Abstract
Guanine bases are sensitive to modification during automated DNA synthesis and processing reactions. Methods for the detection of two types of guanine modifications are described. The first method uses the higher reactivity of the modified G base to KMn04 oxidation than T bases, and thus allows detection by chemical DNA sequencing. The second method makes use of the Escherichia coli nucleotide excision repair enzyme UvrABC endonuclease which can detect "bulky" base modifications at each nucleotide in the synthetic DNA. Though the chemical structures of the two modifications are not known, they may be related. Both types of G modifications are often found in oligonucleotides synthesized by the methoxy-diisopropyl-phosphoramidite (MEDP) chemistry but non-detectable in the products of the beta-cyanoethyl-diisopropyl-phosphoramidite (CEDP) chemistry. The Rubin and Schmid pyrimidine-specific chemical DNA sequencing procedure (Rubin, C.M., and Schmid, C.W. (1980) Nucleic Acids Res. 8, 4613-4619) was found to be applicable to oligonucleotides synthesized by the CEDP chemistry, and to oligonucleotides synthesized by the MEDP chemistry if precautionary measures are taken to destroy the signals produced by the highly KMnO4 sensitive modified guanine bases. We also show how chemical DNA sequencing might be useful for diagnosing other chemical modifications in synthetic oligonucleotides.
Full text
PDF



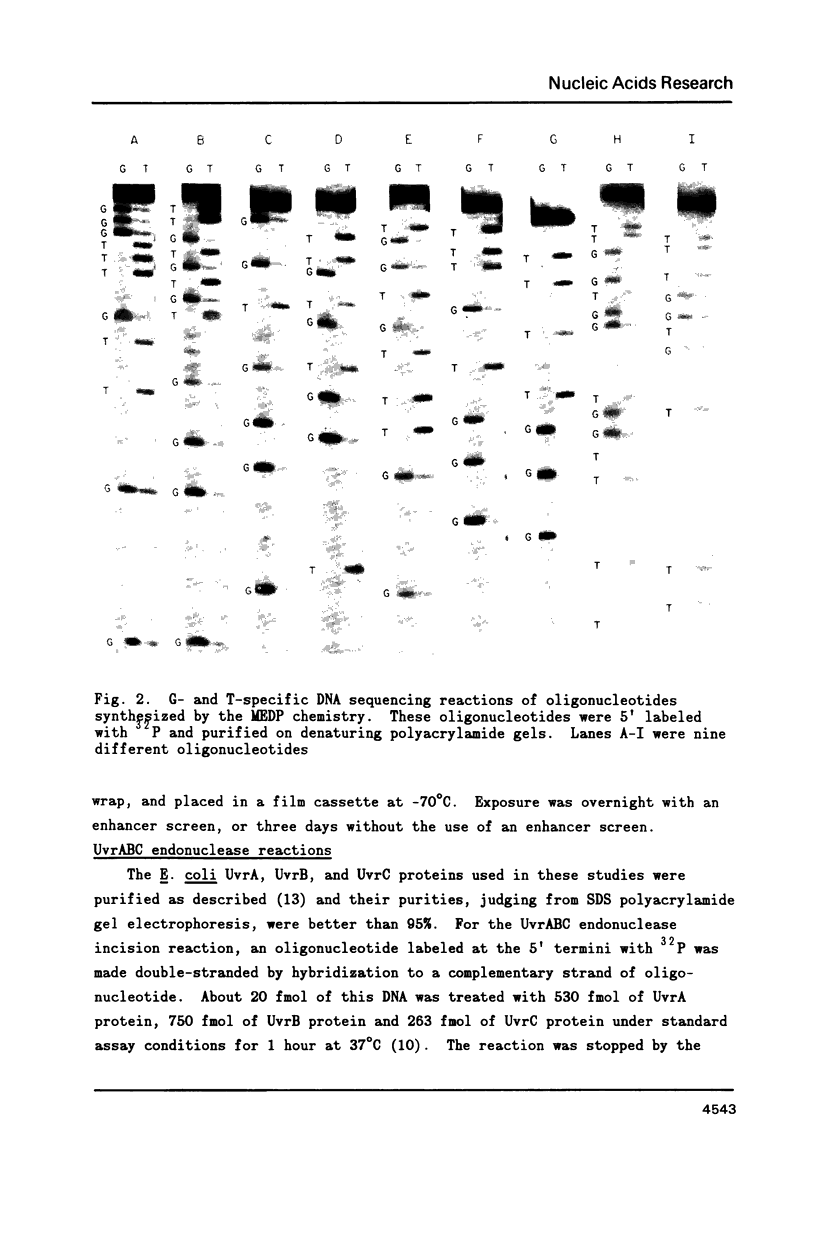










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eadie J. S., Davidson D. S. Guanine modification during chemical DNA synthesis. Nucleic Acids Res. 1987 Oct 26;15(20):8333–8349. doi: 10.1093/nar/15.20.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Gaffney B. L., Senior M., Riddle R. R., Jones R. A. Methylation of thymine residues during oligonucleotide synthesis. Nucleic Acids Res. 1985 Jan 25;13(2):573–584. doi: 10.1093/nar/13.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayatsu H., Ukita T. The selective degradation of pyrimidines in nucleic acids by permanganate oxidation. Biochem Biophys Res Commun. 1967 Nov 30;29(4):556–561. doi: 10.1016/0006-291x(67)90521-9. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair and recombination. Br Med Bull. 1973 Sep;29(3):226–235. doi: 10.1093/oxfordjournals.bmb.a071012. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Rubin C. M., Schmid C. W. Pyrimidine-specific chemical reactions useful for DNA sequencing. Nucleic Acids Res. 1980 Oct 24;8(20):4613–4619. doi: 10.1093/nar/8.20.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Schendel P. F., Defais M., Jeggo P., Samson L., Cairns J. Pathways of mutagenesis and repair in Escherichia coli exposed to low levels of simple alkylating agents. J Bacteriol. 1978 Aug;135(2):466–475. doi: 10.1128/jb.135.2.466-475.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R. Chemistry of guanine and its biologically significant derivatives. Prog Nucleic Acid Res Mol Biol. 1968;8:73–112. doi: 10.1016/s0079-6603(08)60544-9. [DOI] [PubMed] [Google Scholar]
- Singer B. The chemical effects of nucleic acid alkylation and their relation to mutagenesis and carcinogenesis. Prog Nucleic Acid Res Mol Biol. 1975;15(0):219–284. [PubMed] [Google Scholar]
- Urdea M. S., Ku L., Horn T., Gee Y. G., Warner B. D. Base modification and cloning efficiency of oligodeoxyribonucleotides synthesized by the phosphoramidite method: methyl versus cyanoethyl phosphorus protection. Nucleic Acids Symp Ser. 1985;(16):257–260. [PubMed] [Google Scholar]
- Walker G. C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]
- Yeung A. T., Jones B. K., Capraro M., Chu T. The repair of psoralen monoadducts by the Escherichia coli UvrABC endonuclease. Nucleic Acids Res. 1987 Jun 25;15(12):4957–4971. doi: 10.1093/nar/15.12.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Grossman L. Protein complexes formed during the incision reaction catalyzed by the Escherichia coli UvrABC endonuclease. Nucleic Acids Res. 1986 Mar 25;14(6):2567–2582. doi: 10.1093/nar/14.6.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Oh E. Y., Grossman L. Enzymatic properties of purified Escherichia coli uvrABC proteins. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6157–6161. doi: 10.1073/pnas.80.20.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Oh E. Y., Yoakum G. H., Grossman L. The purification of the Escherichia coli UvrABC incision system. Nucleic Acids Res. 1986 Nov 11;14(21):8535–8556. doi: 10.1093/nar/14.21.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon G., Gallo K. A., Samson C. J., Shao K. L., Summers M. F., Byrd R. A. Analytical studies of 'mixed sequence' oligodeoxyribonucleotides synthesized by competitive coupling of either methyl- or beta-cyanoethyl-N,N-diisopropylamino phosphoramidite reagents, including 2'-deoxyinosine. Nucleic Acids Res. 1985 Nov 25;13(22):8181–8196. doi: 10.1093/nar/13.22.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]