGRP1 (siRG) or non-targeting siRNA (NT) were treated for 15 minutes with 100nM PTH or vehicle (DMSO). Cells were then lysed and a Ras-GTP pulldown using the Raf binding domain was performed, followed by immunoblotting for total Ras or H-Ras (21kD). Shown is a representative immunoblot and densitometry (normalized to total Ras). n=4 *p<0.05 as compared to NT.
RasGRP1 Predominately Contributes to PTH Activation of ERK1/2
Previous reports have implicated PKA and PKC in PTH-mediated activation of ERK1/2 [11, 13, 16]. To assess the role of these signaling cascades in our system, mDCT cells were treated for 15 minutes with inhibitors of MEK1/2, PKA, or PKC in the presence or absence of PTH. The resulting cell lysates were immunoblotted as with total ERK1/2 antibodies and with antibodies specific to the active, phosphorylated, ERK1/2 to assess for ERK1/2 activity. As shown in Figure 6, inhibition of MEK1/2 severely decreased ERK1/2 phosphorylation, with a 91±2% reduction (n=4, p<0.05). Inhibition of PKA did not affect ERK1/2 activation by PTH. Inhibiting PKC, however, caused a 42±4% decrease in ERK1/2 phosphorylation (n=4, p<0.05). Conversely, a sharper decrease in ERK1/2 phosphorylation is seen with gene-silencing of RasGRP1, which triggers a 60±6% decrease (n=4, p<0.05). This suggests that while PKC triggers enhanced ERK1/2 activity, RasGRP1 is the predominate pathway by which ERK1/2 is activated in our system. Moreover, as seen in Figure 3 and 4, only the RasGRP1 pathway plays a significant role in PTH-mediated effects on NCC activity. Taken together, these data suggest that PTH stimulation triggers a decrease in NCC activity by activating ERK1/2 in a pathway mediated by RasGRP1.
Figure 6. ERK Phosphorylation in mDCT Cells Treated with PTH.
A. mDCT cells grown as above were treated with 100nM PTH or vehicle (DMSO) for 15 minutes in the presence or absence of a MEK inhibitor (U), PKC inhibitor (B), or PKA inhibitor (H). Cells were then lysed and immunoblotting for phosphorylated or total ERK1/2 was performed. ERK1 appears at 44kD. ERK2 appears at 42kD. n=4, * p<0.05 as compared to control. B. mDCT cells transfected with siRNA for RasGRP1 (siRG) or non-targeting siRNA (NT) were treated for 15 minutes with 100nM PTH or vehicle (DMSO). Cells were then lysed and immunoblotting for phosphorylated or total ERK1/2 was performed. ERK1 appears at 44kD. ERK2 appears at 42kD. n=4, * p<0.05 as compared to NT.
Discussion
Our previous data suggested that the second messenger RasGRP1 was an important mediator of DAG-stimulated suppression of NCC activity and surface expression [9]. Further examination revealed that RasGRP1 activates the H-Ras isoform of Ras resulting in the initiation of the MAPK ERK1/2 cascade of kinases. Activation of the terminal kinase in this pathway, ERK1/2 required RasGRP1. Silencing of RasGRP1 expression and blockade of ERK1/2 activation prevented the functional effect of phorbol esters on NCC [9]. This PKC-independent effect of DAG analogues on NCC engendered an in depth examination of potential upstream “first messengers” or hormones that may trigger this DAG/PE effect. The PTH receptor is expressed in the DCT as well as mDCT cells and there is significant data on the cell signaling initiated by activation of this receptor in the DCT [11, 12, 14–16]. In this segment, PTH is known to not induce calcium signaling but rather acts by activating ERK1/2, making it a possible regulator of NCC function in the DCT [11]. However, effects of PTH on NCC function have not been rigorously examined. Given the uncertainty of NCC regulation by PTH and our data indicating DAG analog suppression of NCC activity we decided to examine this process.
The work described here does indeed demonstrate an effect of PTH on NCC activity in our model, with PTH triggering a 40% reduction on NCC function. This reduction is NCC activity was accompanied by an increase in Ras activitation, with the predominant increase in the isoform H-Ras, as previously seen in PE stimulation [9]. Chemical inhibition of PLC and ERK1/2 prevented PTH action and silencing of RasGRP1 expression rendered PTH ineffective and negated any observed increase in Ras or H-Ras activation. Therefore, PTH does appear to act through PLC, resulting in DAG activation of RasGRP1 and suppression of NCC function.
Prior to this there were no studies examining the effects of PTH on NCC or thiazide-sensitive sodium reabsorption. A previous study in mDCT cells found no change in total sodium reabsorption with PTH treatment. However these studies were done on cells in suspension and presumably included sodium uptake from the Na+K+ATPase pump, ENaC and NCC. [20, 21]. [21]. A microperfusion study of parathyroidectomized animals examining the DCT and CNT didn’t reveal an overall change in sodium reabsorption with PTH [20]. These studies were limited in their ability to evaluate NCC activity as thiazide-sensitive sodium reabsorption was not examined and because of the difficulty in isolating the DCT for microperfusion, isolated DCT sodium handling was not examined. Furthermore, the relatively low PTH doses used in the study did not maximally enhance calcium reabsorption, and the authors state that they cannot rule out an effect on sodium reabsorption with higher doses of PTH [20]. As compensatory alteration of downstream segmental tubular function based on changes in upstream tubular function is a well-established phenomenon in kidney physiology, without isolated segmental data it is difficult to infer the impact on the individual sodium transport pathways along the distal tubule.
The NCC-specific effect of PTH on NCC that we report could have a number of physiologic roles. Overall, PTH has a number of known effects on the kidney. PTH reduces the plasma flow rate and glomerular ultrafiltration coefficient [22]. At the tubular level, PTH is known to have a diuretic effect [17–19]. Infusion of PTH into rats resulted in a decrease in proximal tubule sodium hydrogen exchanger 3 (NHE3) expression and, to a lesser degree, the sodium-potassium-chloride exchange in the thick ascending limb of the Loop of Henle (NKCC2) [18]. Because of this, the diuretic effect has generally been attributed to the proximal tubule, but a 40% reduction in NCC activity could certainly contribute to this effect. It is likely that PTH-induced reductions in NCC activity result in the negative sodium balance (diuresis) seen with elevated levels of PTH. In addition, there is considerable evidence at the whole animal and tubular level that changes in function of NCC result in changes in calcium handling in the DCT [3, 4, 7, 8]. Therefore, a role for PTH in regulating NCC activity may be another mechanism by which PTH regulates calcium. A PTH effect on NCC sodium transport would therefore promote calcium reabsorption. Further work is necessary to explore the role of NCC in these effects
The central role of RasGRP1 activation is of particular interest. PTH receptor activation in the DCT has previously been shown to result in the stimulation of PKC and PKA, but neither of these pathways appears to be significantly involved in PTH-mediated affects on NCC [11, 13, 16]. PKC mediates a portion of the PTH-induced ERK1/2 activation, but PKC’s activation of ERK1/2 does not appear to be involved in the regulation of NCC. Inhibition of PKA or PKC did not change the PTH effect on NCC while silencing of RasGRP1 expression or inhibition of ERK1/2 prevented any PTH-mediated decreases in NCC activity. In summary, this work demonstrates that PTH acts as a regulator of NCC activity. The effect is dependent upon PLC-mediated DAG release, which goes on to trigger RasGRP1, Ras, MEK, and ERK1/2 activation.
Acknowledgements
We thank Eugene Chang and Mark Musch for their advice and discussions. This work was supported by grants from the National Institutes of Health R01 DK-085097 (to R.H.) and K08 DK081728 (to B.K).
Abbreviations
- NCC
sodium chloride cotransporter
- DCT
distal convoluted tubule
- RasGRP1
Ras Guanyl Releasing Protein 1
- DAG
diacylglycerol
- GPCR
G-protein coupled receptor
- PLC
Phospholipase C
- PKC
Protein Kinase C
- PTH
Parathyroid Hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to report.
References
- 1.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, et al. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem. 1994;269:17713–17722. [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 3.Lemann J, Jr., Gray RW, Maierhofer WJ, Cheung HS. Hydrochlorothiazide inhibits bone resorption in men despite experimentally elevated serum 1,25-dihydroxy vitamin D concentrations. Kidney Int. 1985;28:951–958. doi: 10.1038/ki.1985.223. [DOI] [PubMed] [Google Scholar]
- 4.Gesek FA, Friedman PA. Mechanism of calcium transport stimulated by chlorothiazide in mouse distal convoluted tubule cells. The Journal of clinical investigation. 1992;90:429–438. doi: 10.1172/JCI115878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Shaughnessy KM, Karet FE. Salt handling and hypertension. J Clin Invest. 2004;113:1075–1081. doi: 10.1172/JCI21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon DB, Nelson-Williams C, Johnson Bia M, Ellison D, Karet FE, Morey Molina A, et al. Gitelman's variant of Barter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 7.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, et al. Human Hypertension Caused by Mutations in WNK Kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 8.Nicolet-Barousse L, Blanchard A, Roux C, Pietri L, Bloch-Faure M, Kolta S, et al. Inactivation of the Na-Cl co-transporter (NCC) gene is associated with high BMD through both renal and bone mechanisms: analysis of patients with Gitelman syndrome and Ncc null mice. J Bone Miner Res. 2005;20:799–808. doi: 10.1359/JBMR.041238. [DOI] [PubMed] [Google Scholar]
- 9.Ko B, Joshi LM, Cooke LL, Vazquez N, Musch MW, Hebert SC, et al. Phorbol ester stimulation of RasGRP1 regulates the sodium-chloride cotransporter by a PKC-independent pathway. Proceedings of the National Academy of Sciences. 2007;104:20120–20125. doi: 10.1073/pnas.0709506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Springett GM, Kawasaki H, Spriggs DR. Non-kinase second-messenger signaling: new pathways with new promise. Bioessays. 2004;26:730–738. doi: 10.1002/bies.20057. [DOI] [PubMed] [Google Scholar]
- 11.Sneddon WB, Liu F, Gesek FA, Friedman PA. Obligate mitogen-activated protein kinase activation in parathyroid hormone stimulation of calcium transport but not calcium signaling. Endocrinology. 2000;141:4185–4193. doi: 10.1210/endo.141.11.7792. [DOI] [PubMed] [Google Scholar]
- 12.Yang T, Hassan S, Huang YG, Smart AM, Briggs JP, Schnermann JB. Expression of PTHrP, PTH/PTHrP receptor, and Ca(2+)-sensing receptor mRNAs along the rat nephron. Am J Physiol. 1997;272:F751–F758. doi: 10.1152/ajprenal.1997.272.6.F751. [DOI] [PubMed] [Google Scholar]
- 13.Sneddon WB, Yang Y, Ba J, Harinstein LM, Friedman PA. Extracellular signalregulated kinase activation by parathyroid hormone in distal tubule cells. American Journal of Physiology - Renal Physiology. 2007;292:F1028–F1034. doi: 10.1152/ajprenal.00288.2006. [DOI] [PubMed] [Google Scholar]
- 14.Riccardi D, Lee WS, Lee K, Segre GV, Brown EM, Hebert SC. Localization of the extracellular Ca(2+)-sensing receptor and PTH/PTHrP receptor in rat kidney. Am J Physiol. 1996;271:F951–F956. doi: 10.1152/ajprenal.1996.271.4.F951. [DOI] [PubMed] [Google Scholar]
- 15.Dunlay R, Hruska K. PTH receptor coupling to phospholipase C is an alternate pathway of signal transduction in bone and kidney. Am J Physiol. 1990;258:F223–F231. doi: 10.1152/ajprenal.1990.258.2.F223. [DOI] [PubMed] [Google Scholar]
- 16.Friedman PA, Coutermarsh BA, Kennedy SM, Gesek FA. Parathyroid hormone stimulation of calcium transport is mediated by dual signaling mechanisms involving protein kinase A and protein kinase C. Endocrinology. 1996;137:13–20. doi: 10.1210/endo.137.1.8536604. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Norian JM, Magyar CE, Holstein-Rathlou N-H, Mircheff AK, McDonough AA. In vivo PTH provokes apical NHE3 and NaPi2 redistribution and Na-K-ATPase inhibition. American Journal of Physiology - Renal Physiology. 1999;276:F711–F719. doi: 10.1152/ajprenal.1999.276.5.F711. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Li C, Kwon TH, Miller RT, Knepper MA, Frokiaer J, et al. Reduced expression of renal Na+ transporters in rats with PTH-induced hypercalcemia. Am J Physiol Renal Physiol. 2004;286:F534–F545. doi: 10.1152/ajprenal.00044.2003. [DOI] [PubMed] [Google Scholar]
- 19.Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev. 2000;80:277–313. doi: 10.1152/physrev.2000.80.1.277. [DOI] [PubMed] [Google Scholar]
- 20.Costanzo LS, Windhager EE. Effects of PTH, ADH, and cyclic AMP on distal tubular Ca and Na reabsorption. Am J Physiol. 1980;239:F478–F485. doi: 10.1152/ajprenal.1980.239.5.F478. [DOI] [PubMed] [Google Scholar]
- 21.Gesek FA, Friedman PA. On the mechanism of parathyroid hormone stimulation of calcium uptake by mouse distal convoluted tubule cells. The Journal of clinical investigation. 1992;90:749–758. doi: 10.1172/JCI115947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schor N, Ichikawa I, Brenner BM. Mechanisms of action of various hormones and vasoactive substances on glomerular ultrafiltration in the rat. Kidney Int. 1981;20:442–451. doi: 10.1038/ki.1981.160. [DOI] [PubMed] [Google Scholar]