Abstract
Homozygous or compound heterozygous Renin (REN) mutations cause renal tubular dysgenesis (RTD), which is characterized by death in utero due to renal failure and pulmonary hypoplasia. The phenotype resembles the fetopathy caused by angiotensin-converting enzyme inhibitor or angiotensin receptor blocker intake during pregnancy. Recently, heterozygous REN mutations were shown to result in early-onset hyperuricemia, anemia and chronic renal failure. So far, only three different heterozygous REN mutations were reported.
We performed mutation analysis of the REN gene in 39 kindreds with hyperuricemia and chronic kidney disease (CKD) previously tested negative for mutations in the UMOD and HNF1β genes. We identified one kindred with a novel c.28T>C (p.W10R) REN mutation in the signal sequence, concluding that REN mutations are rare events in CKD patients. Affected individuals over four generations were identified carrying the novel REN mutation and were characterized by significant anemia, hyperuricemia and CKD. Anemia was severe and disproportional to the degree of renal impairment. Moreover all heterozygous REN mutations are localized in the signal sequence. Therefore, screening of the REN gene for CKD patients with hyperuricemia and anemia may be focusing on exon 1 sequencing, which encodes the signal peptide.
Introduction
Renin is synthesized as a preproprotein called preprorenin. Preprorenin is targeted to the endoplasmatic reticulum (ER) by a signal sequence where the protein undergoes glycosylation and proteolytic processing, resulting in prorenin (1). Further processing results in storage of renin in electro-dense granules in the juxtaglomerular cells, where renin is secreted upon stimulation by lower blood pressures (baroreceptor reflex) or decresed chloride delivery to the macula densa (2,3).
Mutations in the Renin (REN) gene were reported in patients with renal tubular dysgenesis (RTD) (MIM 267430) (4). Homozygous or compound heterozygous REN mutations were reported in neonates or fetuses, who had died in utero or soon after birth due to anuria and pulmonary hypoplasia (5). The phenotype resembles the fetopathy caused by angiotensin-converting-enzyme (ACE) inhibitor or angiotensin II receptor antagonists (ARB) intake during pregnancy (6,7).
Recently, three different heterozygous REN mutations were found in four European kindreds with autosomal dominant inheritance of anemia, hyperuricemia and chronic kidney disease (CKD) (8,9). With the exception of the severe anemia and low blood pressures this phenotype is almost indistinguishable from patients with Uromodulin (UMOD) mutations causing: (i) familial juvenile hyperuricemia (FJHN) (MIM 162000), (ii) medullary cystic kidney disease type 2 (MCKD2) (MIM 603860) and (iii) glomerulocystic kidney disease (GCKD) (MIM 609886). These disorders have been summarized under the term Uromodulin associated kidney disease (UAKD). Infrequently, mutations in the hepatocyte nuclear factor 1β (HNF1β) were reported with an UAKD phenotype (10).
We performed mutation analysis of the REN gene in 42 individuals from 39 different kindreds with a history of hyperuricemia and CKD, all previously tested negative for UMOD and HNF1β mutations.
Case Report
We detected a novel heterozygous REN mutation in one out of 39 kindreds (for more information about patients and methods see Supplementary Information S1), indicating that heterozygous REN mutations are a rare cause of CKD with hyperuricemia and anemia. The novel REN mutation c.28T>C (p.W10R) is located in exon 1 of the REN gene, which encodes the signal peptide.
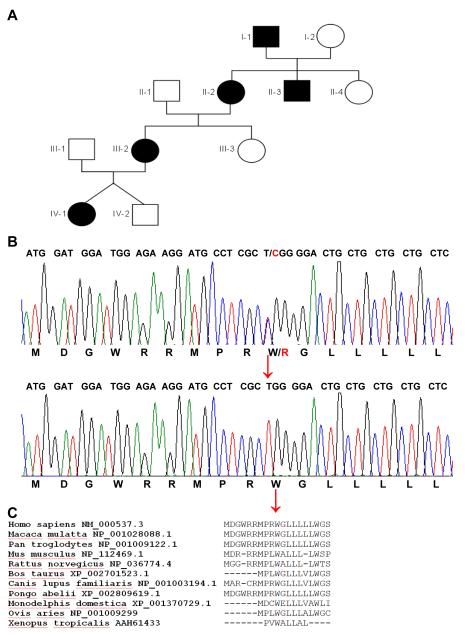
The family carrying the c.28T>C (p.W10R) mutation is of Caucasian descent and has affected individuals over four generations (Fig. 1A). Patient IV-1 is a 3 year old female, who was seen at the age of eleven months due to impaired renal function and significant anemia (Table 1). Her potassium was at the upper level of the reference range with 5.0 mEq/L (5 mmol/L). At the age of 3 years renal function remained stable with a glomerular filtration rate (GFR) of 61 ml/min/1.73m2 and a creatinine of 0.81 mg/dL (71.6 μmol/L). Her uric acid was elevated at 8.3 mg/dL (493.6 μmol/L) and her hemoglobin remained low at 9.2 g/dL (92 g/L). A renal ultrasound showed increased echogenicity but normal kidney size. She has not required any treatment. Her non-identical twin brother (IV-2 in Fig. 1A) is clinically not affected. Her mother (III-2) is a 35 year old female who presented at the age of 16 years with fatigue, impaired renal function, hyperuricemia and anemia (see Table 1). Her blood pressure was low at 90/70 mmHg. She is treated for her anemia with darbopoietin. The maternal grandmother (II-2) is 57 years old and presented at the age of 11 years with fatigue, anemia, and impaired kidney function (Table 1). A kidney biopsy showed tubular atrophy, interstitial fibrosis, and a thickened basement membrane of the Bowman’s capsule, with normal glomeruli. Uric acid was 10.2 mg/dL (606.7 μmol/L) at the age of 52 years. At the age of 55 years, she received a preemptive renal transplant. Her older brother (II-3) presented at the age of 15 years with anemia and fatigue. His renal function was reportedly impaired indicated by an elevated BUN. 15 years after initial presentation he was found to be in end-stage renal disease (ESRD) (Table 1). He was treated with allopurinol. A renal ultrasound 25 years after presentation showed bilaterally small kidneys. He started dialysis in his 40’s. 31 years after initial presentation he received a kidney transplant. The maternal great-grandfather (I-1) presented at the age of 28 years with fainting episodes and was found to be anemic. Due to anemia and impaired kidney function in his daughter (II-2) and son (II-3) he was evaluated at the age of 50 years, showing impaired kidney function, hyperuricemia (Table 1) and hypertension (156/80 mmHg). At the age of 55 years he died due to ESRD and congestive heart failure. Post mortem examination showed small kidneys (size 8 cm bilaterally), small cysts in the renal cortex, and a thickened basement membrane of the Bowman’s capsule.
Fig. 1.
A. Pedgree of the affected kindred with the novel c.28T>C (p.W10R) REN mutation. Males and females are affected in an autosomal dominant pattern over four generations. Affected individuals are indicated by black symbols whereas unaffected individuals are shown with white symbols. Males are indicated by a square, whereas females are indicated by circles.
B. The nucleotide sequence encoding the first 16 amino acids of Renin is shown. The upper sequence shows the heterozygous nucleotide exchange c.28T>C of individual IV-1, resulting in the amino acid exchange from tryptophan to arginine at position 10. Normal reference sequence is shown below. The nucleotide triplets encoding the corresponding amino acids are shown on top of the sequence. The mutation is shown in red and indicated by a red arrow. The corresponding amino acids are shown at the bottom of each sequence.
C. Evolutionary conservation of the signal peptide of Renin is shown. The different species are outlined on the left margin. Strong evolutionary conservation is shown for the affected amino acid at position 10 (p.10W) down to frog (red arrow). At the very end leucine 16 is shown which is affected by two different published mutations which alter the penta-leucine motif (8).
Table 1. Synospsis of the patients’ clinical information.
| I-1 | II-2 | II-3 | III-2 | IV-1 | |
|---|---|---|---|---|---|
|
Age at presentation |
28 yo | 11 yo | 15 yo | 16 yo | 11 mos |
| gender | M | F | M | F | F |
|
Onset of ESRD (years) |
55 yo | ~ 55 yo | ~ 30 yo | NA | NA |
|
Serum creatinine (mg/dl) |
ND | 1.55 | 4.6* | 2.2 | 0.55 |
| BUN (mg/dL) | 46# | 62 | 106* | 46 | 49 |
|
GFR (ml/min/1.73m2) |
ND | ND | ND | 37 | 66 |
|
Serum Uric Acid (mg/dL) |
8.0# | 10.2$ | Tx with allopurinol |
9.2 | 8.3% |
|
Hemoglobin (g/dL) |
↓ § | 10.0 | 9.5* | 10.0 | 9.1 |
|
Pathological findings |
Post mortem: small kidneys B/L, small cortical cysts, thickened Bowman’s capsule |
Bx: TA, IF, TBM |
NA | NA | NA |
| Imaging | Angio: small kidneys |
ND | US: small kidneys B/L |
ND | US: hyperechogenic kidneys, normal kidney size |
Angio, Angiogram; B/L, bilaterally; Bx, kidney biopsy; F, female; IF, interstitial fibrosis; M, male; ND, no data; NA, not applicable; TA, tubular atrophy; TBM, thickened basement membrane; Tx, treatment; US, renal ultrasound;
these lab results were obtained at the first documented lab data at the age of 50 years;
uric acid was first documented at the age of 52 years;
these laboratory results are the first documented lab data at the age of 30 years;
data obtained by history;
laboratory data obtained at age of 3 years
The novel heterozygous REN mutation (c.28T>C, p.W10R) is located in exon 1, which encodes for the signal peptide (Fig. 1B). The mutation segregates with all affected individuals of the kindred. We did not find the REN mutation (c.28T>C, p.W10R) in 100 ethnically matched healthy control individuals. The non-polar amino acid tryptophan is exchanged to the basic amino acid arginine. The tryptophan at position 10 of REN is highly evolutionary conserved in mammals (Fig. 1C). Analysis using the Polyphen-2 software (11) resulted in a significant score of 0.94 (with a maximum score of 1.0) indicating that this mutation is “probably damaging” (sensitivity 0.75, specifity 0.92). We also used the SignalP 3.0 (12) software in order to rule out that the p.W10R mutation would change the cleavage site for the Renin signal sequence. The hydrophobicity score was calculated by using the Kyte and Doolittle method and did not change significantly (13).
Discussion
The occurrence of anemia is surprising because kidney function in our patients was initially not profoundly impaired. Early onset of anemia has also been described by Zivná et al. with a one year old affected child (8). Interestingly, once children advance to adolescence anemia improves with normal hemoglobin levels as long as renal function is not too impaired (9). Increasing testosterone synthesis has been hypothesized to compensate for the childhood anemia (14). Anemia responded well to erythropoietin treatment but not to iron treatment (8,9). Anemia can also be found in patients treated with ACE inhibitors or ARBs (15,16). The RAS influences erythropoiesis mostly via angiotensin II (ANG II). AT1 receptors were identified on erythroid progenitor cells. Treatment of cultured erythroid progenitor cells with ANG II resulted in an increase of blood-forming units, which was blocked by the ARB losartan (17). ANG II also seems to act as a secretagogue for erythropoietin (EPO). Patients with renin-mediated hypertension were found to have higher serum EPO levels than patients with non-renin-mediated hypertension (18,19). This effect is most likely caused by ANG II-mediated vasoconstriction of the renal vessels, thus decreasing postglomerular peritubular blood flow and oxygen delivery to the renal tubulointerstitium (20,21). Moreover, ANG II has a stimulatory effect on proximal tubule sodium reabsorption, which increases oxygen requirement of tubular cells (22). Both events will trigger increased EPO production. Finally, ANG II has been shown to stimulate EPO secretion by direct interaction with hypoxia-inducible-factor 1 (HIF1), which is the master regulator of EPO production signaling hypoxia as a key regulator of erythropoiesis. In cell culture HIF1 expression was increased by adding ANG II (23).
Hyperuricemia is another key characteristic of patients with heterozygous REN mutations and was also previously reported (8,9). Low serum renin and aldosterone levels may result in relative hypotension causing increased proximal tubule sodium reabsorption, which subsequently may result in increased urate reabsorption (24). Impaired kidney function was found as early as three and four years (8,9). Renal function deteriorates very slowly over many years in most patients with progression to ESRD in the fifth or sixth decade. The slow decline may reflect intrinsic blockade of the RAS secondary to the REN mutation.
Computer-based analysis of the novel REN p.W10R did not show any alteration in regard of the signal peptide cleavage site or the hydrophobicity score. It is very likely that the p.W10R mutation is also impairing ER targeting as has been shown for the other heterozygous REN mutations (8,9). Over time this may result in chronic ER stress, triggering apoptosis and inflammation resulting in juxtaglomerular cell loss and nephron loss.
In conclusion, we describe a pedigree in which patients manifested anemia, hyperuricemia, and slowly evolving CKD in association with a heterozygous REN mutation. The incidence of REN mutations is likely to be low in CKD patients with hyperuricemia based on our study of 39 families. Screening of exon 1 may be most efficient as all heterozygous REN mutations are located in exon 1.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by the Maria Pesch Foundation Faculty of Medicine, University of Cologne (to B.B. Beck), NIH (DK088767) (to F. Hildebrandt) and the Pediatric Scientist Development Program (to M.T.F. Wolf). F. Hildebrandt is an investigator of the Howard Hughes Medical Institute, a Doris Duke Distinguished Clinical Scientist and a F.G.L. Huetwell Professor.
References
- 1.Imai T, Miyazaki H, Hirose S, et al. Cloning and sequence analysis of cDNA for human renin precursor. Proc Natl Acad Sci U S A. 1983;80(24):7405–7409. doi: 10.1073/pnas.80.24.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobian L, Tomboulian A, Janecek J. The effect of high perfusion pressures on the granulation of juxtaglomerular cells in an isolated kidney. J Clin Invest. 1959;38(4):605–610. doi: 10.1172/JCI103838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorenz JN, Weihprecht H, Schnermann J, Skøtt O, Briggs JP. Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am J Physiol. 1991;260(4 Pt 2):F486–493. doi: 10.1152/ajprenal.1991.260.4.F486. [DOI] [PubMed] [Google Scholar]
- 4.Gribouval O, Gonzales M, Neuhaus T, et al. Mutations in genes in the reninangiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet. 2005;37(9):964–968. doi: 10.1038/ng1623. [DOI] [PubMed] [Google Scholar]
- 5.Gubler MC, Antignac C. Renin-Angiotens in system in kidney development: renal tubular dysgenesis. Kidney Int. 2010;77(5):400–406. doi: 10.1038/ki.2009.423. [DOI] [PubMed] [Google Scholar]
- 6.Pryde PG, Sedman AB, Nugent CE, Barr M., Jr. Angiotensin-converting enzyme inhibitor fetopathy. J Am Soc Nephrol. 1993;3(9):1575–1582. doi: 10.1681/ASN.V391575. [DOI] [PubMed] [Google Scholar]
- 7.Martinovic J, Benachi A, Laurent N, Daikha-Dahmane F, Gubler MC. Fetal toxic effects and Angiotensin-II-receptor antagonists. Lancet. 2001;358(9277):241–242. doi: 10.1016/S0140-6736(01)05426-5. [DOI] [PubMed] [Google Scholar]
- 8.Zivná M, Hůlková H, Matignon M, et al. Dominant renin gene mutations associated with early-onset hyperuricemia, anemia, and chronic kidney failure. Am J Hum Genet. 2009;85(2):204–213. doi: 10.1016/j.ajhg.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bleyer AJ, Zivná M, Hulková H, et al. Clinical and molecular characterization of a family with a dominant renin gene mutation and response to treatment with fludrocortisone. Clin Nephrol. 2010;74(6):411–422. doi: 10.5414/cnp74411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bingham C, Ellard S, van’t Hoff WG, et al. Atypical familial juvenile hyperuricemic nephropathy associated with a hepatocyte nuclear factor-1beta gene mutation. Kidney Int. 2003;63(5):1645–1651. doi: 10.1046/j.1523-1755.2003.00903.x. [DOI] [PubMed] [Google Scholar]
- 11.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340(4):783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 14.Shahani S, Braga-Basaria M, Maggio M, Basaria S. Androgens and erythropoiesis: past and present. J Endocrinol Invest. 2009;32(8):704–716. doi: 10.1007/BF03345745. [DOI] [PubMed] [Google Scholar]
- 15.Griffing GT, Melby JC. Enalapril (MK-421) and the white cell count and haematocrit. Lancet. 1982;1(8285):1361. doi: 10.1016/s0140-6736(82)92430-8. [DOI] [PubMed] [Google Scholar]
- 16.Olsen MH, Wachtell K, Beevers G, et al. Prognostic importance of hemoglobin in hypertensive patients with electrocardiographic left ventricular hypertrophy: the Losartan intervention for end point reduction in hypertension (LIFE) Study. Am Heart J. 2009;157(1):177–184. doi: 10.1016/j.ahj.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Mrug M, Stopka T, Julian BA, Prchal JF, Prchal JT. Angiotensin II stimulates proliferation of normal early erythroid progenitors. J Clin Invest. 1997;100(9):2310–2314. doi: 10.1172/JCI119769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourgoignie JJ, Gallagher NI, Perry HM, Jr, Kurz L, Warnecke MA, Donati RM. Renin and erythropoietin in normotensive and in hypertensive patients. J Lab Clin Med. 1968;71(3):523–536. [PubMed] [Google Scholar]
- 19.Wiecek A, Kokot F, Kuczera M, Grzeszczak W, Kiersztejn M. Plasma erythropoietin concentrations in renal venous blood of patients with unilateral renovascular hypertension. Nephrol Dial Transplant. 1992;7(3):221–224. doi: 10.1093/oxfordjournals.ndt.a092109. [DOI] [PubMed] [Google Scholar]
- 20.Nakao K, Shirakura T, Azuma M, Mackawa T. Studies on erythropoietin action of Angiotensin II. Blood. 1967;29(5):754–760. [PubMed] [Google Scholar]
- 21.Fisher JW, Samuels AI. Relationship between renal blood flow and erythropoietin production in dogs. Proc Soc Exp Biol Med. 1967;125(2):482–485. doi: 10.3181/00379727-125-32125. [DOI] [PubMed] [Google Scholar]
- 22.Eiam-Ong S, Hilden SA, Johns CA, Madias NE. Stimulation of basolateral Na-HCO3-cotransporter by Angiotensin II in rabbit renal cortex. Am J Physiol. 1993;265(2 Pt 2):F195–203. doi: 10.1152/ajprenal.1993.265.2.F195. [DOI] [PubMed] [Google Scholar]
- 23.Araki-Taguchi M, Nomura S, Ino K, et al. Angiotensin II mimics the hypoxic effect on regulating trophoblast proliferation and differentiation in human placental explants cultures. Life Sci. 2008;82(1-2):59–67. doi: 10.1016/j.lfs.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med. 2005;143(7):499–516. doi: 10.7326/0003-4819-143-7-200510040-00009. [DOI] [PubMed] [Google Scholar]
- 25.Wilcox WD. Abnormal serum uric acid levels in children. J Pediatr. 1996;128(6):731–741. doi: 10.1016/s0022-3476(96)70322-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.