Abstract
Both isotopes of silver [107Ag and 109Ag] were simultaneously polarized by dynamic nuclear polarization (DNP) Large liquid-state NMR enhancements were achieved allowing the Ag NMR characterization of the complexes in mM concentration range. Since both isotopes have long T1, the hyperpolarized NMR signal of one isotope could still be observed even after the magnetization of the other isotope had already been destroyed by rf excitation pulses.
Keywords: Ag, NMR, ligands, isotopes, dynamic nuclear polarization
Silver compounds and complexes are important class of materials due to the extensive inorganic, organometallic, and medicinal chemistry of the Ag+ ion. A number of Ag+ complexes, in particular, the diphosphine complexes Ag(dppe)2+ and Ag(dppp)2+, have been shown to have potent antimicrobial and anticancer properties.[1] Complexes of the β-emitting radionuclide 111Ag such as Ag(dotete)+ have been suggested for the targeted radiotherapy of cancer.[2] Ag has two naturally abundant, spin I=1/2 isotopes: 107Ag (51.83 %, 107γ=1.723 MHz/T) and 109Ag (48.17 %, 109γ=1.9808 MHz/T) and thus, NMR spectroscopy would be a very useful method for the structural characterization of silver complexes as well as for the study of reactions involving Ag+ ions.[3] However, Ag NMR is hampered by two major difficulties which arise from the very low gyromagnetic ratio γ of Ag isotopes: (i) low sensitivity and (ii) long spin-lattice relaxation time T1.[3]
In spite of the major advances in NMR technology, conventional Ag NMR still requires high concentrations and/or prohibitively long experimental acquisition times. For instance, INEPT experiments can only improve the Ag NMR signal enhancement by a factor of 8.7.[4] Here we report much larger 107,109Ag NMR sensitivity enhancement via the fast dissolution dynamic nuclear polarization (DNP) technique.[5, 6] We have found that AgOTf and a number of silver complexes could be polarized via DNP using the free radical 1,3-bisdiphenylene-2-phenylallyl (BDPA) as polarizing agent in sulfolane-based glassing matrices (Table 1).[7] It is worth noting that the other two radicals that we have tried (trityl OX063 and 4-oxo-TEMPO) failed to give any enhancements on AgOTf or AgNO3, probably due to interaction of these radicals with the Ag+ ions as suggested by the color change of these samples prepared for DNP (see Supporting Information).
Table 1.
109Ag NMR chemical shifts, spin-lattice relaxation times, and NMR enhancements of various hyperpolarized Ag-compounds and complexes.
| Compound | Dissolution solvent |
109Ag δ/ppm[d] |
109T1 (107T1)/s |
109ε[e] |
|---|---|---|---|---|
| AgOTf | water | 43 | 370(424) | 6,000 |
| Ag(crypt-222)Otf[a] | methanol | 82 | 108(123) | 8,530 |
| Ag(crypt-222)Otf[a],[c] | methanol | 82 | 91 | 21,000 |
| Ag(en)2OTf[a] | water | 458 | 9 | 630 |
| Ag(d8-bipy)2OTf[b] | methanol | 518 | 28 | 2,650 |
| Ag(bipy)2OTf[b] | methanol | 522 | 27 | 2,520 |
| Ag(dotete)OTf[a] | methanol | 700 | 59(73) | 5,300 |
| Ag(thiourea)3OTf[b] | water | 1022 | 136(203) | 4,030 |
| Ag(CN)2OTf[b] | water | 1185 | 30 | 1,560 |
| Ag(dppe)2OTf[a] | methanol | 1383 | 43 | 1,900 |
| Ag(dppp)2OTf[a] | methanol | 1466 | 47(55) | 7,200 |
Method A.
Method B, see text.
In the presence of Gd(OTf)3 (2.5 mM).
Chemical shifts were referenced to 3 M aqueous solution of AgNO3.
The enhancements were measured 8 s (transfer time) after dissolution.
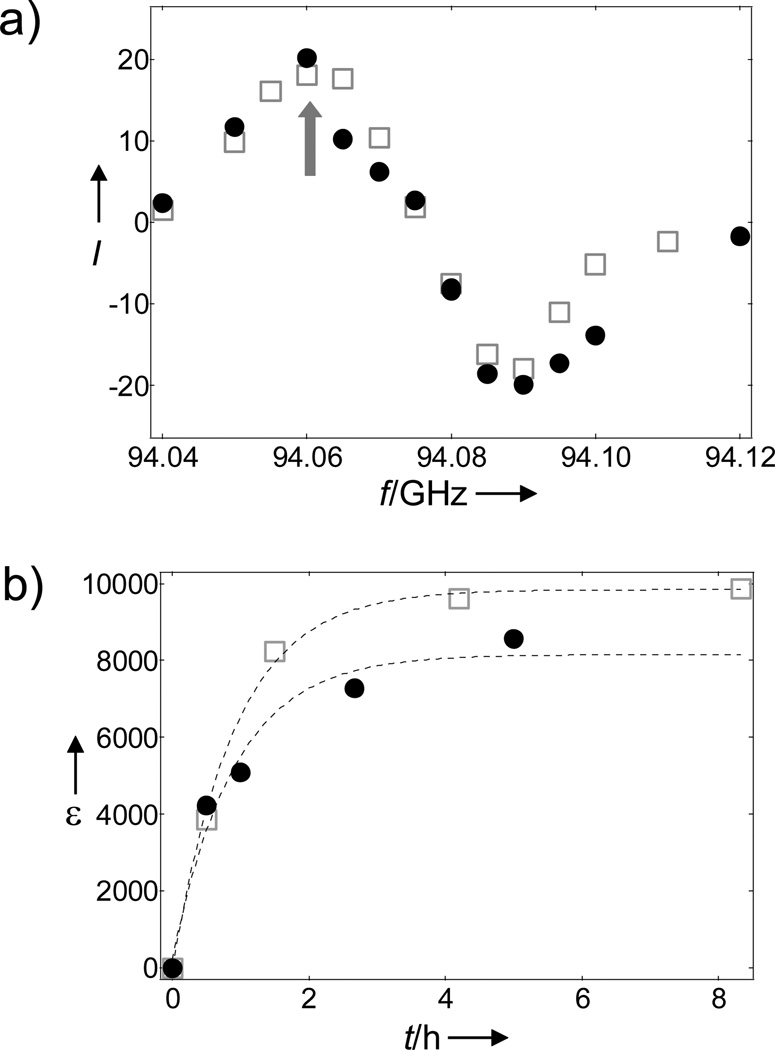
The 107,109Ag microwave DNP spectra (Figure 1a) were obtained by measuring the liquid-state 107,109Ag NMR signal enhancement of Ag(cryp-222)OTf samples polarized at different microwave frequencies in the HyperSense commercial polarizer after dissolution with methanol and transfer (8 s). We were not able to record the microwave DNP spectra at cryogenic conditions in the polarizer because of spurious NMR ringing signals.[6] Considering the very low γ of 107,109Ag nuclei, the DNP process is expected to proceed predominantly via the thermal mixing process[8] and the nearly identical DNP spectrum of the two Ag isotopes supports this mechanism. A characteristic feature of DNP by thermal mixing is that all isotopes are polarized simultaneously as they acquire the same spin temperature Ts at the same microwave frequency.[8] Figure 1b shows the liquid-state NMR enhancement (9.4 T, 298 K) of 107,109Ag(crypt-222)OTf as a function of irradiation time of frozen samples (3.35 T, 1.4 K) at the positive polarization peak.
Figure 1.
a) Relative liquid-state 107Ag (□) and 109Ag (●) NMR intensities (MeOH, 9.4 T and 298 K) of hyperpolarized Ag(crypt-222)OTf as a function of microwave irradiation frequency. The arrow indicates the positive polarization peak where the subsequent 107,109Ag DNP experiments were performed. b) Polarization buildup curves of 107Ag (□) and 109Ag (●) taken at the positive polarization peak. Each point represents a separate DNP experiment.
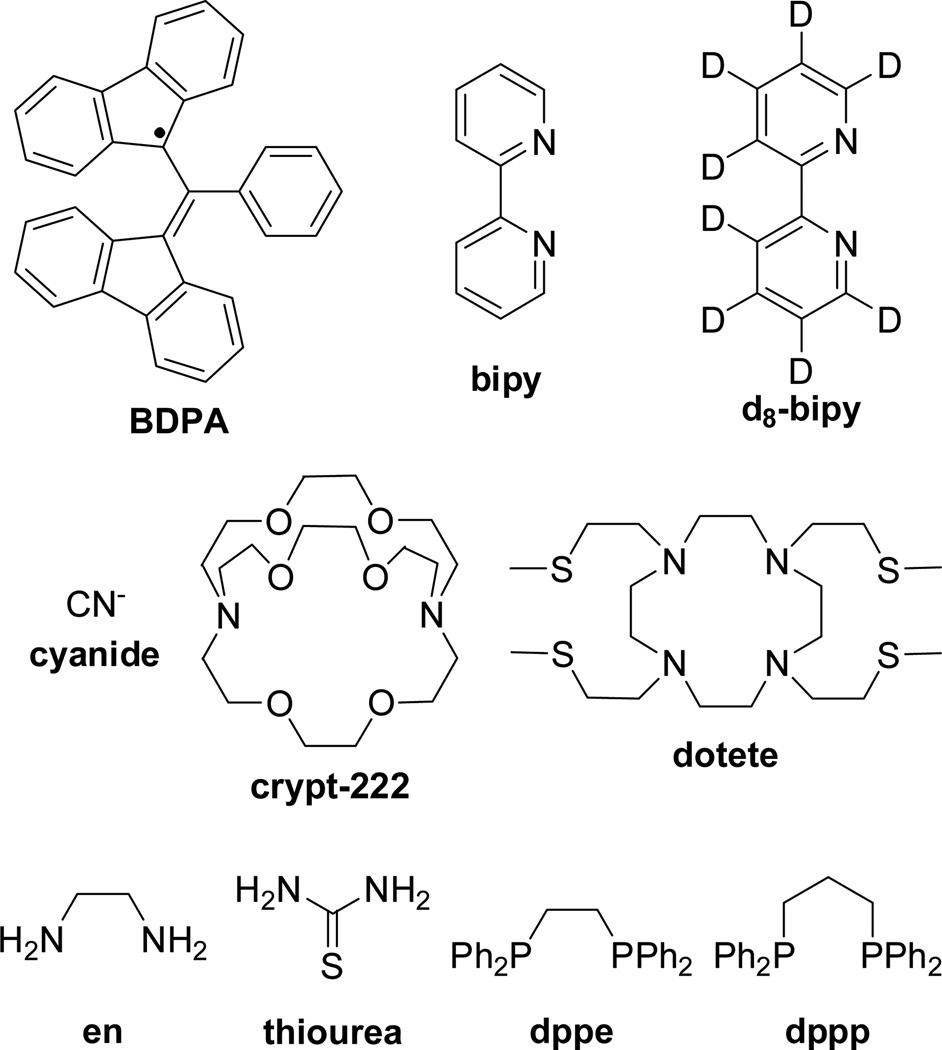
Ag+ is a soft (type B) metal ion and accordingly, we have selected ligands with C−, N−, P−, and S− donor atoms for Ag+-complexation (Figure 2). Hyperpolarized Ag-complexes were produced using two different methods: (i) directly polarizing the Ag-complexes with BDPA in sulfolane-based glassing matrix (method A) and (ii) complexing hyperpolarized AgOTf with a ligand during the dissolution phase (method B) (Table 1). The concentration of the silver complexes was in the mM range (2–15 mM) in the dissolution liquid. The measured 109Ag chemical shifts are shown in Table 1; we chose to perform most of our measurements with 109Ag since traditionally, 109Ag is the preferred isotope for Ag NMR because it has 1.4 times the receptivity of 107Ag.[3]
Figure 2.
BDPA free radical polarizing agent and the ligands used for Ag+-complexation
NMR signal from uncomplexed Ag+ was not observed with any of ligands used in method B indicating that the complexation process was very fast even with the rigid macrocylic ligands dotete and crypt-222. This is in agreement with the reported fast formation kinetics of silver complexes.[9] Due to the high lability of silver complexes, only one signal was observed for Ag(CN)2−, Ag(CN)32−, and Ag(CN)43− species when excess KCN was used and for the same reason, no 107Ag-13C coupling could be detected with 13C-labeled KCN (Supporting Information). Analogously, only one signal was observed with thiourea (Figure 3b) although a variety of species with ligand to Ag+ ratios of 1:1, 2:1, 3:1, 4:1, and 3:2 exist in equilibrium in this system.[10] On the other hand, with bisphosphine ligands that form 5 or 6 membered chelate rings, the exchange processes were slow enough to directly observe 109Ag-31P coupling (Figure 3c).
Figure 3.
a) Hyperpolarized (HP) and thermal (TH) 109Ag NMR signals of a) AgOTf, b) Ag(thiourea)3OTf, and c) Ag(dppp)2OTf. The HP-NMR spectra were taken with 1 scan at 9.4 T and 298 K 8 s after dissolution of the polarized samples. The insets show the corresponding hyperpolarized NMR signal decay due to rf excitation and T1 relaxation.
As seen from Table 1, not all silver complexes have long T1 value. The 109Ag T1 values ranged from 9 s to 370 s. For the Ag(bipy)2+ complex, deuteration of the ligand did not have an effect on the T1 value, which suggests that dipolar interactions are not the dominant mechanism for relaxation. Chemical shift anisotropy has previously been identified as the major pathway for T1 relaxation of silver complexes.[11] The very different T1 values measured for these complexes likely reflect different chemical shift anisotropy contributions to the T1 relaxation. 107Ag has a slightly longer T1 than 109Ag due to its lower γ.
Similarly to 13C and 89Y DNP, addition of trace amount of T1 relaxation agent [2.5 mM Gd(OTf)3] resulted in a significant improvement in the 109Ag NMR enhancement of Ag(crypt-222)OTf Ag (from 8,530 to 21,000, see Table 1) although this came at the expense of a slightly shorter T1 (Supporting Information)[6].
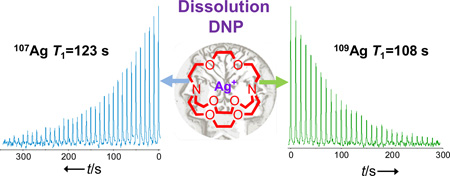
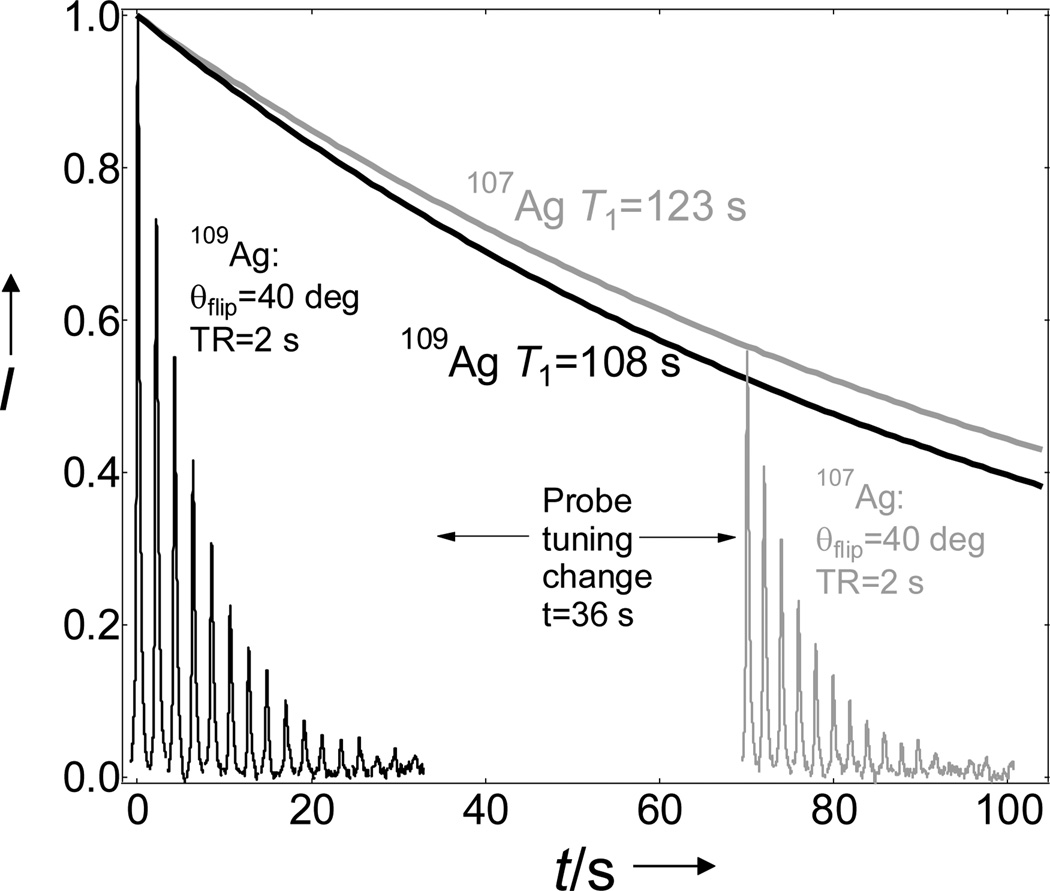
Silver is the only element in the periodic table that has an almost 1:1 composition of two spin I=1/2 isotopes with very similar NMR properties (107Ag 51.8%, 109Ag 48.2%). This allowed us to perform an interesting NMR experiment where one isotope could still be observed after the NMR signal of the other already decayed due to pulsing and T1 relaxation. The difference between the 109Ag and 107Ag resonances is Δν=B(109γ-107γ)=2.4236 MHz in a 9.4 T magnet. In our setup, it takes about 30–40 seconds to tune the probe from the Larmor frequency of 109Ag to that of 107Ag. Using hyperpolarized 107,109Ag(crypt-222)OTf, the magnetization of 109Ag was destroyed by rf pulsing, but this did not affect the NMR signal of 107Ag, which could still be observed with a slightly lower polarization as dictated by its T1 decay (Figure 4). This also illustrates how two consecutive spectroscopy or imaging experiments could be performed with 109,107Ag-based NMR probes. It is worth noting that such experiments cannot be performed by conventional NMR.
Figure 4.
Consecutive detection of 109Ag and 107Ag NMR signals of hyperpolarized Ag(crypt-222)OTf at 9.4 T and 298 K.
In summary, we have demonstrated that silver complexes can be characterized by Ag NMR in mM concentration range either by complexing hyperpolarized Ag+-ions with ligands or polarizing preformed Ag-complexes. Several thousand fold 107,109Ag NMR signal enhancements were achieved using BDPA radical as the polarizing agent. The unique isotopic composition of silver allowed us to perform NMR experiments in which the hyperpolarized signal of one isotope could still be observed after the hyperpolarized state of the other had already been destroyed by rf excitation pulses.
Experimental Section
Small aliquots of samples (40–100 µL) were polarized in the HyperSense commercial polarizer at 3.35 T and 1.4 K with a 100 mW microwave source. After 2–4 hours of irradiation, the samples were dissolved with 4 mL superheated water or methanol and the dissolution liquid was rapidly transferred (t=8 s) to a 10 mm NMR tube inside a 9.4 T high resolution Varian VNMRS magnet for NMR measurements at 298 K. The NMR measurements of hyperpolarized samples were referenced to a 3 M aqueous solution of AgNO3. See Supporting Information for detailed sample preparation and experimental setup.
Supplementary Material
Footnotes
This work is supported by the NIH (grant number R21EB009147).
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Berners-Price SJ, Johnson RK, Giovenella AJ, Faucette LF, Mirabelli CK, Sadler PJ. J. Inorg. Biochem. 1988;33:285. doi: 10.1016/0162-0134(88)80007-2. [DOI] [PubMed] [Google Scholar]
- 2.Gyr T, Macke HR, Hennig M. Angew. Chem., Int. Ed. Engl. 1998;36:2786. [Google Scholar]
- 3.Penner GH, Liu X. Prog. Nucl. Magn. Reson. Spectrosc. 2006;49:151. [Google Scholar]
- 4.Price SJB, Brevard C, Pagelot A, Sadler PJ. Inorg. Chem. 1985;24:4278. [Google Scholar]
- 5.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10158. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lumata L, Jindal AK, Merritt ME, Malloy CR, Sherry AD, Kovacs Z. J. Am. Chem. Soc. 2011;133:8673–8680. doi: 10.1021/ja201880y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lumata L, Ratnakar SJ, Jindal A, Merritt M, Comment A, Malloy C, Sherry AD, Kovacs Z. Chem. Eur. J. 2011;17:10825–10827. doi: 10.1002/chem.201102037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abragam A, Goldman M. Rep. Prog. Phys. 1978;41:395. [Google Scholar]
- 9.Cox BG, Schneider H. Pure Appl. Chem. 1990;62:2259. [Google Scholar]
- 10.Henrichs PM, Ackerman JJH, Maciel GE. J. Am. Chem. Soc. 1977;99:2544. [Google Scholar]
- 11.Zangger K, Armitage IM. Met.-Based Drugs. 1999;6:239. doi: 10.1155/MBD.1999.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.