Abstract
Propylene Glycol (PG) is a common solvent used in medical preparations. It is “generally recognized as safe” at regulated concentrations, however its apoptotic potential is unknown. C57BL/6 mice (P4–30) were exposed to PG to examine whether PG could produce apoptosis in the developing CNS. PG triggered widespread apoptotic neurodegeneration with the greatest damage at P7. Significant apoptosis was observed at doses as low as 2mL/kg. These findings have implications for the safety of drug preparations used in pediatric medicine. The anticonvulsant phenobarbital (PB), which alone produces apoptosis in the immature CNS, is prepared in 68% PG and 10% Ethanol (EtOH). We assessed whether PG contributes to the neurotoxic potential of PB. The agents (both at sub-toxic doses) produce significantly more apoptosis when used in combination. In conclusion, finding an alternative non-apoptotic solvent that can be used as a substitute for PG may be beneficial to patients.
Keywords: Brain, Propylene Glycol, Solvent, Apoptotic Neurodegeneration, Synergistic Interactions, Drug preparations, Toxicity, Phenobarbital
Introduction
Apoptosis is a genetically regulated endogenous cellular process, in which a variety of extrinsic and intrinsic signals can activate multiple independent pathways resulting in cell death (1). Ethanol (EtOH), a commonly abused solvent, produces widespread apoptotic neurodegeneration in the developing mammalian brain (2, 3). Recently, we reported that dimethyl sulfoxide (DMSO), a widely used solvent in industry and biological research that is routinely given to children as part of a transplantation procedure, is able to mimic this neurodegenerative process in the developing mouse brain (4). This solvent-induced apoptosis appears to be most severe during the period of synaptogenesis (the brain growth spurt). In rodents the period of vulnerability occurs during the first several weeks of post-natal life, whilst in humans it extends from the second trimester of gestation to several years after birth (5).
Propylene Glycol (PG; 1, 2-Propanediol) is another solvent frequently used in the preparation of pharmaceuticals, as well as in foods and cosmetics. The US Food and Drug Administration have identified PG as being “generally recognized as safe” (6), with an acceptable daily intake of up to 25mg/kg (7). Although it is considered to be a nontoxic “inactive” ingredient in pharmaceuticals, the American Academy of Pediatrics Committee on Drugs recognized PG as a potential toxicant over 20 years ago (8). These concerns developed due to early studies linking PG to CNS disturbances in children, including depression and seizures (9, 10). During the last decade, PG toxicity has been increasingly reported in human infants and adults. Adverse effects associated with PG exposure include hyperosmolality, metabolic abnormalities, hypotension, renal dysfunction and multi-organ failure (11–18); however there are no recent studies directly linking PG exposures to CNS disturbances in humans.
Many agents used in pediatric neurology are prepared in a liquid medium containing from 30–80% PG, and often also containing 10% EtOH. The use of PG as a vehicle for intravenous drugs is of major concern for two reasons. Firstly, infants can become exposed to extremely high doses of PG, as is the case in the Pediatric Intensive Care Unit (PICU), where sedatives are administered as long-term i.v. infusions (19, 20). Secondly, many clinically used agents can alone produce apoptosis in the immature CNS, including phenobarbital (PB) (21, 22), which is prepared in 68% (v/v) PG and 10% EtOH. The combined effects of chemicals in these mixtures are unclear and have not been widely studied. Collectively, they may have some adverse effects that are greater than expected, even if their individual doses are sub-toxic.
Little information is available about the direct neurotoxic potential of PG. The purpose of this study was therefore to examine whether PG could induce apoptosis in the developing CNS. Since PG is used as the vehicle for compounds known to produce apoptosis, we also assessed whether the combined effect of PB dissolved in PG would produce significantly more neuroapoptosis than PB dissolved in saline.
Methods
Animals and drugs
Research procedures were approved by Washington University in St Louis' Institutional Animal Care and Use Committee. C57BL/6 mice (Harlan, Indianapolis) were used in all experiments. For single-dose exposure studies, animals received a single intraperitoneal injection of 100% (v/v) PG at various doses (1–10mL/kg; laboratory grade (USP/FCC; >99% pure); Fisher Scientific, Fair Lawn, NJ), or 10mL/kg body weight of saline. To detect apoptotic cell death in the developing brain, postnatal-day (P) seven mice (n=12) were exposed to a one-time 10mL/kg dose of PG. To determine the dose-response effects, P7 mice (n=36) were exposed to various doses of PG (0, 1, 2, 3, 5, and 10mL/kg). To determine whether animals of different ages are sensitive to PG-induced apoptosis, P4, P7, P14, P17, P24 and P30 mice (n=72) were exposed to a one-time 10mL/kg dose of PG. To determine whether PG would augment the apoptotic potential of PB, combination studies were conducted. P7 mice (n=26) were exposed to a one-time dose of PB (5mg/kg; 5-Ethyl-5-phenyl-2,4,6-trioxohexa-hydropyrimidine; Sigma-Aldrich, St. Louis, MO), which was prepared as a solution dissolved in either saline or 68% (v/v) PG (0.68mL/kg administered intraperitoneally). The 5mg/kg dose of PB was previously determined to be non-toxic (i.e. exposure does not increase apoptosis when compared to saline). Treatment conditions were distributed equally between sexes and in pre-weaning animals across litters in order to control for potential litter or gender effects. At various intervals between 2 and 24h following treatment, animals were anesthetized with sodium pentobarbital and perfused as previously described (4). Brains were processed with one of three histological procedures.
Histological Procedures
For Activated Caspase-3 (AC-3) Immunohistochemistry and De Olmos silver staining, brains were cut into 70μm transverse sections on a vibratome. Sections were processed according to protocols previously described (4). Staining was visualized with light microscopy. Areas of damage were identified using an atlas of the developing mouse brain (23). Plastic sections for electron microscopy were prepared as previously described (4) and viewed in a JEOL 100 CX transmission electron microscope.
Stereology
Cell counts were performed using Stereoinvestigator 7.5 (MicroBrightField, Colchester, VT). Every 8th section was chosen from each brain (excluding brainstem and cerebellum) and processed for AC-3 immunohistochemistry. The optical fractionator technique was used to obtain unbiased estimates of the total number of apoptotic cells in the brain. Guard volume was set to 5.0μm, whilst inter-section interval, counting frame size, and distance between counting frames were adjusted to sample approximately 200 degenerating cells. The counter was blind to litter, gender and treatment.
Statistical Analysis
Data are presented as mean (± SEM). Dose-response analysis data were evaluated using a four-parameter logistic equation (GraphPad Prism Software Inc., San Diego, CA). A one-way ANOVA with subsequent post-hoc comparisons was used to judge significance of the observed effect. For mixture effect studies, comparison of data was calculated by Student's two-tailed t-test following tests to determine normality of data. Differences were considered significant at P<0.05.
Results
Pattern and time course of caspase-3 activation
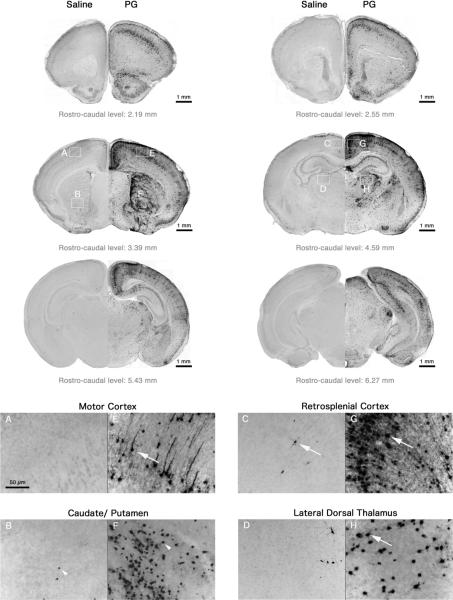
Caspase-3 is the major effector caspase in the CNS (24) and immunohistochemical detection of AC-3 immunoreactivity has proven to be a sensitive measure of apoptosis (3). Brains were initially examined at 8h after injection since this time point was found to produce the peak levels of damage of intact cells in our previous studies (4). In saline brains, AC-3 positive neurons were sparse and sporadically distributed within various regions, showing a pattern typical of physiological cell death (Figure 1). PG produced a striking increase in AC-3 positive cells throughout the brain (Figure 1), as compared to saline-treated animals. The most severely affected areas were the caudate/putamen (CPu) and cortical regions (especially the retrosplenial, cingulate, and motor cortex). The nucleus accumbens, superior colliculus, pyramidal cell layer of the hippocampus, subiculum, and specific areas of the thalamus (lateral-dorsal (LD Th), lateral-rostral, reticular, lateral-geniculate nucleus) were also damaged. Affected cells were relatively intact in most of these regions, with staining in the nucleus, cell body, and entire dendritic tree. However, staining in the CPu was localized to cell bodies (Figure 1F) and cells appeared to be in an advanced apoptotic stage, suggesting the onset of degeneration in this region begins at an earlier time-point or that degeneration progresses at a faster rate. Furthermore, damage appeared to be more intense in the lateral part of the CPu.
Figure 1.
Sections from P7 mouse brain following 8h treatment with saline or PG stained immunohistochemically with antibodies to AC-3. Saline brains (A–D) showed a pattern of sparse AC-3 positive neurons (white arrows) that is typical of physiological cell death, whilst PG triggered widespread neuroapoptosis in many brain regions (E–H). In the CPu, cells appeared to be in a later stage of the apoptotic process (staining localized to intact condensed cell bodies; white arrow heads). Top images were viewed at 10× magnification. High-powered photomicrographs were viewed at 20×.
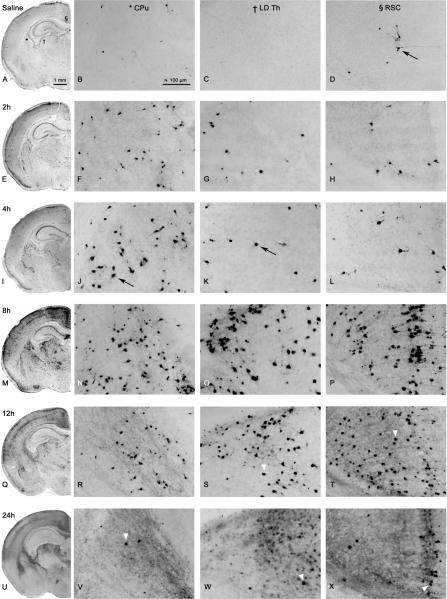
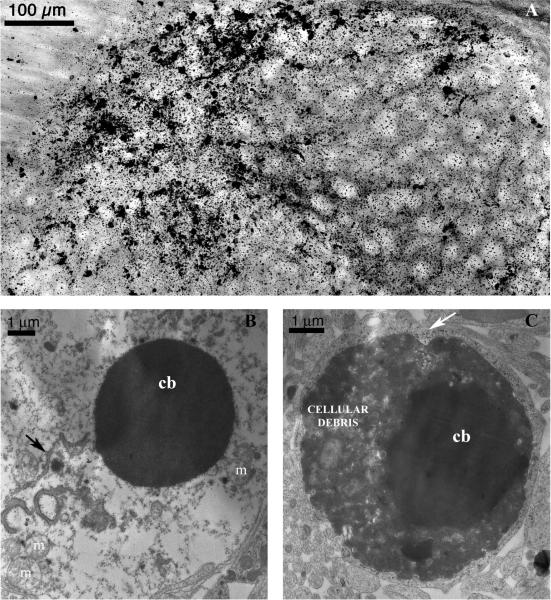
The pattern of degeneration was then examined following other survival intervals – 2h, 4h, 12h, and 24h after injection (n=24). These time course experiments (Figure 2) revealed the cell death process was relatively rapid with cells beginning to degenerate as early as 2h (Figure 2E–H). At 2h, the majority of damage was concentrated in the CPu (Figure 2F), however there was sporadic scattering of AC-3 positive cells all over most cortical regions, with more intense damage to layer 2 neurons. Pyramidal cells in the ventral aspects of the hippocampus were also damaged. There was an overall increase in apoptotic cell death at 4h (Figure 2I–L), where dendrite staining was now visible. Peak levels of damage were reached by 8h (Figure 2M–P). Sporadic damage was still present, however damage to specific regions was more obvious, and cortical damage had progressed to involve layer 4 neurons. At 12h, overall AC-3 staining was decreased (Figure 2Q–T). At 24h, staining of intact AC-3 positive cells in affected brain regions was dramatically reduced. In most of these animals the AC-3 levels were similar to those seen in saline controls. However, the most severely affected animal after 24h treatment is shown in Figure 2 (U–X), where AC-3 positive cells are clearly elevated from controls. Furthermore, an extensive diffuse non-specific type of staining was present in these regions at 24h, possibly due to the presence of cellular debris. De Olmos silver staining at 24h shows severely fragmented cells that display the characteristics of late-stage apoptosis, including condensed cell bodies and compact debris of the degeneration products (Figure 3A). To confirm whether PG produced degeneration via an apoptotic mechanism, electron microscopy was used. Cell death triggered by PG was found to have morphological characteristics of apoptosis (25, 26), including chromatin balls, cytoplasmic condensation, and disintegration of the nuclear membrane (Figure 3B–C).
Figure 2.
Histological sections from P7 mouse brain treated with saline (A–D) or PG (E–X) at various time points after exposure. Black arrows depict AC-3 positive-cells. Hemi-sections are taken from the same rostro-caudal level (10× magnification). High-powered photomicrographs (20× magnification) are taken from the CPu (*), LD Th (†), and retrosplenial cortex (RSC; §), as indicated by a symbol in panel A. Onset of damage appeared at 2h in the CPu (F). Peak levels of damage occurred at 8h in most regions (M–P). Overall AC-3 staining was decreased at 12h (Q–T) and 24h (U–X), where staining was specific to intact cell bodies (white arrow heads). In addition to localized staining, a non-specific diffuse staining was present at 24h.
Figure 3.
De Olmos silver staining at 24h shows advanced-stage degeneration (A - image taken from LD Th of a P7 animal exposed to 10mL/kg PG (10× magnification). Cell bodies are amorphous, axons and dendrites are no longer visible, and cellular debris is scattered throughout the region. Electron Microscopy photomicrographs (10,000× magnification) (B–C) demonstrate cells with classical hallmarks of apoptosis (8h exposure to 10mL/kg PG). Image (B) shows the nuclear membrane is no longer intact (broken membrane remnant: black arrow), allowing nuclear and cytoplasmic compartments to mix. Mitochondria appear to be swollen (m), and a condensed spherical chromatin ball (cb) has formed. A very late stage apoptotic cell (C) shows migration of nuclear chromatin masses into the cytoplasm and disintegration of material inside the cell. The apoptotic cell can be distinguished from adjacent tissue cells; however the surrounding phagocyte has already started to encroach into the degenerating cell (white arrow).
Dose-dependency of PG-induced apoptosis
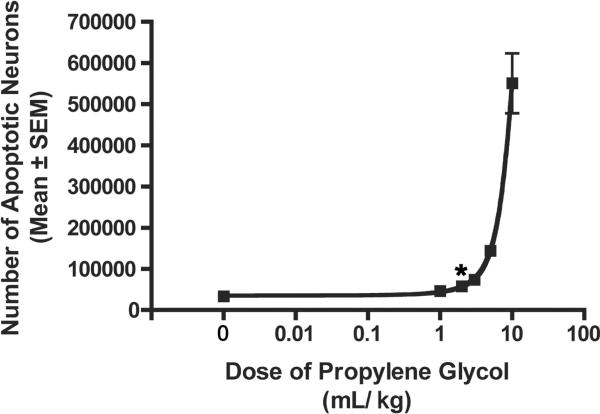
PG produces apoptotic neurodegeneration in a dose dependent manner (F[5,35]=42.55, P<0.0001; Figure 4). At 1mL/kg there was no significant difference between AC-3 cell counts for PG-treated and saline animals. However, significantly greater damage was observed at 2mL/kg compared to saline (P=0.0042). At this dose, approximately 60,000 neurons died by apoptosis compared to background levels of 30,000 as seen in controls. At 10mL/kg over 500,000 neurons were undergoing apoptosis. Considerable animal death occurred at doses above 10mL/kg. Consequently, complete dose-response data was not developed and an ED50 for PG could not be calculated.
Figure 4.
P7 animals were exposed for 8h to various doses of PG. Total AC-3 positive neurons were estimated using stereology. Number of degenerating cells increased with increasing doses of PG (F[5,35]=42.55, P<0.0001; R2 of 0.99; n=6 at each dose tested). Significantly greater damage was observed at 2mL/kg compared to saline (*P=0.0042). Error bars are too small to be seen.
Assessment of the combined effect of PB and PG
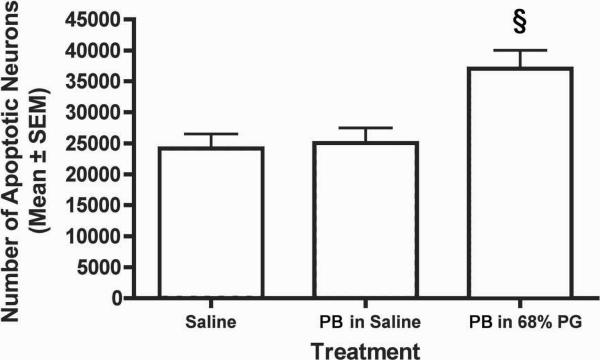
PB (5 mg/kg) prepared in saline produced similar levels of apoptosis to saline controls (Figure 5). However, PB prepared in a sub-toxic dose of 68% (v/v) PG (as given clinically) produced significantly more apoptosis than PB in saline alone (P<0.05). Approximately 12,000 more neurons died when using PG as a solvent, an amount almost 50% greater than the damage observed when using saline. Due to concern that this observed effect between PG and PB could be a reflection of a pharmacokinetic interaction in which PG were raising the concentration of PB, we conducted additional experiments using higher doses of PB in saline. We found that 20mg/kg of PB in saline did not produce any detectable apoptosis but 40mg/kg did. Thus, if a pharmacokinetic interaction were to underlie the observed effect, PG would need to be producing between a 4- to 8-fold increase in the concentration of PB in order to account for the observed effect.
Figure 5.
The combined effect of PB and PG, both used at sub-toxic doses. 5mg/kg PB prepared in saline (n=8) produces similar levels of apoptosis to saline alone (n=8). PB prepared in 68% PG (n=10) produces significantly more apoptosis than PB prepared in saline (§P<0.05).
Age-dependency of PG-induced apoptosis
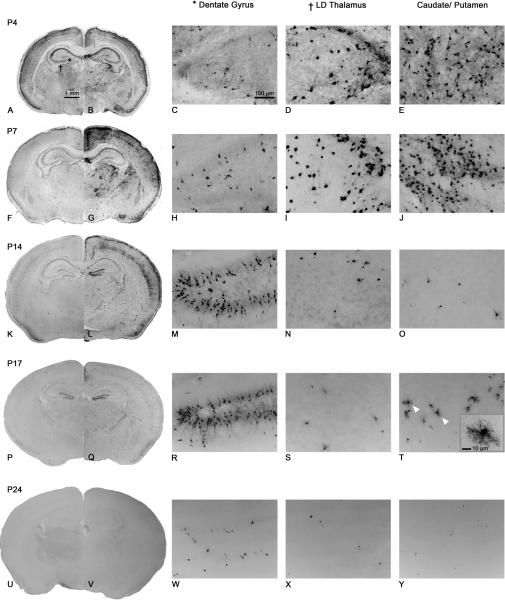
Severity and location of damage varied between different ages (Figure 6). The greatest amount of PG-induced apoptosis occurred in P7 animals (Figure 6F–J). At P4 (Figure 6A–E), the pattern of degeneration was similar to that of P7, however the overall damage was less severe. The sporadic pattern of background staining was present, and some of the same regions were damaged including the CPu, most cortical regions and thalamic nuclei. However, there were some subtle differences between P4 and P7 treated animals. Some regions that were damaged at P7 did not appear to be affected at P4: dorsal lateral geniculate nucleus, superior colliculus, subiculum, auditory cortex and visual cortex. The most striking difference was the distribution of AC-3 cells within the cortex. At P4, damage was limited to layer 4 neurons, whereas at P7 there was damage to layer 2 and layer 4 neurons. At P14 (Figure 6K–O), the overall damage was dramatically reduced. Sporadic background staining was still present and most cortical regions were damaged, but very little damage was observed in the CPu or in the LD Th. Apoptosis also occurred in new regions that were not damaged at P4 and P7, the most noticeable area being the granule cell layer of the dentate gyrus (Figure 6M). In addition, the neuroepithelium, anterior olfactory nucleus, and dorsal aspects of the hippocampus were also damaged at P14. At P17 (Figure 6P–T) there was still damage present in the dentate gyrus (Figure 6R) and anterior olfactory nucleus. The specific layers of damage in the cortex as previously seen at P4, P7 and P14 could not be seen at this age. Damage could now be seen in the CPu, however closer examination of the cells showed that damaged cells appeared to be oligodendrocytes (Figure 6T). No damage was detected at P24 (Figure 6U–Y) and P30 (not shown).
Figure 6.
Histological sections from P4–24 mouse brain following 8h treatment with saline (left hemi-sections) or PG (right hemi-sections); viewed at 10×. Dentate gyrus (*) and LD Th (†) photomicrographs are taken from the same section as those indicated by a symbol in panel A. Caudate/putamen photomicrographs are taken from a more anterior section of the brain to demonstrate the extent of damage in this region. High-powered images were viewed at 20×. At P4 (A–E), severe damage was seen in the LD Th and CPu. These same regions were damaged at P7 (F–J), the age at which overall damage peaked. At P14 (K–O), overall damage was decreased and both LD Th and CPu showed similar levels of AC-3 positive cells as saline. The granule cell layer of the dentate gyrus was severely damaged at P14 (M), whereas this region was not damaged at P4 (C) or P7 (H). At P17 (P–T), AC-3 positive cells were still present in the dentate gyrus (R), however overall damage was further reduced. A new population of dying cells appeared in the CPu (white arrow heads). Closer examination of these cells showed morphology typical of oligodendrocytes (T; box at 40× magnification). At P24 (U–Y), damage was similar to saline. Staining was extremely sparse making it difficult to see at low magnification.
Discussion
PG is used as a solvent for topical, oral and injectable medications, and is generally considered to be safe. However, here we report that PG produces neurodegeneration in the developing mouse CNS. The degenerative process was relatively rapid with cells beginning to fragment within 12 hours of drug exposure. The degeneration is AC-3 positive and has morphological characteristics of apoptosis, as confirmed by electron microscopy. To our knowledge this is the first report to show that PG produces apoptosis in the CNS of any species. The pattern of degeneration seen with PG was largely similar to that of EtOH (2) and DMSO (4). Likewise, the observed damage was dependent on age at the time of exposure. Peak sensitivity was at P7, where damage was widespread but appeared to affect specific populations of neurons. The damage was most striking in the CPu, thalamus and layers 2 and 4 of the cortex. The specific cell populations undergoing degeneration also varied at different ages tested. The most noticeable difference being that damage was consistently seen in the dentate gyrus at P14 and P17, whereas it was not seen at earlier ages.
EtOH-induced neuroapoptosis is believed to be mediated by a duel mechanism; blockade of NMDA receptors and hyperactivation of GABAA receptors (2). While the pattern of PG-induced neuroapoptosis has a close resemblance to that of EtOH, there was no damage in the anterodorsal nucleus of the thalamus (AD). Damage in this area by EtOH has been attributed to its GABAergic activity. Lack of damage in the AD following PG exposure suggests that PG is not producing damage through GABA receptors. DMSO has been reported to inhibit NMDA receptors (27), and NMDA antagonists also produce apoptosis (28). Anticonvulsants that inhibit voltage-gated sodium channels are also known to induce apoptosis (21). It is unknown whether PG has any of these properties that could account for its apoptogenic liability. Further work is needed to determine the mechanism underlying the observed damage.
Infants are readily exposed to PG in foods and medicines. The use of PG as a vehicle for pediatric i.v. medications (e.g. diazepam, lorazepam, phenytoin, and PB) means that infants are exposed to PG during a critical period of neural development, when their brains would be sensitive to apoptogenic effects of the solvent. The apoptotic effect of PG was found to be dose dependent, with significant apoptosis detected at doses as low as 2mL/kg. However, the effect was not significant at 1mL/kg. Based on these results we conclude that the toxic threshold is between 1 and 2mL/kg. The amount of PG a child would receive following a single loading dose of 40mg/kg PB is 0.2mL/kg. A 5–10 fold difference between routine clinical exposure and the toxic threshold would suggest that a one-time exposure to PG is unlikely to produce apoptosis. However, it is unknown whether humans and mice produce similar blood PG levels following the same mg/kg dose. Due to potential interspecies differences in pharmacokinetics, the true outcome in humans is difficult to predict. Pharmacokinetic studies in both newborn mice and human neonates determining the blood PG levels achieved following PG exposure are needed to assess if our initial conclusion about the safety of a single exposure is correct.
More concerning are instances when human infants are exposed to PG over a prolonged period, since the toxic threshold is likely to be surpassed. For example, significant PG accumulation in serum (9-fold increase from baseline levels to the end of infusion) has been reported in infants receiving continuous infusion of lorazepam for sedation (19). A more recent study reported that critically ill neonates administered medications via continuous infusion were exposed to doses of PG that were 180 times the acceptable daily intake (20). Furthermore, for treatment of certain clinical conditions such as status epilepticus, it is not uncommon to use multiple drugs, many of which are prepared as solutions containing from 30–80% PG. Typically, lorazepam (prepared in 80% PG and 10% EtOH) is administered intravenously at approximately 0.1mg/kg. If seizures are not immediately arrested, PB (prepared in 68% PG and 10% EtOH) is administered intravenously at a dose of up to 40mg/kg over a brief period of time (< 1 hr usually). Unfortunately at least 50% of patients are still seizing after receiving the lorazepam/PB regimen. For these patients, phenytoin (an anticonvulsant also dissolved in PG and EtOH), fosphenytoin (an anticonvulsant that is not dissolved in PG or EtOH) or additional PB is given until seizures are arrested. Since unmitigated seizure activity results in widespread excitotoxic damage in the brain, our findings do not argue that clinicians should not aggressively treat status epileticus but rather suggest that treatments utilizing a non-toxic solvent are needed.
PG is not given in isolation but it is typically used in conjunction with another agent. To determine the true effect of using PG as a solvent, we investigated whether a mixture of PB dissolved in PG could produce more neuroapoptosis than PB dissolved in saline. Individual agents were used at doses that alone do not produce apoptosis: 5mg/kg PB (standard clinical maintenance dose) and 68% PG (0.68mL/kg; Figure 4). Our results indicate that PB dissolved in PG produces significantly greater apoptosis than PB dissolved in saline, raising additional concerns about the safety of PG when used as a solvent. In these cases where mixtures of compounds are used, the toxic threshold dose of PG would be lower than 1–2mL/kg. Assessing the combined effect of chemicals is complex and often unpredictable (29). Potential effects include: additivity, where agents are no more and no less effective in combination than they are separately and synergism, where the effectiveness of agents is increased when in combination (30). It is uncertain whether the observed effects in our study represent synergism or additivity. However, it is clear that the combination of 2 agents at non-toxic doses can lead to a significant toxic effect. To differentiate between these 2 effects, more rigorous testing is required such as the “dose additivity” and “effect additivity” methods of analyzing interactions (29).
In human infants PG exposure has been associated with many acute toxic effects including CNS disturbances such as drowsiness and confusion (9, 10, 12, 31, 32). Rigorous behavioral experiments were not conducted in our study, but we noticed that sedation and drowsiness were seen in animals treated with higher PG doses. However, at lower doses where significant apoptosis was still detectable, there was no notable drowsiness. This finding suggests that apoptosis could be occurring in humans who have accumulated high enough levels of PG to develop CNS disturbances acutely. More importantly, it is still unknown whether apoptosis results in long-term cognitive and behavioral abnormalities. This issue has not been addressed in either humans or animals for PG. For EtOH, a single several hour exposure in P7 mice results in substantial apoptosis and produces detectable impairments in spatial learning and memory when animals are tested as juveniles (33). Furthermore, EtOH-induced apoptosis is considered to be a potential mechanism accounting for some of the CNS sequelae associated with Fetal Alcohol Syndrome in humans (34). Further studies are needed to clarify the long-term CNS consequences of PG exposure, including potential cognitive and behavioral outcomes.
Acknowledgments
STATEMENT OF FINANCIAL SUPPORT: This work was supported by: the National Institutes of Health (P30HD062171, R01ES012443 to N.B.F. and K01MH083046 to K.K.N.).
ABBREVIATIONS
- AC-3
activated caspase-3
- CPu
caudate/putamen
- EtOH
ethanol
- LD Th
lateral-dorsal thalamus
- P
postnatal day
- PB
phenobarbital
- PG
propylene glycol
REFERENCES
- 1.Lockshin RA, Zakeri Z. Programmed cell death and apoptosis: origins of the theory. Nat Rev Mol Cell Biol. 2001;2:545–550. doi: 10.1038/35080097. [DOI] [PubMed] [Google Scholar]
- 2.Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- 3.Olney JW, Tenkova T, Dikranian K, Muglia LJ, Jermakowicz WJ, D'Sa C, Roth KA. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis. 2002;9:205–219. doi: 10.1006/nbdi.2001.0475. [DOI] [PubMed] [Google Scholar]
- 4.Hanslick JL, Lau K, Noguchi KK, Olney JW, Zorumski CF, Mennerick S, Farber NB. Dimethyl sulfoxide (DMSO) produces widespread apoptosis in the developing central nervous system. Neurobiol Dis. 2009;34:1–10. doi: 10.1016/j.nbd.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 6.Food and drug administration GRAS status of propylene glycol and propylene glycol monostearate Fed Regist. 1982;47:27810. 21 CFR 184.1666. [Google Scholar]
- 7.Joint FAO/WHO Expert Committee on Food Additives (JECFA) Toxicological evaluation of some food additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. WHO Food Additives; Geneva: 1974. (Series, No. 5). [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics “Inactive” ingredients in pharmaceutical products Committee on Drugs. Pediatrics. 1985;76:635–643. [PubMed] [Google Scholar]
- 9.Martin G, Finberg L. Propylene glycol: a potentially toxic vehicle in liquid dosage form. J Pediatr. 1970;77:877–878. doi: 10.1016/s0022-3476(70)80253-0. [DOI] [PubMed] [Google Scholar]
- 10.Arulanantham K, Genel M. Central nervous system toxicity associated with ingestion of propylene glycol. J Pediatr. 1978;93:515–516. doi: 10.1016/s0022-3476(78)81183-4. [DOI] [PubMed] [Google Scholar]
- 11.Demey HE, Daelemans RA, Verpooten GA, De Broe ME, Van Campenhout CM, Lakiere FV, Schepens PJ, Bossaert LL. Propylene glycol-induced side effects during intravenous nitroglycerin therapy. Intensive Care Med. 1988;14:221–226. doi: 10.1007/BF00717993. [DOI] [PubMed] [Google Scholar]
- 12.Guillot M, Bocquet G, Eckart P, Amiour M, el-Hachem C, Garnier R, Galliot-Guilley M, Haguenoer JM. Home environment and acute propylene glycol intoxication in a two-year old. An unusual case report. Arch Pediatr. 2002;9:382–384. doi: 10.1016/s0929-693x(01)00797-7. [DOI] [PubMed] [Google Scholar]
- 13.Yaucher NE, Fish JT, Smith HW, Wells JA. Propylene glycol-associated renal toxicity from lorazepam infusion. Pharmacotherapy. 2003;23:1094–1099. doi: 10.1592/phco.23.10.1094.32762. [DOI] [PubMed] [Google Scholar]
- 14.Wilson KC, Reardon C, Farber HW. Propylene glycol toxicity in a patient receiving intravenous diazepam. N Engl J Med. 2000;343:815. doi: 10.1056/nejm200009143431115. [DOI] [PubMed] [Google Scholar]
- 15.Wilson KC, Reardon C, Theodore AC, Farber HW. Propylene glycol toxicity: a severe iatrogenic illness in ICU patients receiving IV benzodiazepines: a case series and prospective, observational pilot study. Chest. 2005;128:1674–1681. doi: 10.1378/chest.128.3.1674. [DOI] [PubMed] [Google Scholar]
- 16.Bledsoe KA, Kramer AH. Propylene glycol toxicity complicating use of barbiturate coma. Neurocrit Care. 2008;9:122–124. doi: 10.1007/s12028-008-9065-z. [DOI] [PubMed] [Google Scholar]
- 17.Miller MA, Forni A, Yogaratnam D. Propylene glycol-induced lactic acidosis in a patient receiving continuous infusion pentobarbital. Ann Pharmacother. 2008;42:1502–1506. doi: 10.1345/aph.1L186. [DOI] [PubMed] [Google Scholar]
- 18.Food and Drug Administration [accessed July 22, 2011];Drug Safety Communication: Serious health problems seen in premature babies given Kaletra (lopinavir/ritonavir) oral solution. 2011 Mar 8; http://www.fda.gov/Drugs/DrugSafety/ucm246002.htm#safety_announcement,
- 19.Chicella M, Jansen P, Parthiban A, Marlowe KF, Bencsath FA, Krueger KP, Boerth R. Propylene glycol accumulation associated with continuous infusion of lorazepam in pediatric intensive care patients. Crit Care Med. 2002;30:2752–2756. doi: 10.1097/00003246-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Shehab N, Lewis CL, Streetman DD, Donn SM. Exposure to the pharmaceutical excipients benzyl alcohol and propylene glycol among critically ill neonates. Pediatr Crit Care Med. 2009;10:256–259. doi: 10.1097/PCC.0b013e31819a383c. [DOI] [PubMed] [Google Scholar]
- 21.Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farber NB, Olney JW. Drugs of abuse that cause developing neurons to commit suicide. Brain Res Dev Brain Res. 2003;147:37–45. doi: 10.1016/j.devbrainres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Halliday G, Watson C, Koutcherov Y, Wang H. Atlas of the Developing Mouse Brain. Elsevier; Amsterdam: 2007. [Google Scholar]
- 24.Kuan CY, Roth KA, Flavell RA, Rakic P. Mechanisms of programmed cell death in the developing brain. Trends Neurosci. 2000;23:291–297. doi: 10.1016/s0166-2236(00)01581-2. [DOI] [PubMed] [Google Scholar]
- 25.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishimaru MJ, Ikonomidou C, Tenkova TI, Der TC, Dikranian K, Sesma MA, Olney JW. Distinguishing excitotoxic from apoptotic neurodegeneration in the developing rat brain. J Comp Neurol. 1999;408:461–476. [PubMed] [Google Scholar]
- 27.Lu C, Mattson MP. Dimethyl sulfoxide suppresses NMDA- and AMPA-induced ion currents and calcium influx and protects against excitotoxic death in hippocampal neurons. Exp Neurol. 2001;170:180–185. doi: 10.1006/exnr.2001.7686. [DOI] [PubMed] [Google Scholar]
- 28.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 29.Lau K, McLean WG, Williams DP, Howard CV. Synergistic interactions between commonly used food additives in a developmental neurotoxicity test. Toxicol Sci. 2006;90:178–187. doi: 10.1093/toxsci/kfj073. [DOI] [PubMed] [Google Scholar]
- 30.Berenbaum MC. What is synergy? Pharmacol Rev. 1989;41:93–141. [PubMed] [Google Scholar]
- 31.Glasgow AM, Boeckx RL, Miller MK, MacDonald MG, August GP, Goodman SI. Hyperosmolality in small infants due to propylene glycol. Pediatrics. 1983;72:353–355. [PubMed] [Google Scholar]
- 32.Fligner CL, Jack R, Twiggs GA, Raisys VA. Hyperosmolality induced by propylene glycol. A complication of silver sulfadiazine therapy. JAMA. 1985;253:1606–1609. [PubMed] [Google Scholar]
- 33.Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T, Young C, Olney JW, Muglia LJ. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol Dis. 2004;17:403–414. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Olney JW, Wozniak DF, Farber NB, Jevtovic-Todorovic V, Bittigau P, Ikonomidou C. The enigma of fetal alcohol neurotoxicity. Ann Med. 2002;34:109–119. doi: 10.1080/07853890252953509. [DOI] [PubMed] [Google Scholar]