Abstract
Targeting hyperphosphorylated tau by immunotherapy is emerging as a promising approach to treat tauopathies such as Alzheimer’s disease and frontotemporal dementia. We have previously reported that active tau immunization clears tau aggregates from the brain and attenuates or prevents functional impairments in two different tangle mouse models. Here, we assessed the efficacy of passive immunization with the PHF1 antibody, which targets a phospho-epitope within one of our active immunogens. Homozygous female tangle mice (JNPL3, 2–3 months) were injected intraperitoneally once per week with PHF1 or pooled mouse IgG (250 μg/ 125 μL; n = 10 per group) for a total of 13 injections. Their behavior was assessed at 5–6 months of age and brain tissue was subsequently harvested for analyses of treatment efficacy. The treated mice performed better than controls on the traverse beam task (p < 0.03), and had 58% less tau pathology in the dentate gyrus of the hippocampus (p = 0.02). As assessed by western blots, the antibody therapy reduced the levels of insoluble pathological tau by 14–27% (PHF1, p < 0.05; PHF1/total tau, p < 0.0001) and 34–45% (CP13 or CP13/total tau, p < 0.05). Levels of soluble tau and sarkosyl soluble tau were unchanged, compared with controls, as well as total tau levels in all the fractions. Plasma levels of PHF1 correlated inversely with tau pathology in the brainstem (p < 0.01), with a strong trend in the motor cortex (p < 0.06) as well as with insoluble total tau levels (p < 0.02), indicating that higher dose of antibodies may have a greater therapeutic effect. Significant correlation was also observed between performance on the traverse beam task and PHF1 immunoreactivity in the dentate gyrus (p < 0.05) as well as with insoluble PHF1/total tau ratio on western blots (p < 0.04). These results show that passive immunization with tau antibodies can decrease tau pathology and functional impairments in the JNPL3 model. Future studies will determine the feasibility of this approach with other monoclonals and in different tangle models in which thorough cognitive assessment can be performed.
Keywords: behavior, immunotherapy, mice, PHF1, tau, tangles
An emerging therapy for Alzheimer’s disease (AD) is immune modulation to clear amyloid-β (Aβ) (Schenk et al. 1999), which is likely to be antibody-mediated (Solomon et al. 1997; Bard et al. 2000; DeMattos et al. 2001; Sigurdsson et al. 2001, 2004; Bacskai et al. 2002; Das et al. 2003; Lemere et al. 2003), and improves cognition in animal models (Dodart et al. 1999; Janus et al. 2000; Morgan et al. 2000; Kotilinek et al. 2002). Unfortunately, the first clinical trial on this approach was halted because of encephalitis in 6% of patients (Schenk 2002), but it is currently being refined in animal models and in several new clinical studies. Some degree of cognitive stabilization was observed in the first trial (Hock et al. 2003; Gilman et al. 2005) and autopsies suggested removal of Aβ plaques (Nicoll et al. 2003, 2006; Ferrer et al. 2004; Masliah et al. 2005a). However, recent findings from this trial indicate that plaque clearance did not halt or slow the progression of dementia, emphasizing the need for alternative targets (Holmes et al. 2008).
Another important target for immunization in AD patients is pathological tau protein that is also the primary target in various tauopathies. Our published findings indicate that active immunization with an AD specific phosphorylated tau epitope, in JNPL3 P301L tangle model mice (Lewis et al. 2000), reduces brain levels of aggregated tau and slows progression of the tangle-related behavioral phenotype (Asuni et al. 2007). Clearance of extracellular tau/tangles may reduce associated damage and prevent the spread of tau pathology (Sigurdsson et al. 2002; Clavaguera et al. 2009; Frost et al. 2009; Sigurdsson 2009). Our findings (Asuni et al. 2007) and numerous reports of neuronal uptake of antibodies suggest that intracellular tau aggregates are also being cleared (Sigurdsson 2009).
Specifically, we have shown that these antibodies enter the brain and bind to pathological tau within neurons based on their colocalization with AD specific tau antibodies (Asuni et al. 2007). Furthermore, we have demonstrated that this approach reduces tau aggregates and prevents cognitive decline in three different tests in another tangle model (Boutajangout et al. 2010b). Others have reported that immunization with α-synuclein in transgenic mice clears these intraneuronal aggregates (Masliah et al. 2005b), and that Aβ antibodies are internalized in cultured neurons and clear intracellular Aβ aggregates (Tampellini et al. 2007). These studies support our findings and interpretations. Most recently, the promise of tau immunotherapy has been confirmed by others (Boimel et al. 2010). Although the active approach has certain advantages, it may have autoimmune side effects that can be avoided with passive immunization.
Here, we determined in the JNPL3 P301L mouse model, whether the repeated administration of a monoclonal tau antibody, PHF1, would have a therapeutic effect as assessed by functional, histological and biochemical measures. A part of this work was reported previously at the Alzheimer’s Association International Conference on Alzheimer’s Disease 2010 (Boutajangout et al. 2010a).
Materials and methods
Animals and antibody injections
Homozygous female JNPL3 mice (n = 10; obtained from Taconic, USA) were injected intraperitoneally (i.p.) with PHF1, a monoclonal tau antibody generously provided by Dr. Peter Davies, that recognizes neurofibrillary tangles and pre-tangles in Alzheimer’s disease and various tangle mouse model, including the JNPL3 model (Lewis et al. 2000). It recognizes tau that is phosphorylated on serine amino acids 404 and 396 on C-terminus of tau (Greenberg et al. 1992). The antibody dose was 250 μg/125 μL, dissolved in phosphate buffered saline. Identical controls (n = 11) were injected i.p. with the same dose of mouse IgG in phosphate buffered saline (Equitech-Bio Inc., Kerrville, TX, USA). The mice received their first injection at 9–12 weeks of age and then at 7 day intervals for a total of 13 injections, followed by behavioral testing at 5–6 months and subsequent tissue analysis at 6–7 months. One control mouse died prior to behavioral analysis. All animals housed at NYU School of Medicine animal facilities are cared for by the Division of Laboratory Animal Resources veterinary staff in an AAALAC approved facilities. All the animal procedures were approved prior to experimentation by the IACUC committee of the university, and are in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals. These guidelines meet or exceed the issued ARRIVE guidelines.
Antibody levels in plasma
The mice were bled before the commencement of the study and periodically throughout the experiment. Plasma levels of PHF1 were detected by ELISA in which 0.5 μg of a peptide, Tau379–408[PSer396, 404], that contains the phosphoepitope recognized by the antibody, was coated per well (Immulon 2HB; Thermo Electron Corp., Milford, MA, USA). The phospho-peptide was synthesized at the Keck Foundation at Yale University. For detection, goat antimouse IgG linked to a horseradish peroxidase (GE Healthcare, Little Chalfont, UK) was used at 1 : 3000 dilution. Tetramethyl benzidine (Pierce, Rockford, IL, USA) was the substrate.
Behavioral studies
At 5–6 months of age, animals went through several behavior tests 1 month prior to tissue harvesting to determine if therapy diminished age-related motor deficits that develop as tau pathology advances in the JNPL3 model. Sensorimotor tests that were performed were traverse beam, rotarod and locomotor activity.
Sensorimotor tests
The mice were adapted to the room with lights on for 15 min prior to testing.
Traverse beam
This task tests balance and general motor coordination and function integration. Mice were assessed by measuring their ability to traverse a graded narrow wooden beam to reach a shaded goal box. A bright light near the starting position on the beam guided the mice towards the goal box. The beam (1.1 cm wide and 50.8 cm long) was placed between two identical columns at 30 cm in height, and under the beam was a foam cushion to prevent injury. Mice were placed on the beam in perpendicular position for habituation, and were then monitored for 60 s. The number of foot slips each mouse had before falling or reaching the goal box were recorded for each of four successive trials and defined as errors. If the mouse fell of the beam before reaching the goal box, the animal was placed back in its position before falling.
Rotarod
Rotarod is used to measure forelimb and hind limb motor coordination and balance. This procedure was designed to assess motor behavior without a practice confound. Mice were first habituated in two trials to reach a baseline level of performance, and subsequently tested in three additional trials with 15 min between trials (Rotarod 7650 accelerating model; Ugo Basile, Biological Research Apparatus, Varese, Italy). Animals were placed on the rod (3.6 cm in diameter) with initial speed set at 1.5 rpm that was then raised every 30 s by 0.5 rpm. A soft foam cushion was placed under the rod to prevent injury from falling. The rod was cleaned with 30% ethanol after each session. To assess the performance, the speed of the rod was recorded when the mouse fell or inverted (by clinging) from the top of the rotating barrel.
Locomotor activity
Mouse activity was recorded over 15 min in a circular open field activity chamber (70 cm in diameter). Mice were first habituated in the chamber few at a time for 15 min and then each mouse was tested for 15 min. After each session, the field was cleaned with 30% ethanol. A camera above the chamber automatically recorded horizontal movements in each dimension (i.e. x, y and two z planes) by measuring movement of the animal (white) relative to black background (San Diego Instruments, San Diego, CA, USA). Results are reported as distance traveled (cm), mean resting time and velocity (average and maximum) of the mouse.
Tissue processing and histology
Following behavioral testing, mice were deeply anesthetized with ketamine/xylazine (250 mg/50 mg per kg body weight, i.p.). The brain was subsequently removed without perfusion and processed as previously described (Sigurdsson et al. 1996). The left hemisphere was snap frozen and stored at −80°C until processed for western blots. Coronal brain sections (40 μm) of the right hemisphere were saved for histological staining with (i) mouse monoclonal tau antibody that stains pathological tau the [PHF1 (1 : 1000, recognizes phosphorylated serines 396 and 404 of the tau protein) (Otvos et al. 1994), generously provided by Peter Davies], (ii) rabbit polyclonal antibody against glial fibrillary acidic protein (GFAP 1 : 500; Dako, Carpinteria, CA, USA) in astrocytes, and (iii) tomatolectin (10 μg/mL; Vector Laboratories, Burlingame, CA, USA) to detect microglia. The sectioned series were placed in ethylene glycol cryoprotectant and stored at −20°C until used for immunohistochemistry. Staining was performed as previously described (Sigurdsson et al. 1996, 2001; Asuni et al. 2006), using mouse-on-mouse immunodetection kit (Vector) for the monoclonals, with every 10th section stained. To verify that reduced tau immunoreactivity in immunized mice was not caused by epitope masking by the injected PHF1 antibody, unmasking procedure was performed as described previously (Asuni et al. 2007).
Image analysis
Tau pathology in brain sections was quantified blindly with the Bioquant program (Bioquant Image Analysis Corp., Nashville, TN, USA) as described previously (Asuni et al. 2007). Every 10th section, randomly chosen, was stained with each antibody. For quantitative image analysis of immunohistochemistry, we initially selected the granular layer of the dentate gyrus, which consistently contained intraneuronal tau aggregates (pre-tangles and tangles). We analyzed the motor cortex and brainstem in these animals as well because tau pathology in those regions may relate to the motor abnormalities observed in this model. An individual blind to the experimental conditions of the study performed all procedures. For the PHF1 stained sections, we sampled every 10th section randomly from the mouse brain. In the dentate gyrus, the measurement was the percentage of area in the measurement field at 200× magnification occupied by reaction product with the tip of the dentate gyrus at the left edge of the field. In the motor cortex, the measurement was the percentage of neuronal staining in the field at 100× magnification with the thickest region of the cingulum positioned at the lower left edge of the field. In the brainstem, the measurement was the percentage of neuronal staining in the field at 100× magnification with the center of the top edge of the field positioned below the Aqueduct of Sylvius. Five sections were analyzed per animal for each brain regions.
Rating of microgliosis and astrogliosis
Semi-quantitative analysis was based on the severity of microgliosis throughout the brain (0, predominantly resting microglia; 1+, a few ramified and/or phagocytic microglia; 2+, moderate number of ramified/phagocytic microglia; and 3+, numerous ramified/phagocytic microglia). The rating of the GFAP sections was based on the complexity of astrocytic branching throughout the brain (1+, resting astrocytes, few processes; 2+, reactive astrocytes, moderate branching; and 3+, reactive astrocytes, extensive branching).
Western blots
Brains were weighed and homogenized (10% w/v) in Tris-buffered saline, pH 7.4 [TBS: 10 mM Tris/150 mM NaCl, containing protease inhibitors (one tablet of complete protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN, USA) per 50 mL of TBS, 1 mM NaF, 0.4 μM Na3 VO4 and 0.5 μM okadaic acid)]. The homogenate was centrifuged at 20 800 g for 5 min (4°C) and supernatants collected. For sarkosyl extraction, 11% sarkosyl solution was added to 300 μL supernatant for a final concentration of 1% and then incubated for 1 h at 37°C. Sarkosyl extracted supernatant and supernatant without sarkosyl were then centrifuged at 100 000 g for 1 h at 4°C in Beckman TL-100 ultracentrifuge, and the high-speed supernatants were collected and used for western blot analysis. Sarkosyl extraction results in dissociation of insoluble proteins including aggregated tau proteins. However, paired helical filaments, the end stage of tau pathology are not soluble in sarkosyl (Greenberg et al. 1990).
For the insoluble fraction, the pellet was re-suspended in the same volume of buffer without protease and phosphatase inhibitors, but that contained 1% (v/v) Triton X-100 and 0.25% (w/v) deoxycholate sodium. It was then ultracentrifuged at 50 000 g for 30 min to obtain a detergent extracted supernatant that was analyzed as an insoluble fraction (Boutajangout et al. 2002, 2004).
The supernatants from these three fractions were heated at 100°C for 5 min and the same amount of protein was electrophoresed on 12% (w/v) polyacrylamide gel. The blots were blocked in 5% nonfat milk with 0.1% Tween-20 in TBS and incubated with different antibodies overnight, and then washed and incubated at 21–23°C for 1 h with peroxidase-conjugated, anti-mouse or anti-rabbit IgG. Subsequently, the bound antibodies [monoclonal PHF1, monoclonal CP13 (P-Ser202) generously provided by Peter Davies (Albert Einstein College of Medicine, Bronx, NY) and polyclonal B19 (total tau raised against bovine tau) (Brion et al. 1991), generously provided by Jean-Pierre Brion (Free University of Brussels, Belgium)] were detected by enhanced chemiluminescence (ECL) (Pierce). Densitometric analysis of immunoblots was performed by the National Institutes of Health ImageJ program and the levels of pathological tau were normalized relative to total tau protein.
Data analysis
All data were analyzed with GraphPad Prism 4.3. Locomotor activity (distance traveled, maximum velocity, average speed and resting time), tau aggregates on western blots and immunoreactivity on brain sections within the dentate gyrus, motor cortex and brainstem were analyzed with unpaired t-test. Welch correction was used if the data failed a test of equal variance. When data failed two out of three normality tests (KS, D’Agostino & Pearson omnibus, and Shapiro–Wilk normality tests) non-parametric Mann–Whitney test was used. The tests were one-tailed as it was assumed based on our past findings that the immunotherapy would lead to clearance of tau pathology that would slow the progression of behavioral impairments. Total tau levels were not expected to change based on our previous studies and, therefore, were analyzed with two-tailed analysis. Two of the behavioral test, the traverse beam test and the rotarod test were analyzed by unpaired t-test or Mann–Whitney (trials combined) and two-way ANOVA (treatment x trials). Correlation between behavioral tests and tau pathology and between the plasma levels of PHF1 antibodies versus tau pathology or behavior was analyzed with Pearson r correlation or Spearman rank correlation when the data failed two out of three normality tests described above.
Results
To determine the feasibility of passive immunotherapy, homozygous P301L mice received 13 weekly i.p. injections with PHF1, a monoclonal tau antibody that recognizes neurofibrillary tangles and pre-tangles in various tangle mouse models, including the JNPL3 model employed in the present study, as well as in AD and other tauopathies (Lewis et al. 2000). PHF1 recognizes tau that is phosphorylated on serine amino acids 396 and 404 on the C-terminus of tau (Greenberg et al. 1992). It is, therefore, a monoclonal analog of the prototype of our active immunization approach (Asuni et al. 2007; Boutajangout et al. 2010b), Tau379–408[PSer396, 404] that contains the PHF1 antibody epitope.
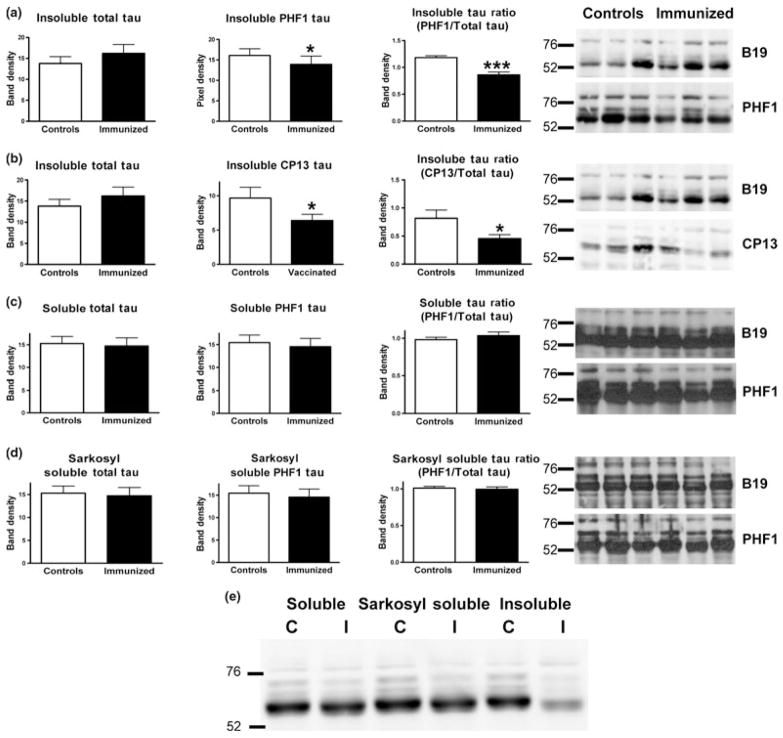
As assessed by western blots at the end of the study (Fig. 1a–d), the antibody therapy reduced the ratio of pathological to total tau by 27% (PHF1/B19; p < 0.0001) and 45% (CP13/B19; p < 0.05), but the levels of soluble tau and sarkosyl soluble tau were unchanged, compared with controls. Total tau levels did not differ significantly between the groups in any of the three fractions. In concord with the ratio analysis, levels of PHF1 tau only differed between the groups in the insoluble fraction (14% reduction, p < 0.05). Likewise, CP13 tau levels were decreased in the immunized mice in the insoluble fraction (34% reduction, p < 0.05). For comparison, representative samples from the control and immunized groups were run on the same gel, clearly showing PHF1-mediated clearance of the insoluble tau fraction (Fig. 1e)
Fig. 1.
PHF1 immunotherapy reduced levels of insoluble tau in the brain. As assessed by western blots of the left hemisphere, the antibody therapy reduced insoluble pathological tau protein (a) PHF1/B19, 27% reduction, p < 0.0001; PHF1, 14% reduction, p < 0.05. (b) CP13/ B19, 45% reduction, p < 0.05; CP13, 34% reduction, p < 0.05, but levels of soluble (c) and sarkosyl soluble tau (d) were unchanged compared with controls. n = 10 per group. (e) Shows representative samples from the control and immunized groups run on the same gel, clearly showing PHF1-mediated clearance of the insoluble tau fraction. *p < 0.05; ***p < 0.001.
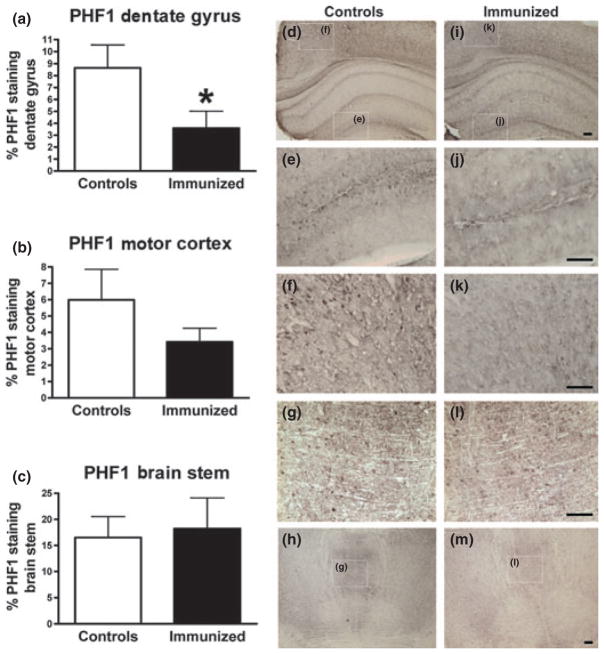
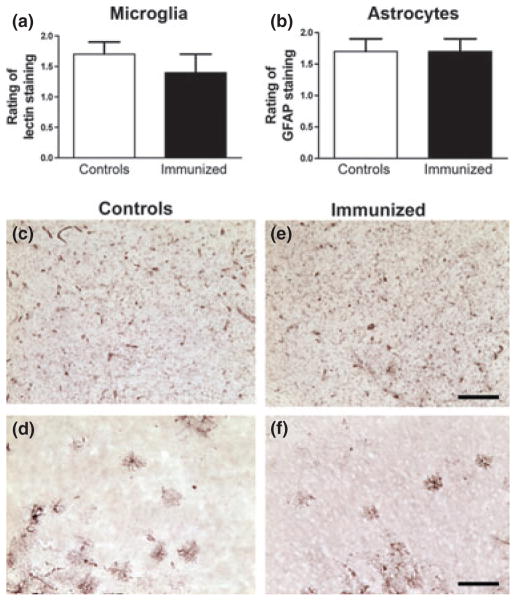
Histological analysis indicated that the treated mice had 58% less PHF1 tau pathology in the dentate gyrus of the hippocampus (p = 0.02), and there was a strong trend for a decrease in PHF1 tau in the motor cortex (43% reduction, p = 0.12), but not in the brainstem that has more advanced tau pathology (Fig. 2a–m). These findings are in line with the western blots. Similar degree of micro and astrogliosis was observed in both groups (Fig. 3), which is analogous to our prior studies with active tau immunotherapy (Asuni et al. 2007; Boutajangout et al. 2010b).
Fig. 2.
(a–c) PHF1 immunized P301L mice had less tau pathology in the dentate gyrus with a strong trend for decrease in the motor cortex, but not in the brainstem. Immunized animals had 58% and 43% less PHF1 stained tau pathology than controls in the dentate gyrus (p = 0.02) and motor cortex (p < 0.12), respectively. The degree of tau pathology was similar between the groups in the brainstem. (d–m) Representative images from a control (d–h) and an immunized mouse (i–m) show clearance of tau pathology with immunization. Higher magnifications of the boxed areas in d, h, i and m are shown in e, f, g, j, k and l, respectively. n = 10 per group and five sections were analyzed per animal. *p < 0.05. Scale = 100 μm.
Fig. 3.
(a and b) Similar degree of micro and astrogliosis was observed in both groups. Representative cortical images for a control (c and d) and an immunized mouse (e and f) are shown. n = 10 per group, 15–20 sections were analyzed per animal (every 10th section of the brain). Scale = 100 μm.
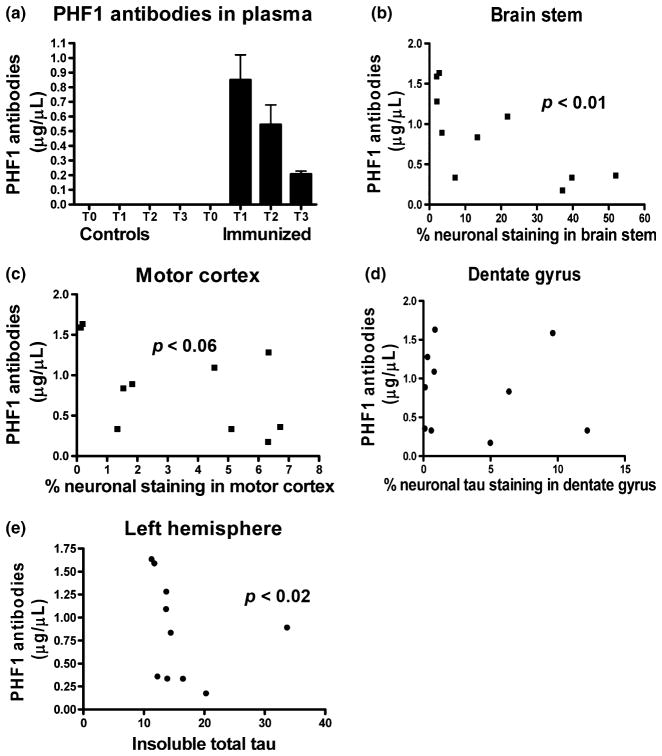
Plasma levels of PHF1 were measured prior to the first injection (T0), 24 h after the 12th injection (T1), then 7 (T2) and 14 (T3) days after the 13th injection. As expected, time dependent decline in plasma PHF1 was observed, and the rate of decline seemed to fit with typical half-life of injected IgG (Fig. 4a). Interestingly, T1 plasma levels of PHF1 correlated inversely with tau pathology in the brainstem (r = −0.72; p < 0.01), with a strong trend for a similar pattern in the motor cortex (r = −0.53; p < 0.06), but not in the dentate gyrus (Fig. 4b–d), indicating that a higher dose of antibodies may have a greater therapeutic effect. In support of the histological analysis, insoluble total tau levels correlated inversely with T1 plasma levels (r = −0.68, p < 0.02, Fig. 4e). Significant correlation was also observed between performance on the traverse beam task and PHF1 immunoreactivity in the dentate gyrus (r = 0.39; p < 0.05) as well as insoluble PHF1/total tau ratio on western blots (r = 0.40; p < 0.04).
Fig. 4.
(a) PHF1 antibodies were cleared relatively quickly from plasma. No detectable antibodies were observed in controls, whereas the levels in immunized animals decreased over time. The rate of clearance appeared to be faster than for endogenous IgG (typical half-life of IgG is 21–28 days), as is often observed for therapeutic monoclonals (Keizer et al. 2010). Each bar represents the average values for the immunized mice + SEM. T0: prior to first immunization, T1: 24 h after the 12th injection, T2: 7 days after the 13th and last injection, T3: 14 days after last injection. The ELISA plates were coated with Tau379–408[P-Ser396, 404]. n = 10 per group per each time point. (b–e) Plasma levels of PHF1 antibodies correlated inversely with tau pathology. Significant correlation was observed in the brainstem (b; p < 0.01), and a strong trend for correlation in the motor cortex (c; p = 0.06). A similar pattern, albeit with an outlier, was observed in the dentate gyrus (d). Similar pattern was detected when antibody levels were compared with levels of insoluble total tau on western blots (e; p < 0.02). n = 10 per group.
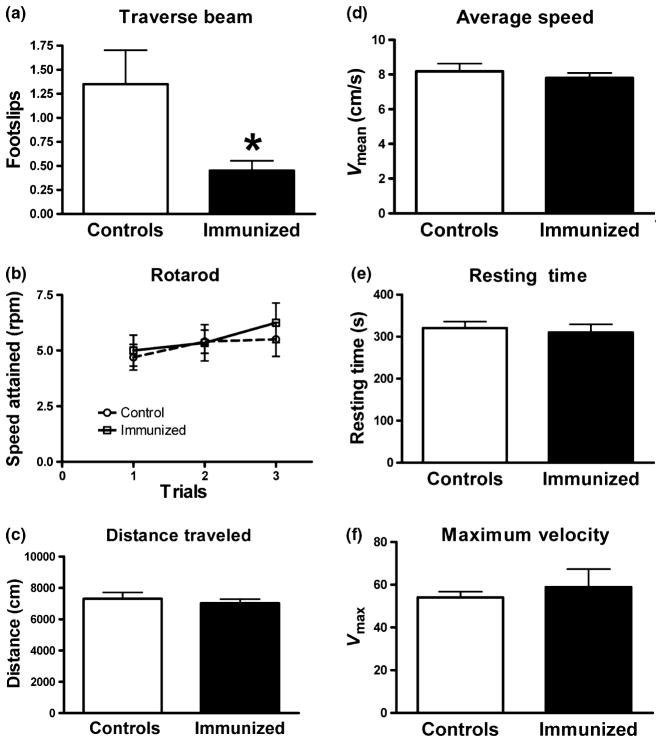
On the behavioral tasks, the treated mice performed better than controls on the traverse beam task (p < 0.03; Fig. 5a), but the groups did not differ on the rotarod or on the locomotor activity measures (Fig. 5b–f). As reflected by the behavioral analysis and lack of increased astrogliosis, there were no obvious detrimental effects of the antibody injections. One of the control mice died during the study for unknown reasons.
Fig. 5.
(a) PHF1 immunized P301L mice performed better on the traverse beam compared with controls. There was a significant difference between IgG injected controls and PHF1 immunized animals on the number of foot slips on the traverse beam with control animals having more foot slips when crossing the beam (trials combined, p = 0.03). (b–f) The groups did not differ significantly on the rotarod (b) or on various locomotor activity measures (c–f). n = 10 per group. *p < 0.05.
These results indicate that passive immunization with tau antibodies can decrease tau pathology and functional impairments in the JNPL3 P301L model. Future studies will determine the feasibility of this approach with other monoclonals and in different tangle models that more closely resembles AD.
Discussion
Our previous findings indicate that active immunization with a disease-related phosphorylated tau epitope, in two different tangle mouse models reduces brain levels of aggregated tau proteins and slows progression of the tauopathy-related behavioral phenotype (Asuni et al. 2007; Boutajangout et al. 2010b). Antibody-mediated clearance of extracellular tau aggregates/tangles may prevent spread of tau pathology (Sigurdsson et al. 2002; Clavaguera et al. 2009; Frost et al. 2009; Sigurdsson 2009), and reduce associated toxicity. Furthermore, numerous reports of neuronal uptake of antibodies suggest that intracellular tangles and pre-tangles may also be directly affected (Sigurdsson 2009). In support of that notion, we have previously shown that purified antibodies from high titer immunized mice enter the brain and bind to pathological tau based on their colocalization with tauopathy-specific tau antibodies (Asuni et al. 2007). Likewise, these antibodies generated in response to the vaccine stain both pathological tau in human AD brain and tangle Tg mouse brain (Asuni et al. 2007).
PHF1 is a monoclonal antibody that recognizes phosphoserine in positions 396 and 404 of the tau molecule (Greenberg et al. 1992), both of which are contained within our prototype phospho-tau immunogen, Tau379–408[PSer396, 404] (Asuni et al. 2007; Boutajangout et al. 2010b). In the present study, multiple injections of this antibody were associated with clearance of tau pathology as assessed by immunohistochemistry and western blots. Some functional improvements were observed as well. Analogous to our previous active tau immunization studies, tau pathology correlated well with antibody levels (Asuni et al. 2007) and behavioral outcome (Asuni et al. 2007; Boutajangout et al. 2010b), further supporting the validity of this approach.
When these findings are compared with our prior report using active immunization encompassing the same epitope in the same homozygous JNPL3 model (Asuni et al. 2007), the passive approach appears to be less efficacious. For example, PHF1 mediated clearance of tau pathology was more prominent in less affected regions such as the dentate gyrus compared with regions with more advanced tau pathology such as the brainstem. Likewise, soluble PHF1 tau levels were not altered with the passive immunotherapy, likely reflecting a more gradual clearance of tau aggregates. Western blots of individual brain regions might have clarified this further but at the same time complicated analysis of insoluble tau, which is limited in this model at the age analyzed. With regard to functional measures, therapeutic improvements were only observed in the traverse beam test, whereas with the active approach in our prior study in this model, benefits were also detected on the rotarod task. However, those previous improvements were less pronounced at 8 months compared with 5 months of age. Here, the mice were only tested at 5–6 months of age and appeared to have less functional impairments than the active immunization study group. Hence, it may be more difficult to detect therapeutic benefits when functional deficits are more modest in the control group.
One reason for less efficacy of PHF1 passive immunotherapy compared with our previous comparable active approach is likely the presumably greater fluctuation in antibody levels following weekly i.p. injections, compared with multiple active immunizations. Furthermore, the polyclonal response to the tau immunogen results in generation of antibodies that target a larger portion of the tau molecule than PHF1. These endogenous antibodies are of various classes, isotypes and affinities that change over time with repeated immunizations. All these differences may have added therapeutic benefits. Prior studies in the Aβ field have shown that efficacy of antibody-mediated clearance of Aβ can be isotype-dependent (Sigurdsson 2006). It is also conceivable that certain type of T-cell activation may have beneficial effects, analogous to when Aβ is being targeted by immunotherapy (Fisher et al. 2010; Schwartz and Shechter 2010). However, a key safety feature of passive immunization is that it avoids a potentially detrimental T-cell response. Such adverse effects halted the first Aβ immunotherapy trial (Orgogozo et al. 2003), which makes the passive approach attractive for proof-of-concept clinical trials. For chronic therapy, the active approach would be more appropriate and it can be tailored to minimize autoimmune side effects.
There are two known pathways for clearance of tau within cells. The proteosome system handles soluble normal tau and misfolded soluble tau. Aggregates of misfolded tau are too large for proteosomal degradation and should be removed via the autophagy/lysosomal system. Macroautophagy has been shown to be likely involved in tau clearance (Berger et al. 2006; Hamano et al. 2008), and lysosomal processing influences tau aggregation and clearance in an inducible cellular model of tauopathy (Wang et al. 2009). Other studies have demonstrated that inhibition of lysosomes increase tau levels (Bendiske and Bahr 2003), lysosomal tau is detected in AD and control brains (Ikeda et al. 1998) and numerous autophagic and lysosomal vesicles are observed by electromicroscopy in the JNPL3 model (Lin et al. 2003), and in neuronal cultures with tau mutations (Lim et al. 2001). It is interesting to note as well that early pathological changes occur in the lysosomal system in AD (Nixon 2007), that may be associated with attempted clearance of tau and/or Aβ aggregates. Antibody-mediated disassembly of these aggregates should facilitate access and subsequent degradation by lysosomal enzymes, and thereby promote neuronal health. Importantly, antibodies have been visualized in lysosomes by immunoelectroscopy (Meeker et al. 1987). Our preliminary findings in a brain slice tangle model support this scenario. We have observed in this slice model with confocal imaging and biochemical measurements that anti-tau antibodies applied to the culture colocalize with tau aggregates and endosomal/ lysosomal markers within neurons (Krishnamurthy et al. 2010).
No obvious toxic effects were observed following the multiple injections of the PHF1 antibody. The only mouse that died during the study belonged to the control group. Astrogliosis, which is a sensitive indicator of neurotoxicity, was comparable in the treated and control mice. The antibody-mediated clearance of the tau aggregates is likely to be gradual as reflected in similar degree of microgliosis in the immunized mice versus controls. These results mirror our previous active tau immunization studies (Asuni et al. 2007; Boutajangout et al. 2010b). Likewise, as in those prior studies, normal tau is not affected following PHF1 therapy. This is not particularly surprising as this pool of tau is soluble in the cytosol and should not be accessible to the antibodies based on the proposed mechanism of action. Furthermore, PHF1 and the polyclonal antibodies generated towards the disease-related phospho-tau epitope, should primarily target pathological tau protein.
In summary, these findings show for the first time that disease-specific tau antibodies can clear tau aggregates from the brain which reduces related functional impairments. Overall, passive or active tau immunotherapy is promising for various tauopathies and should be assessed in clinical trials in the near future.
Acknowledgments
This work was supported by AG032611 (EMS), AG020197 (EMS), AG022102 (PD), MH38623 (PD), the Alzheimer’s Association (ES, AB) and Applied Neurosolutions Inc (PD).
Abbreviations used
- Aβ
amyloid-β
- AD
Alzheimer’s disease
- TBS
Tris-buffered saline
Footnotes
Conflict of interest
Patent on tau immunotherapy pending.
References
- Asuni AA, Boutajangout A, Scholtzova H, Knudsen E, Li YS, Quartermain D, Frangione B, Wisniewski T, Sigurdsson EM. Vaccination of Alzheimer’s model mice with Aβ derivative in alum adjuvant reduces Aβ burden without microhemorrhages. Eur J Neurosci. 2006;24:2530–2542. doi: 10.1111/j.1460-9568.2006.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27:9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT. Non-Fc-mediated mechanisms are involved in clearance of amyloid-β in vivo by immunotherapy. J Neurosci. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Bendiske J, Bahr BA. Lysosomal activation is a compensatory response against protein accumulation and associated synaptopathogenesis – An approach for slowing Alzheimer disease? J Neuropathol Exp Neurol. 2003;62:451–463. doi: 10.1093/jnen/62.5.451. [DOI] [PubMed] [Google Scholar]
- Berger Z, Ravikumar B, Menzies FM, et al. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15:433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- Boimel M, Grigoriadis N, Lourbopoulos A, Haber E, Abramsky O, Rosenmann H. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp Neurol. 2010;224:472–485. doi: 10.1016/j.expneurol.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Boutajangout A, Leroy K, Touchet N, Authelet M, Blanchard V, Tremp G, Pradier L, Brion JP. Increased tau phosphorylation but absence of formation of neurofibrillary tangles in mice double transgenic for human tau and Alzheimer mutant (M146L) presenilin-1. Neurosci Lett. 2002;318:29–33. doi: 10.1016/s0304-3940(01)02461-2. [DOI] [PubMed] [Google Scholar]
- Boutajangout A, Authelet M, Blanchard V, Touchet N, Tremp G, Pradier L, Brion JP. Characterisation of cytoskeletal abnormalities in mice transgenic for wild-type human tau and familial Alzheimer’s disease mutants of APP and presenilin-1. Neurobiol Dis. 2004;15:47–60. doi: 10.1016/j.nbd.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive tau immunotherapy diminishes functional decline and clears tau aggregates in a mouse model of tauopathy. Alzheimers & Dement. 2010a;6:S578. [Google Scholar]
- Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci. 2010b;30:16559–16566. doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion JP, Hanger DP, Bruce MT, Couck AM, Flamentdurand J, Anderton BH. Tau in Alzheimer neurofibrillary tangles – N-terminal and C-terminal regions are differentially associated with paired helical filaments and the location of a putative abnormal phosphorylation site. Biochem J. 1991;273:127–133. doi: 10.1042/bj2730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Howard V, Loosbrock N, Dickson D, Murphy MP, Golde TE. Amyloid-β immunization effectively reduces amyloid deposition in FcRγ−/ − knock-out mice. J Neurosci. 2003;23:8532–8538. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodart JC, Meziane H, Mathis C, Bales KR, Paul SM, Ungerer A. Behavioral disturbances in transgenic mice overexpressing the V717F β-amyloid precursor protein. Behav Neurosci. 1999;113:982–990. doi: 10.1037//0735-7044.113.5.982. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Boada RM, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-β immunization in Alzheimer’s disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher Y, Nemirovsky A, Baron R, Monsonego A. T cells specifically targeted to amyloid plaques enhance plaque clearance in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e10830. doi: 10.1371/journal.pone.0010830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, et al. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Greenberg SG, Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA. 1990;87:5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SG, Davies P, Schein JD, Binder LI. Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau. J Biol Chem. 1992;267:564–569. [PubMed] [Google Scholar]
- Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, DeTure M, Ko LW. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wildtype tau expression. Eur J Neurosci. 2008;27:1119–1130. doi: 10.1111/j.1460-9568.2008.06084.x. [DOI] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Streffer JR, et al. Antibodies against β-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, et al. Long-term effects of Aβ (42) immunisation in Alzheimer’s disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Akiyama H, Arai T, Kondo H, Haga C, Iritani S, Tsuchiya K. Alz-50/Gallyas-positive lysosome-like intraneuronal granules in Alzheimer’s disease and control brains. Neurosci Lett. 1998;258:113–116. doi: 10.1016/s0304-3940(98)00867-2. [DOI] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, et al. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493–507. doi: 10.2165/11531280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J Neurosci. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy PK, Deng Y, Mathews PM, Sigurdsson EM. Mechanistic studies of antibody mediated clearance of tau aggregates using an ex vivo brain slice model. Alzheimers & Dement. 2010;6:S276. doi: 10.3389/fpsyt.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Spooner ET, LaFrancois J, et al. Evidence for peripheral clearance of cerebral Aβ protein following chronic, active Aβ immunization in PSAPP mice. Neurobiol Dis. 2003;14:10–18. doi: 10.1016/s0969-9961(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Lim F, Hernandez F, Lucas JJ, Gomez-Ramos P, Moran MA, Avila J. FTDP-17 mutations in tau transgenic mice provoke lysosomal abnormalities and tau filaments in forebrain. Mol Cell Neurosci. 2001;18:702–714. doi: 10.1006/mcne.2001.1051. [DOI] [PubMed] [Google Scholar]
- Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. Ultrastructural neuronal pathology in transgenic mice expressing mutant (P301L) human tau. J Neurocytol. 2003;32:1091–1105. doi: 10.1023/B:NEUR.0000021904.61387.95. [DOI] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Aβ vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005a;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Adame A, et al. Effects of asynuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005b;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Meeker ML, Meeker RB, Hayward JN. Accumulation of circulating endogenous and exogenous immunoglobulins by hypothalamic magnocellular neurons. Brain Res. 1987;423:45–55. doi: 10.1016/0006-8993(87)90823-7. [DOI] [PubMed] [Google Scholar]
- Morgan D, Diamond DM, Gottschall PE, et al. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Barton E, Boche D, et al. Aβ species removal after Aβ42 immunization. J Neuropathol Exp Neurol. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- Nixon RA. Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci. 2007;120:4081–4091. doi: 10.1242/jcs.019265. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- Otvos L, Jr, Feiner L, Lang E, Szendrei GI, Goedert M, Lee VM. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994;39:669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- Schenk D. Amyloid-β immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Shechter R. Systemic inflammatory cells fight off neurodegenerative disease. Nat Rev Neurol. 2010;6:405–410. doi: 10.1038/nrneurol.2010.71. [DOI] [PubMed] [Google Scholar]
- Sigurdsson EM. Immunotherapy for conformational diseases. Curr Pharm Des. 2006;12:2569–2585. doi: 10.2174/138161206777698837. [DOI] [PubMed] [Google Scholar]
- Sigurdsson EM. Tau-focused immunotherapy for Alzheimer’s disease and related tauopathies. Curr Alzheimer Res. 2009;6:446–450. doi: 10.2174/156720509789207930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson EM, Lorens SA, Hejna MJ, Dong XW, Lee JM. Local and distant histopathological effects of unilateral amyloid-β 25–35 injections into the amygdala of young F344 rats. Neurobiol Aging. 1996;17:893–901. doi: 10.1016/s0197-4580(96)00169-8. [DOI] [PubMed] [Google Scholar]
- Sigurdsson EM, Scholtzova H, Mehta PD, Frangione B, Wisniewski T. Immunization with a non-toxic/non-fibrillar amyloid-β homologous peptide reduces Alzheimer’s disease associated pathology in transgenic mice. Am J Pathol. 2001;159:439–447. doi: 10.1016/s0002-9440(10)61715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson E, Wisniewski T, Frangione B. Infectivity of amyloid diseases. Trends Mol Med. 2002;8:411. doi: 10.1016/s1471-4914(02)02403-6. [DOI] [PubMed] [Google Scholar]
- Sigurdsson EM, Knudsen E, Asuni A, Fitzer-Attas C, Sage D, Quartermain D, Goni F, Frangione B, Wisniewski T. An attenuated immune response is sufficient to enhance cognition in an Alzheimer’s disease mouse model immunized with amyloidβ derivatives. J Neurosci. 2004;24:6277–6282. doi: 10.1523/JNEUROSCI.1344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon B, Koppel R, Frankel D, Hanan-Aharon E. Disaggregation of Alzheimer β-amyloid by site-directed mAb. Proc Natl Acad Sci USA. 1997;94:4109–4112. doi: 10.1073/pnas.94.8.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampellini D, Magrane J, Takahashi RH, Li F, Lin MT, Almeida CG, Gouras GK. Internalized antibodies to the Aβ domain of APP reduce neuronal Aβ and protect against synaptic alterations. J Biol Chem. 2007;282:18895–18906. doi: 10.1074/jbc.M700373200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Martinez-Vicente M, Kruger U, Kaushik S, Wong E, Mandelkow EM, Cuervo AM, Mandelkow E. Tau fragmentation, aggregation and clearance: The dual role of lysosomal processing. Hum Mol Genet. 2009;18:4153–4170. doi: 10.1093/hmg/ddp367. [DOI] [PMC free article] [PubMed] [Google Scholar]