Summary
Mice deficient in caspase-8, FADD, or cFLIP, present defects in yolk sac vascularization and embryonic lethality at E10.5. Ablation of RIPK3, a kinase that promotes a form of necrotic cell death, has recently been shown to rescue embryonic lethality in caspase-8 deficient animals. Here we show that while FADD, RIPK3 double knockouts develop normally, the lethal effects of cFLIP deletion are not rescued by RIPK3 deficiency. Remarkably, embryos lacking FADD, cFLIP, and RIPK3 develop normally. Distinct regions of apoptosis were observed in E9.5 FLIP, RIPK3 double knockout embryos, but not in caspase-8−/− or FADD−/− embryos. In vitro studies using death receptor stimulation show that the FADD-caspase-8-cFLIPL complex blocks RIPK3-dependent necrosis, while cFLIPL blocks RIPK3-independent apoptosis promoted by the FADD-caspase-8 complex. Together, these results suggest the cross-regulation of two distinct processes in development and death-receptor signaling: RIPK3-dependent signaling (including necrosis) controlled by the enzymatic activity of the FADD-caspase-8-cFLIPL complex, and cFLIPL control of RIPK3-independent apoptosis by FADD-caspase-8.
Introduction
Apoptosis, or programmed cell death, is an essential process for development and homeostasis of multicellular organisms. Insight into the important roles apoptosis plays in these processes has come from knockout animals ablated for genes in cell death pathways (Weinlich et al., 2011). While knockout animals of pro-apoptotic genes such as Apaf-1, caspase-9, and caspase-3 all demonstrate phenotypes consistent with failure to eliminate cells, animals ablated for key components of the death receptor pathway suffer early embryonic lethality more consistent with a failure of development (Green et al., 2011; Weinlich et al., 2011). Embryos deficient in caspase-8, the adaptor molecule FADD, or the non-catalytically active caspase-8 homologue cFLIP, die around E10.5 (Varfolomeev et al., 1998; Yeh et al., 2000; Yeh et al., 1998; Zhang et al., 1998) with similar defects in vascularization of the yolk sac (Oberst and Green, 2011; Sakamaki et al., 2002), which suggests that these proteins perform significant non-apoptotic roles in development. Caspase-8, FADD, and cFLIPL (herein called “FLIP”) have also been implicated cell cycle regulation and NF-kB activation (Budd et al., 2006; Oberst and Green, 2011; Tourneur and Chiocchia, 2010) in various tissues post-development. Recent work has shown that caspase-8 deficiency can be rescued by concurrent deletion of RIPK3 (Kaiser et al., 2011; Oberst et al., 2011), a kinase that promotes a form of programmed necrotic cell death (sometimes called “necroptosis” (Galluzzi et al., 2012)), indicating a more specific and limited role for caspase-8 in development. Biochemical evidence demonstrates that a caspase-8-FLIP heterodimer acts to inhibit the function of RIPK3 in vitro, suggesting that this complex might play the same role in vivo (Oberst et al., 2011). In this work, we address the survival functions of FADD and FLIP in the context of RIPK3-dependent necrosis and apoptosis during development.
Results and Discussion
FADD−/− embryonic lethality is rescued by RIPK3 ablation
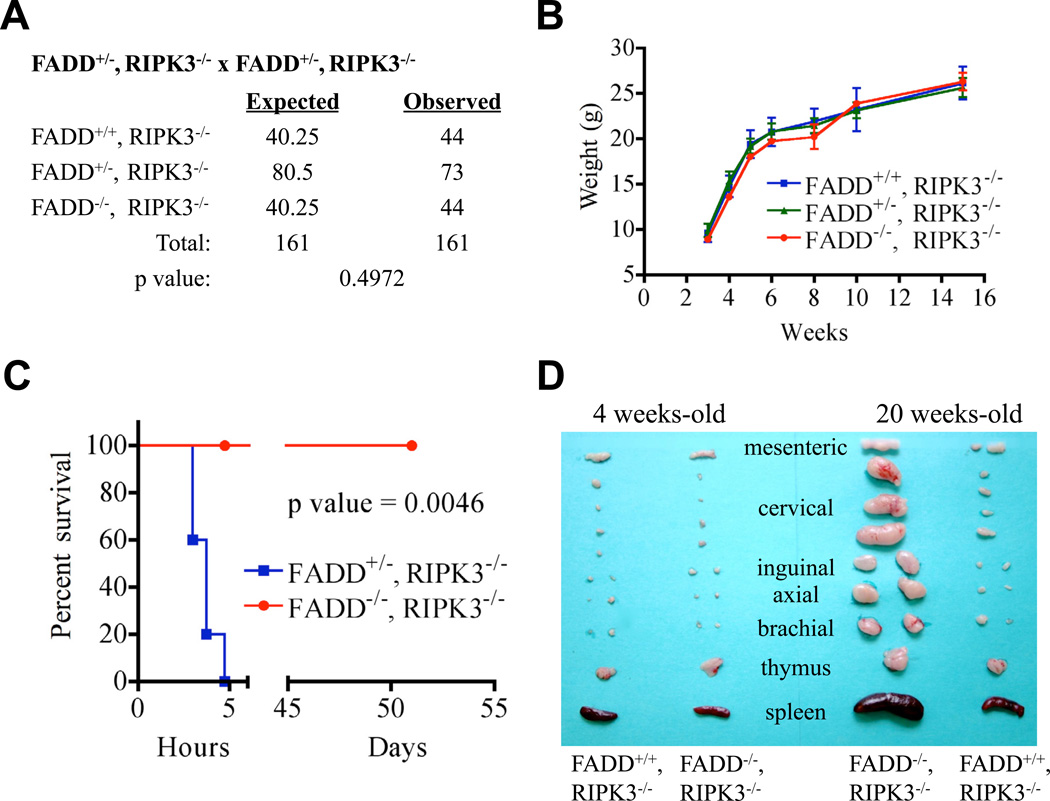
The activation of caspase-8 depends on the adapter molecule FADD (Oberst and Green, 2011). We therefore hypothesized that deletion of RIP kinases might rescue the embryonic lethality of FADD−/− mice similar to caspase-8−/− animals (Kaiser et al., 2011; Oberst et al., 2011). However, in one study, the lethal effects of FADD deletion were only partially rescued by ablation of RIPK1 (Zhang et al., 2011), as these animals died perinatally, as is also seen in RIPK1−/− mice (Kelliher et al., 1998). Because RIPK1 can promote necrosis that depends on RIPK3 (Cho et al., 2009), we asked if deletion of RIPK3 rescues development in FADD−/− mice. We found that FADD, RIPK3 double knockout (DKO) mice were weaned at expected frequencies (Figure 1A) and grew with kinetics identical to those of their FADD+/+ and +/− littermates (Figures 1B, S1A). As seen in caspase-8, RIPK3 DKO mice (Kaiser et al., 2011; Oberst et al., 2011), FADD, RIPK3 DKO mice were completely resistant to lethal hepatic injury induced by injection of agonistic anti-CD95 antibody (Ogasawara et al., 1993) (Figures 1C, S1B–D). Also, as seen in caspase-8, RIPK3 DKO mice, FADD, RIPK3 DKO animals accumulated over time a population of B220+CD3+ T lymphocytes (Figure S1E), resulting in a lymphoaccumulative disease (Figure 1D), resembling that seen in mice or humans lacking CD95 or its ligand (Wilson et al., 2009). Therefore, like caspase-8 (Kaiser et al., 2011; Oberst et al., 2011), FADD is required for prevention of RIPK3-mediated embryonic lethality and for the function of CD95.
Figure 1. FADD−/−, RIPK3−/− mice are viable and overtly normal, functionally deficient for FADD and display severe progressive lymphoaccumulation.
(A) Expected and observed frequency of FADD status in offspring from crosses of FADD+/−, RIPK3−/− animals. The resulting offspring were genotyped at weaning (p=0.4972). (B) Plot of weight in grams of littermate FADD+/+, RIPK3−/−, FADD+/−, RIPK3−/−, and FADD−/−, RIPK3−/− animals. (C) Effect of anti-CD95 in vivo. 15 µg of agonist anti-CD95 antibody Jo2 was injected intravenously into FADD+/−, RIPK3−/− or FADD−/−, RIPK3−/− animals. Animals were monitored and euthanized when moribund. (D) Lymphoid organs removed from young (4 wk) and old (20 wk) littermate mice of the indicated genotypes. Scale bar is 1 cm. See also Figure S1.
FADD, RIPK1 DKO mice display defects in B cell activation-induced proliferation (Zhang et al., 2011). Because FADD had been previously suggested to play a role in cell cycle progression (Tourneur and Chiocchia, 2010), we examined lymphocytes from young (5 wk) FADD, RIPK3 DKO mice, and observed no differences in activation-induced proliferation of T or B cells between these and FADD sufficient cells (Figures S1F–G). Therefore, the defect observed in the FADD, RIPK1 DKO (Zhang et al., 2011) is likely to be a consequence of RIPK1-deficiency, rather than due to the absence of FADD.
Elimination of RIPK3 does not rescue FLIP-deficient embryos
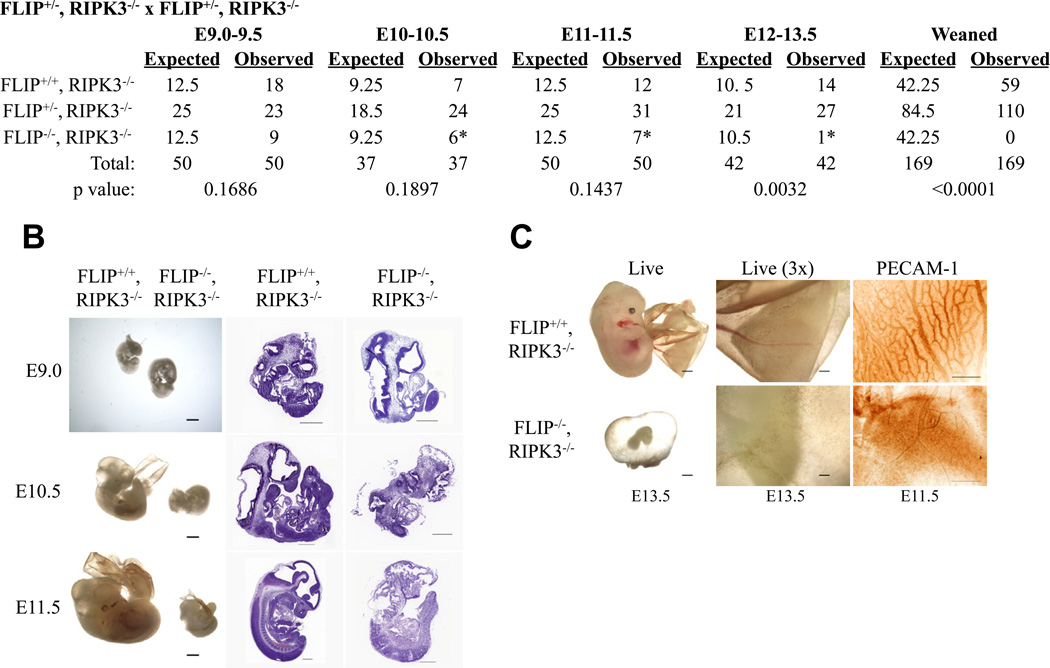
To investigate the role of RIPK3 in the embryonic lethality observed in FLIP deficient mice, we crossed FLIP+/−, RIPK3−/− animals (Figure 2A). No FLIP−/−, RIPK3−/− mice were detected at weaning. Examination of embryos from timed matings showed developmental abnormalities beginning around E10–10.5 (Figures 2A, 2B). As seen in animals lacking caspase-8, FADD, or FLIP (Sakamaki et al., 2002; Varfolomeev et al., 1998; Yeh et al., 2000; Yeh et al., 1998; Zhang et al., 1998), the vasculature of the yolk sacs of FLIP, RIPK3 DKO mice showed severe defects, readily observable in live embryos or by staining for the endothelial marker, PECAM-1 (Newman et al., 1990) (Figure 2C).
Figure 2. FLIP−/−, RIPK3−/− mice are embryonic lethal.
(A) Expected and observed frequency of FLIP status in offspring from crosses of FLIP+/−, RIPK3−/− animals. The resulting offspring were genotyped at the indicated developmental time points or at weaning. Asterisks reflect malformed embryos. (B) Embryos (left) and H&E stained sections of fixed embryos (right) of the indicated genotypes at the indicated timepoints. Representative images are presented, n ≥ 3 for each genotype. Scale bars for images on left are 1 mm and for sections on right are 500 µm. (C) Embryos of the indicated genotypes at E13.5 (left and middle) showing vascularization of the embryos and yolk sacs. Left scale bar is 1 mm, middle scale bar is 333 µm. Right panels show PECAM-1 immunostaining on sections from E11.5 embryos of the indicated genotypes. Representative images are presented, n ≥ 3 for each genotype. Scale bar is 250 µm.
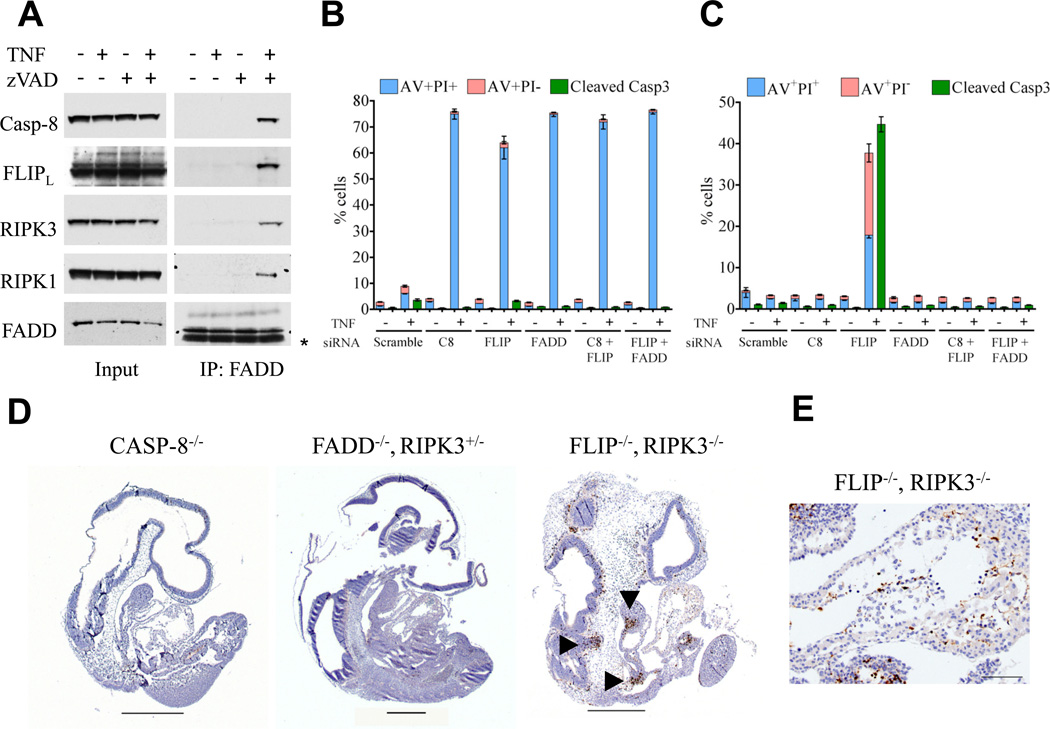
Formally, the ability of RIPK3 ablation to rescue development of caspase-8−/− (Kaiser et al., 2011; Oberst et al., 2011) or FADD−/− mice (Figure 1), but not that of FLIP−/− mice (Figure 2), indicates that FLIP has a function distinct from those of FADD and caspase-8 in embryogenesis. To gain more insight into these interrelated functions, we turned to an established in vitro system in which ligation of TNF Receptor-1 induces RIPK1-RIPK3-dependent necrosis that is inhibited by the activity of the caspase-8-FLIP heterodimer (Oberst et al., 2011). Cells were treated with TNF with or without the caspase inhibitor zVAD-fmk. In the presence of the inhibitor, TNF induced the formation of a complex, identified upon immunoprecipitation of FADD, containing FADD, caspase-8, FLIP, RIPK1, and RIPK3 (Figure 3A). In contrast, in the absence of caspase inhibition, no components of the complex were found associated with FADD (Figure 3A). This is consistent with findings suggesting that caspase-8 (presumably in the form of the caspase-8-FLIP heterodimer) cleaves RIPK1 and RIPK3 (Feng et al., 2007; Rebe et al., 2007). Furthermore, additional findings suggest that caspase-8-FLIP is rapidly degraded in cells (Feoktistova et al., 2011) and therefore this instability may extend to the entire complex to control both apoptosis and necrosis (Green et al., 2011).
Figure 3. FLIP-deficient cells and embryos undergo apoptosis in the absence of RIPK3.
(A) Immunoprecipitation of a FADD containing complex from SVEC4–10 cells treated with or without 20 ng/mL TNF and 50 µM zVAD-fmk. NIH3T3 cells, with (B) or without (C) stably expressed RIPK3 were transfected with the indicated siRNAs for 48 hr, followed by treatment with TNF for 9 hr. At harvest, cultures were split and cell death was assessed by AnnexinV-APC and propidium iodide (PI) staining (with AnnexinV+,propidium iodide− as apoptotic [pink] and AnnexinV+,propidium iodide+ as necrotic or late apoptotic [blue]), while the presence of cleaved caspase-3 (green) was assessed by intracellular staining. (D) Cleaved caspase-3 immunostaining in sections from E9.5–10 embryos of the indicated genotypes. Arrowheads mark areas of focal cleaved caspase-3 staining. Scale bars are 500 µm. (E) Cleaved caspase-3 immunostaining in heart section from an E9.5 FLIP−/−, RIPK3−/− embryo. Scale bar is 100 µm. For D and E, representative images are presented, n ≥ 3 for each genotype. See also Figure S2.
Ablation of RIPK3 in FLIP-deficient cells and embryos converts death from necrosis to apoptosis
We then examined the interplay of FLIP and FADD in cells with or without RIPK3. In the presence of RIPK3, knockdown of FLIP, FADD, or both sensitized cells to TNF-induced cell death with characteristics of necrosis (i.e., rapid loss of plasma integrity as detected by propidium iodide (PI) staining without downstream effector caspase activation) (Figures 3B, S2A–B). However, in the absence of RIPK3, knockdown of FLIP sensitized cells to TNF-induced apoptosis (Annexin V+, PI− and downstream effector caspase activation as detected by intracellular cleaved caspase-3 staining), while knockdown of FADD did not (Figures 3C, S2A–B). Importantly, knockdown of both FADD and FLIP did not sensitize cells to apoptosis (Figures 3C, S2A–B). These results are consistent with the interpretation, supported here by caspase-8 knockdown (Figure 3C) and extensively by other studies (Wilson et al., 2009), that FADD promotes caspase-8-mediated apoptosis that is inhibited by FLIP. However, in the presence of RIPK3, loss of function of the FADD-caspase-8-FLIP complex results in RIPK3-dependent necrosis (Figure 3B).
Based on these observations, we reasoned that in embryos, a lack of both FLIP and RIPK3 would result in uncontrolled activation of caspase-8, as we observed (Figure 3C) and as described in cell lines (Oberst et al., 2011). We therefore examined apoptosis in embryos at E9.5–10, lacking caspase-8, FADD, or both FLIP and RIPK3 (Figures 3D–E, S2C–D). Caspase-8−/− embryos at this stage did not display obvious apoptotic cell death, consistent with previous observations (Sakamaki et al., 2002), and this was also the case for embryos lacking FADD (Yeh et al., 1998) (Figures 3D,S2C–D). In contrast, FLIP, RIPK3 DKO embryos showed apoptosis in the endothelium, the first branchial arch, and other regions (Figure 3DE). Thus, while embryonic lethality in caspase-8−/− or FADD−/− animals may occur due to unregulated RIPK3-necrosis (supported by the survival of caspase-8, RIPK3 DKO (Kaiser et al., 2011; Oberst et al., 2011) and FADD, RIPK3 DKO mice [Figure 1A]), apoptotic cell death may be responsible for lethality in FLIP, RIPK3 DKO mice.
Mice lacking FADD, FLIP, and RIPK3 are developmentally normal
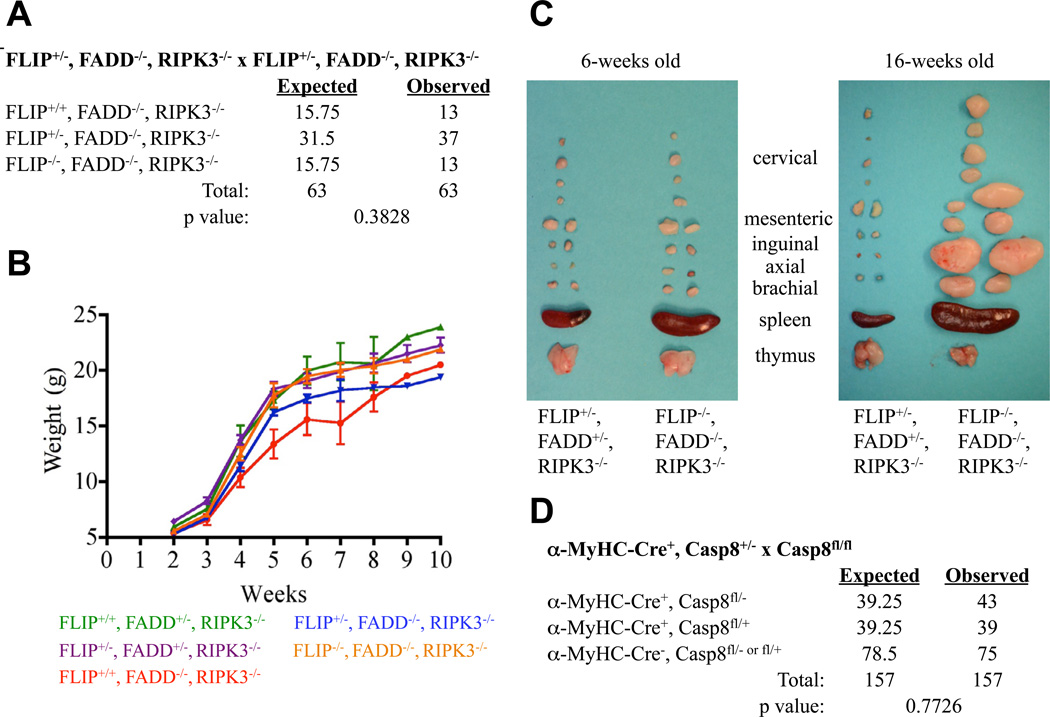
Because knockdown of FADD protected cells lacking FLIP and RIPK3 from TNF-induced apoptosis (Figure 3C), we examined the consequences of the triple knockout (TKO) of FADD, FLIP, and RIPK3. Using two different breeding strategies, we found that animals lacking all three genes were born at expected frequencies (Figures 4A, S3A–D). Here we have found a novel situation in which a combination of two lethal genotypes (FADD KO; FLIP, RIPK3 DKO) yields a TKO with normal development. Young TKO animals were grossly normal (Figure S3B), and gained weight with age indistinguishably from littermates (Figure 4B). Further, because FADD (Tourneur and Chiocchia, 2010) and FLIP (Budd et al., 2006) have been implicated in proliferation and activation of NF-kB in activated T cells, we examined proliferation and phosphorylation of p65RelA in activated TKO T lymphocytes. No abnormalities in activation-induced proliferation (Figure S3E) or activation of NF-kB (Figure S3F) were observed. Finally, as in caspase-8, RIPK3 DKO (Kaiser et al., 2011; Oberst et al., 2011) and FADD, RIPK3 DKO (Figure 1D) mice, these animals accumulated over time a population of B220+CD3+ T cells (Figure S3G), resulting in severe lymphoaccumulation (Figure 4C).
Figure 4. FLIP−/−, FADD−/−, RIPK3−/− mice are viable with overtly normal development, but display severe progressive lymphoaccumulation.
(A) Expected and observed frequency of FLIP and FADD status in offspring from crosses of FLIP+/−, FADD−/−, RIPK3−/− animals. The resulting offspring were genotyped for FLIP status at weaning (p=0.3828). (B) Plot of weight in grams of littermate animals of the indicated genotypes at different ages. (C) Lymphoid organs removed from young (6 wk) and old (16 wk) littermate mice of the indicated genotypes. Scale bar is 1cm. (D) Expected and observed frequency of caspase-8 status in offspring from crossing of αMyHC-Cre+Casp8+/− with Casp8flox/flox animals. See also Figures S3 and S4.
The normal development of FADD, FLIP, RIPK3 TKO mice provides strong support for the emerging model (Figure S4A) that the FADD-caspase-8-FLIP complex inhibits RIPK3-mediated embryonic lethality while not promoting apoptosis in affected cells. In the absence of FLIP, the function of FADD promotes lethality, most likely via the action of caspase-8 (Oberst and Green, 2011). Unfortunately, due to the close linkage of FLIP and caspase-8, this could not be readily tested in vivo. However, this idea is strongly supported by the normal development of caspase-8, RIPK3 DKO mice (Kaiser et al., 2011; Oberst et al., 2011), but not FLIP, RIPK3 DKO mice (Figure 2). Further, because FADD, RIPK1 (Zhang et al., 2011) and FADD, RIPK3 (Figure 1) DKO mice develop normally in utero, both RIPK1 and RIPK3 mediate embryonic lethality, most likely by the process of RIPK1- RIPK3-mediated programmed necrosis (Weinlich et al., 2011).
Mice lacking FADD (Yeh et al., 1998; Zhang et al., 1998), FLIP (Yeh et al., 2000), or caspase-8 (Sakamaki et al., 2002; Varfolomeev et al., 1998) are embryonic lethal around E10.5 due to, at least partially, a failure in yolk sac vascularization, an effect we also observed in FLIP, RIPK3 DKO mice (Figure 2C). According to the model (Figure S4A), this lethality in FLIP, RIPK3 DKO mice is predicted to be caused by FADD-caspase-8-dependent apoptosis. Indeed, we observed early, focal apoptosis in endothelium and other structures in these embryos that was not observed at this stage in embryos lacking caspase-8 or FADD (Figures 3D–E,S2C–D). An early study, using mixed chimeras, suggested that embryonic lethality in FADD−/− mice correlates with the null allele associated with the region of the embryonic heart (Yeh et al., 1998). We found, however, that conditional deletion of caspase-8 in the heart (alpha-myosin heavy chain-Cre (Agah et al., 1997)) did not cause embryonic lethality (Figures 4D, S4B), and weaned animals showed no differences in body weight (WT 22.6 ± 6.4 g. vs. KO 25.2 ± 4.3 g., n = 5) or heart weight (WT 0.14 ± 0.02 g. vs. KO 0.14 ± 0.03 g. n = 5). Previous studies using conditional deletion of caspase-8, however, have shown that E10.5 lethality is observed upon ablation of this gene in endothelium (TIE1-Cre) (Kang et al., 2004). Caspase-8 ablation in liver (Albumin-Cre) (Kang et al., 2004), skin (Keratin 5 or 14-Cre) (Kovalenko et al., 2009; Lee et al., 2009), or intestinal epithelium (Villin-Cre) (Gunther et al., 2011), while having effects, did not cause E10.5 lethality, nor did conditional deletion of FADD in the intestine or skin (Bonnet et al., 2011; Welz et al., 2011). It is possible that the precursors of definitive hematopoiesis, which arise in the endothelium of the aorta, move to the yolk sac, and are required for remodeling of the yolk sac vasculature (Dzierzak and Speck, 2008), are the targets of RIPK3-dependent necrosis in the absence of FADD or caspase-8, and of caspase-8-dependent apoptosis in the absence of FLIP and RIPK3.
Therefore, there is a close interplay between two cell death pathways, one leading to caspase-8-dependent apoptosis, and one leading to RIPK3-dependent necrosis. The presence of FLIP prevents the formation of FADD-dependent, active caspase-8 homodimers, required for apoptosis, while the resulting caspase-8-FLIP heterodimers prevent RIPK3 activation, required for programmed necrosis. In the absence of the FADD-caspase-8-FLIP complex, RIPK3 drives necrosis, resulting in embryonic lethality. In the presence of FADD, but in the absence of FLIP, caspase-8 drives apoptosis, also resulting in embryonic lethality at the same stage. Thus, either death pathway disrupts the proper development of the yolk sac vasculature, unless both are held in check through the interactions we have described (Figure S4A).
If so, why is the system “built” in this complex manner? One possibility relates to the dissemination of DNA viruses, which often carry endogenous inhibitors of caspase-8 (Weinlich et al., 2011). Such viruses may thereby trigger RIPK3-dependent necrosis, which would limit the infection. Indeed, cytomegalovirus (Weinlich et al., 2011) has been shown to cause such necrosis. Therefore, the developmental regulation of RIPK3-dependent necrosis by FADD-caspase-8-FLIP may represent a “failsafe” mechanism to prevent viral dissemination if the apoptotic pathway is compromised, resulting in the untoward consequences of deletion of any of the latter three components. A more direct role for these death processes in embryonic development may only be unveiled when the specific cell types and triggers that initiate both caspase-8-apoptosis and RIPK3-necrosis are elucidated.
Methods
Mice, treatments, and timed matings
Mice with a deleted allele of caspase-8 were generated by germ line deletion of a caspase-8flox allele described previously (Salmena et al., 2003). A distinct set of caspase-8flox animals were generated as previously described (Kang et al., 2004) and were crossed to alpha-myosin heavy chain-Cre animals (Agah et al., 1997). RIPK3-deficient animals were obtained from V. Dixit (Newton et al., 2004). FADD and FLIPdeficient animals have been previously described (Yeh et al., 2000; Yeh et al., 1998). Genotypes were confirmed by tail snip PCR as described previously. For anti-CD95 injections, animals were injected via tail vein with 15 µg purified Jo2 antibodies in lipopolysaccharide-free PBS per animal. Liver enzymes were assayed using a Trilogy Multi-Purpose Analyzer System from Drew Scientific, and liver sections were created and stained with haematoxylin and eosin, in the St. Jude Veterinary Pathology Core facility. Timed matings were performed by mating animals and then verifying developmental age through palpation and ultrasound, with post-dissection staging performed by the St. Jude Veterinary Pathology Core facility. Dissections were performed using a Leica M844/F40 surgical microscope scope. Image capture of embryos were performed using a Nikon SMZ1500 Epi-fluorescence Stereoscopic Zoom Microscope with a DS-Fi1 Camera and Nikon Elements Imaging Software. Sections from embryos were generated and stained with haematoxylin and eosin, anti-cleaved caspase-3 (BioCare), or anti-CD31/PECAM-1 antibody (BD Pharmingen) and biotinylated rabbit anti-rat antibody (Vector). The St. Jude Institutional Animal Care and Use Committee approved all procedures in accordance with the Guide for the Care and Use of Animals.
Knockdown experiments
NIH 3T3 cells that do not express endogenous RIPK3 were stably transduced with a multicistronic pBabe-PURO vector containing fulllength, untagged murine RIPK3 followed by a T2A ribosome skipping sequence, followed by eGFP. Control cells received the same vector containing eGFP alone. Cells were treated with siRNAs as described in Supplementary Experimental Procedures.
Immunoprecipitation of FADD
SVEC 4–10 cells were treated with or without 20 ng ml−1 recombinant murine TNF-α (Peprotech) and 50 uM zVAD (SM Biochemicals) for 90 min. Immunoprecipitation of DISC-associated complexes was carried out using buffer and lysis conditions previously described (Geserick et al., 2009). FADD was immunoprecipitated using the M19 polyclonal anti-FADD antibody conjugated to Protein A/G-PLUS Agarose beads, both from Santa Cruz.
Immune cell staining, cell death, and activation assays
For immune cell staining, the spleen, thymus and lymph node were harvested from animals and single cell suspensions were generated. For immune cell staining from the blood, blood was harvested from the retrorbital sinus from animals anesthetized with 2–2.5% isoflurane in 1 l oxygen. Red blood cells were lysed in hypotonic buffer and samples were stained with the appropriate antibodies as described in Supplementary Experimental Procedures. Data was acquired using a FACsCalibur or LSRII using FlowJo Collectors or FACsDiva software, respectively. Data analysis was performed using FlowJo (Tree Star). For cell death assays, cells were harvested at 9 hr, stained with AnnexinV-APC (Invitrogen) and propidium iodide (Sigma), and assayed for viability using flow cytometry. For cleaved caspase-3 intracellular staining, cells were harvested, fixed, permeabilized, and stained per manufacturer’s instructions (eBioscience). Proliferation assays and culture conditions were performed per standard protocols as detailed in Supplementary Experimental Procedures.
Highlights.
FADD−/−, but not cFLIP−/− embryonic lethality is rescued by RIPK3 ablation.
Ablation of RIPK3 in FLIP-deficient cells and embryos reveals apoptotic cell death
FADD, FLIP, RIPK3 TKO mice are developmentally normal.
Therefore, FADD and FLIP function to control caspase-8 and RIPK3 in development.
Supplementary Material
Acknowledgements
The authors thank Patrick Fitzgerald and Laura L. McCormick for providing essential logistical and administrative support, and maintenance of the mouse colony, respectively. We also thank the St. Jude Animal Resource Center, Veterinary Pathology core, and Hartwell Center, as well as Melissa Johnson and Michael Taylor for their assistance. This work was supported by ALSAC and the NIH (grant AI44828 to D.R.G). C.P.D. was supported by a fellowship grant from the Sass Foundation for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest. 1997;100:169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, Haase I, Pasparakis M. The Adaptor Protein FADD Protects Epidermal Keratinocytes from Necroptosis In Vivo and Prevents Skin Inflammation. Immunity. 2011;35:572–582. doi: 10.1016/j.immuni.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Budd RC, Yeh W-C, Tschopp Jr. cFLIP regulation of lymphocyte activation and development. Nat Rev Immunol. 2006;6:196–204. doi: 10.1038/nri1787. [DOI] [PubMed] [Google Scholar]
- Cho Y, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FKM. Phosphorylation-Driven Assembly of the RIP1-RIP3 Complex Regulates Programmed Necrosis and Virus-Induced Inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, Castanares M, Wu M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Hacker G, Leverkus M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geserick P, Hupe M, Moulin M, Wong WW, Feoktistova M, Kellert B, Gollnick H, Silke J, Leverkus M. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J Cell Biol. 2009;187:1037–1054. doi: 10.1083/jcb.200904158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Oberst A, Dillon CP, Weinlich R, Salvesen GS. RIPK-Dependent Necrosis and Its Regulation by Caspases: A Mystery in Five Acts. Mol Cell. 2011;44:9–16. doi: 10.1016/j.molcel.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, Waisman A, Brenner O, Haffner R, Gustafsson E, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–2984. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger B, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- Kovalenko A, Kim J-C, Kang T-B, Rajput A, Bogdanov K, Dittrich-Breiholz O, Kracht M, Brenner O, Wallach D. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. The Journal of Experimental Medicine. 2009;206:2161–2177. doi: 10.1084/jem.20090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Lee D-J, Chan C, Chen S-W, Ch'en I, Jamora C. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature. 2009;458:519–523. doi: 10.1038/nature07687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ, Berndt MC, Gorski J, White GC, 2nd, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Green DR. It cuts both ways: reconciling the dual roles of caspase 8 in cell death and survival. Nat Rev Mol Cell Biol. 2011;12:757–763. doi: 10.1038/nrm3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, KKitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Rebe C, Cathelin S, Launay S, Filomenko R, Prevotat L, L'Ollivier C, Gyan E, Micheau O, Grant S, Dubart-Kupperschmitt A, et al. Caspase-8 prevents sustained activation of NF-kappaB in monocytes undergoing macrophagic differentiation. Blood. 2007;109:1442–1450. doi: 10.1182/blood-2006-03-011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamaki K, Inoue T, Asano M, Sudo K, Kazama H, Sakagami J, Sakata S, Ozaki M, Nakamura S, Toyokuni S, et al. Ex vivo whole-embryo culture of caspase-8-deficient embryos normalize their aberrant phenotypes in the developing neural tube and heart. Cell Death Differ. 2002;9:1196–1206. doi: 10.1038/sj.cdd.4401090. [DOI] [PubMed] [Google Scholar]
- Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, Berry DM, Tamblyn L, Shehabeldin A, Migon E, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourneur L, Chiocchia G. FADD: a regulator of life and death. Trends Immunol. 2010;31:260–269. doi: 10.1016/j.it.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM, Kemper OC, Kollet O, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- Weinlich R, Dillon CP, Green DR. Ripped to death. Trends Cell Biol. 2011;21:630–637. doi: 10.1016/j.tcb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol. 2009;10:348–355. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A, Mirtsos C, Suzuki N, Bonnard M, Goeddel DV, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–642. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, et al. FADD: essential for embryo development and signaling from some, but not all inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.