Abstract
Background aims
GATA-4 is a cardiac transcription factor and plays an important role in cell lineage differentiation during development. We investigated whether overexpression of GATA-4 increases adult mesenchymal stromal cell (MSC) transdifferentiation into a cardiac phenotype in vitro.
Methods
MSC were harvested from rat bone marrow (BM) and transduced with GATA-4 (MSCGATA-4) using a murine stem cell virus (pMSCV) retroviral expression system. Gene expression in MSCGATA-4 was analyzed using quantitative reverse transcription–polymerase chain reaction (RT-PCR) and Western blotting. Native cardiomyocytes (CM) were isolated from ventricles of neonatal rats. Myocardial transdifferentiation of MSC was determined by immunostaining and electrophysiologic recording. The transdifferentiation rate was calculated directly from flow cytometery.
Results
The expression of cardiac genes, including brain natriuretic peptide (BNP), Islet-1 and α-sarcomeric actinin (α-SA), was up-regulated in MSCGATA-4 compared with control cells that were transfected with Green Fluorescent Protein (GFP) only (MSCNull). At the same time, insulin-like growth factor-binding protein (IGFBP)-4 was significantly up-regulated in MSCGATA-4. A synchronous beating of MSC with native CM was detected and an action potential was recorded. Some GFP + cells were positive for α-SA staining after MSC were co-cultured with native CM for 7 days. The transdifferentiation rate was significantly higher in MSCGATA-4. Functional studies indicated that the differentiation potential of MSCGATA-4 was decreased by knockdown of IGFBP-4.
Conclusions
Overexpression of GATA-4 significantly increases MSC differentiation into a myocardial phenotype, which might be associated with the up-regulation of IGFBP-4.
Keywords: cardiomyocyte, co-culture, GATA-4, genetic engineering, insulin-like growth factor-binding protein-4, stem cell
Introduction
Regenerative medicine in cardiology is a new field based on the possibility of replacing lost or damaged cardiac cells during ischemia and improving cardiac function by cell transplantation. The bone marrow (BM) is an easily accessible source of autologous adult stem and progenitor cells. BM-derived mesenchymal stromal cells (MSC) are multipotent and have been demonstrated to improve cardiac function following intramyocardial transplantation into infarcted myocardium (1–4). Their ability to repair the myocardium depends on transdifferentiation into myocardial progenitor cells and the protection of the native myocardium under ischemic insult.
Although there is great enthusiasm for repairing the ischemic heart by using cell therapy, simple cell implantation cannot reinstate cardiac function because of the relatively low rate of stem cell differentiation (5,6). Genetic engineering of MSC represents a useful strategy for boosting the therapeutic potency of MSC (7,8). GATA-4 is highly expressed in cardiomyocytes (CM) throughout embryonic development, postnatal growth and adulthood, during which it functions as a critical regulator of cardiac differentiation (9). In postnatal CM, GATA-4 is essential for maintaining the cardiac genetic program (10) and for the adaptive response of the heart to numerous stimuli, including hormones and work overload (11,12). Increased expression of GATA-4 inhibits doxorubicin (DOX)-induced autophagy and reduces CM death, whereas GATA-4 gene silencing triggers autophagy and renders DOX more toxic (13). GATA-4 has also been shown to be essential for cardiac differentiation of pluripotent (P19) stem cells to form beating CM (14,15). Depletion of GATA-4 prevents terminal differentiation and induces apoptosis of the pre-cardiac cells, whilst gain-of-function studies induce ectopic beating CM (15). We have reported that previously overexpression of GATA-4 in MSC significantly increases MSC survival in ischemic myocardium (16). Recently, Rysa et al. (17) reported that the reversal of reduced GATA-4 activity prevents adverse post-infarction remodeling through myocardial angiogenesis, anti-apoptosis and stem cell recruitment. However, it is unclear whether and how GATA-4 can increase the adult BM-sourced MSC transdifferentiation into cardiac phenotypes. Our preliminary mRNA microarray study indicated that many mRNA were up-regulated or down-regulated in MSC transduced with GATA-4 (MSCGATA-4) compared with MSC transduced with GFP only (MSCNull). Several mRNA related to anti-oxidative stress and myocardial markers are listed in supplementary Table 1. The expression of insulin-like growth factor-binding protein (IGFBP)-4 was significantly higher in MSCGATA-4 than that in MSCNull (9.21 ± 0.60 fold, P < 0.00091 versus MSCNull). IGFBP-4 is a member of the IGFBP family (18–20). Recently, several studies have suggested that IGFBP-4 has insulin-like growth factor (IGF)-independent actions on cellular functions (21,22). It has been reported that IGFBP-4 enhances P19CL6 cell differentiation into a cardiac phenotype (23). Our results indicate that GATA-4 promotes MSC transdifferentiation into myocardial phenotypes, which may be associated with up-regulating IGFBP-4.
Methods
All protocols conformed to the Guidelines for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH) (NIH publication number 85–23, revised 1996) and was approved by the University of Cincinnati (Cincinnati, OH, USA) Animal Care and Use Committee.
MSC culture and transduction with GATA-4 plasmid
MSC were isolated according to a method described previously (24), with some modifications. In brief, femurs and tibias were obtained from Sprague Dawley (SD) rats. BM cells were flushed and cultured with Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 20% fetal bovine serum and penicillin (100 U/mL)/streptomycin (100 μg/mL) at 37°C in humid air with 5% CO2.
The second passage of MSC was used to transduce recombinant GATA-4, as described previously (16). In brief, a retrovirus expressing GATA-4 was constructed using a murine stem cell virus (pMSCV) retroviral expression system (Clontech, Mountain View, CA, USA). IRES-EGFP was cloned into pMSCV vectors at XhoI and EcoRI sites, and then GATA-4 was excised from pcDNA-GATA-4 (25) with HindIII and XhoI restriction enzymes and cloned into pMSCV-IRES-EGFP at BglII and SalI sites. GP2-293 cells (Clontech) were co-transfected with pMSCV-GATA-4-IRES-EGFP and pVSVG; control GP2-293 cells were co-transfected with pMSCV-IRES-EGFP and pVSVG. After 48 h, supernatants were filtered and incubated with MSC in the presence of 10 μg/mL polybrene (Sigma, St. Louis, MO, USA) for 12 h. Stable GATA-4- and Green Fluorescent Protein (GFP)-expressing clones were acquired by selecting with puromycin (3 μg/mL; Sigma) for 5 days. The expression of GATA-4 was verified by immunostaining, quantitative real-time–polymerase chain reaction (PCR) and Western blot. pMSCV-IRES-EGFP was transfected into MSC and served as a transfection control (MSCNull).
Quantitative reverse transcription–PCR
Total RNA from MSC was isolated using an RNeasy mini kit (Qiagen, Valencia, CA, USA). Quantitative reverse transcription–PCR (RT-PCR) was carried out on an iQ5 real-time system with iQ SYBR Super-mix (Bio-Rad, Hercules, CA, USA) as described previously (26). In brief, complementary DNA was synthesized using SuperScript™ III First-Strand Synthesis for RT-PCR (Invitrogen, Carlsbad, CA, USA) and cDNA was amplified using Taq DNA polymerase in the presence of primers (Table I). The expression of each target mRNA relative to Glycer-aldehyde-3-phosphate dehydrogenase (GAPDH) was calculated based on the threshold cycle (CT) as r = 2−Δ (ΔCT), where ΔCT = CT target − CT GAPDH and Δ(ΔCT) = ΔCT experimental − ΔCT control.
Table I.
Sequence for each primer.
| Primers | Sense | Anti-sense |
|---|---|---|
| IGFBP-4 | 5′-GAG CCG TAC CCA CGA AGA C | 5′-GAC TCA GGC CAA GAC TCC AT |
| BNP | 5′-TTC AGC CTC GGA CTT GGA AAC | 5′-CCT TGT GGA ATC AGA AGC AGG |
| α-SA | 5′-TCA TCC TCC TGG GCC ATG T | 5′-TAT CAC GCG GCG AAC CA |
| Islet-1 | 5′-CAG CTG CAC ACC TTG CGG AC | 5′-GTG TAT CTG GGA GCT GCG AG |
| GAPDH | 5′-ATG GGA GCT GGT CAT CAA C | 5′-CCA CAG TCT TCT GAG TGG CA |
Electroimmunoblotting
The protein of different cardiac markers was quantified using Western blotting. Cells were washed in phosphate-buffered saline (PBS) and homogenized in lysis buffer. Protein concentrations were quantified with Bio-Rad DC-Protein Assay Reagent (Bio-Rad). Denatured proteins (25 and 50 μg) were separated using 12% sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE), transferred to a nitrocellulose membrane (Bio-Rad), and immunoblotted overnight at 4°C with primary antibodies against IGFBP-4 (Abcam, Cambridge, MA, USA), anti-brain natriuretic peptide (BNP; Abcam), Islet-1 (Santa Cruz, CA, USA), α-sarcomeric actinin (α-SA; Sigma) and β-actin (Cell Signaling, Danvers, MA, USA). Membranes were then incubated for 1 h with Horseradish peroxidase (HRP)-conjugated secondary antibody at room temperature, washed and developed with an ECL plus kit (GE Healthcare, Pittsburgh, PA, USA). Blots were analyzed by densitometry with NIH image software (AlphaEase FC, version 6.0.0).
Immunocytochemistry
Cells cultured on glass coverslips were fixed in 4% paraformaldehyde, and incubated with mouse monoclonal anti-α-SA (Sigma) and rabbit polyclonal anti-GATA-4 (Abcam). After thorough washing, secondary antibodies of goat anti-mouse IgG or goat anti-rabbit IgG conjugated with Alexa Fluor-568 (Invitrogen) were applied. Nuclei were stained with 4′,6-diamino-2-phenylindole (DAPI). Confocal images were obtained with a Zeiss LSM 510 confocal/two-photon microscope equipped with Zeiss AIM Version 4.2.
CM culture and co-culture with MSC
Native CM were isolated from ventricles of 1–3-day-old SD rats using a commercially available neonatal CM isolation kit (Worthington Biochemical Co., Lakewood, NJ, USA), in accordance with the supplier’s protocol. To investigate transdifferentiation, MSC were plated with CM at a ratio of 1:40 in either mixed co-cultures or a dual-chamber system. In some experiments, neonatal CM were labeled with a red fluorochrome membrane dye, PKH26 (Sigma), before co-culture with MSC. Cell beating was recorded using a charge-coupled device (CCD) camera connected to an inverted phase–contrast microscope equipped with fluorescent filters (Olympus, Center Valley, PA, USA). To quantify the transdifferentiation rate of MSC, co-cultured cells were trypsinized and incubated with anti-α-SA antibody (Sigma) conjugated with allophycocyanin (APC). At least 20 000 cells were analyzed on a BD FACS-Calibur cell sorter (BD Biosciences, San Jose, CA, USA).
Action potential recordings
Intracellular potential changes were measured in cultured cells with glass microelectrodes filled with solution containing (in mM) cesium aspartate 115, CsCl 20, ethylene glycol tetraacetic acid (EGTA 11), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 10, MgCl2 2.5 and Mg-adenosine triphosphate (ATP 2) (pH 7.2), and had a resistance of 1.5–2.5 MΩ (27,28). Isolated single cells, including GFP− (native CM) and MSC (GFP+) either cultured alone or co-cultured with CM, were maintained at room temperature (24°C) and perfused with Tyrode’s solution containing (in mM) NaCl 140, KCl 5.4, MgCl2 1, CaCl2 1.8, HEPES 5 and glucose 10 (pH 7.4). The action potential (AP) was recorded using the whole-cell patch clamp technique with an Axon-patch-200B amplifier (Axon Instruments, Foster City, CA, USA).
Statistical analysis
Quantitative data were expressed as mean ± SEM. One-way analysis of variance (SigmaStat 3.1; Systat Software, San Jose, CA, USA) with the Holm–Sidak method and/or Bonferroni correction was used to determine the significance of differences. Differences were considered significant if the P-value was less than 0.05.
Results
MSC differentiation into cardiac phenotype
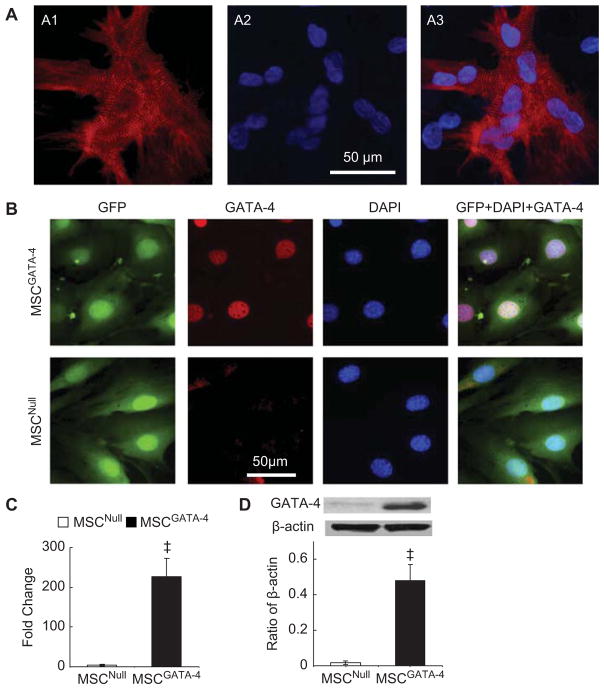
Native myocytes were isolated from neonatal rat ventricles. After being cultured for 24 h, native CM began to beat spontaneously. Immunostaining showed that all CM were positive for α-SA and myofibers were seen with clear Z-lines in sarcomeres. Nuclei were centrally located and homogeneous (Figure 1A).
Figure 1.
Characterization of cultured CM and MSC transduced with GATA-4 (MSCGATA-4) or empty vector (MSCNull). (A) Primary cultured CM (5 days) were stained with α-SA antibody. CM were positive for α-SA (red) and myofibers were seen with clear Z-lines in sarcomeres. Nuclei were counterstained with DAPI. (A1) α-SA (red); (A2) DAPI; (A3) merge of A1 and A2. (B) MSC were stained with GATA-4 antibody. Both MSCGATA-4 and MSCNull were GFP+, only MSCGATA-4 stained intensely for GATA-4 in nuclei (red). (C) Quantitative real-time PCR of GATA-4 expression. (D) Western blot of GATA-4 and corresponding semi-quantitative data. ‡P < 0.05 versus MSCNull.
Both MSCGATA-4 and MSCNull were GFP immunopositive; only MSCGATA-4 stained intensely for GATA-4 (Figure 1B). Quantitative real-time PCR data indicated that the expression of GATA-4 was significantly increased in MSCGATA-4 (Figure 1C). MSC-GATA-4 also exhibited higher levels of GATA-4 protein (Figure 1D).
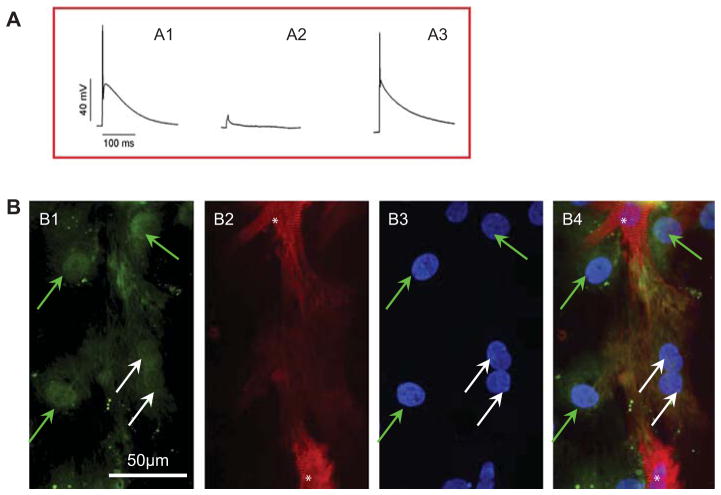
MSC were distinguished from CM on the basis of GFP positivity when they were co-cultured with CM that were pre-labeled with PKH26. We did not observe spontaneous beating from MSC. However, a synchronous contraction of MSC with CM was noted (supplementary video A–D). Furthermore, the beating MSC (GFP+) was connected with rat CM and shown to be PKH26+, which suggested that PKH26 had been transferred from native CM into MSC. To determine whether these cells had a CM-like function, the AP of single isolated cells was recorded. MSC co-cultured with CM showed similar AP to that of neonatal CM (Figure 2A), indicating electrical activity and the existence of functional sarcolemmal ion channels in these cells. Co-cultured cells were characterized further by immunostaining with α-SA. Some GFP+ MSC were also α-SA+. These cells had GFP+ nuclei, which excluded the possibility of GFP+ cell overlay with α-SA+ CM (Figure 2B). However, compared with native CM, differentiated cells were immature CM with weak α-SA staining.
Figure 2.
MSCGATA-4 differentiated into cardiac phenotypes after MSCGATA-4 were co-cultured with CM for 7 days. (A) AP recorded from various cells: (A1) MSCGATA-4 co-cultured with CM; (A2) MSCGATA-4 culture alone; (A3) neonatal CM. (B) Representative micrographs showing GFP, α-SA immunolabeling and DAPI staining. (B1) GFP; (B2) α-SA; (B3) DAPI; (B4) merge of B1–B3. White arrows, transdifferentiated MSCGATA-4 (GFP+/α-SA+ cell); green arrows, non-differentiated MSCGATA-4 (GFP+/α-SA− cell); white stars, native CM.
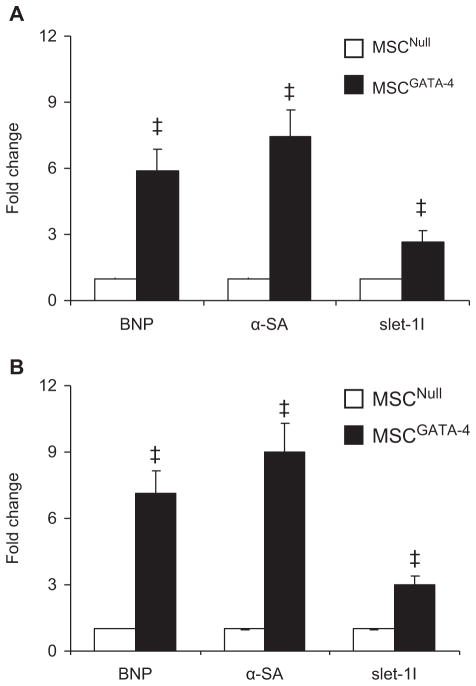
Cardiac gene expression in MSC was analyzed using quantitative RT-PCR. BNP, α-SA and Islet-1 were significantly up-regulated in MSCGATA-4 compared with MSCNull, either cultured alone (Figure 3A) or co-cultured with CM in a dual-chamber system for 7 days (Figure 3B).
Figure 3.
Expression of cardiac markers in MSC analyzed with quantitative real-time PCR. (A) MSC alone; (B) MSC co-cultured with CM in dual-chamber system for 7 days. ‡P < 0.05 versus MSCNull.
GATA-4 up-regulated IGFBP-4 and enhanced MSC myocardial differentiation
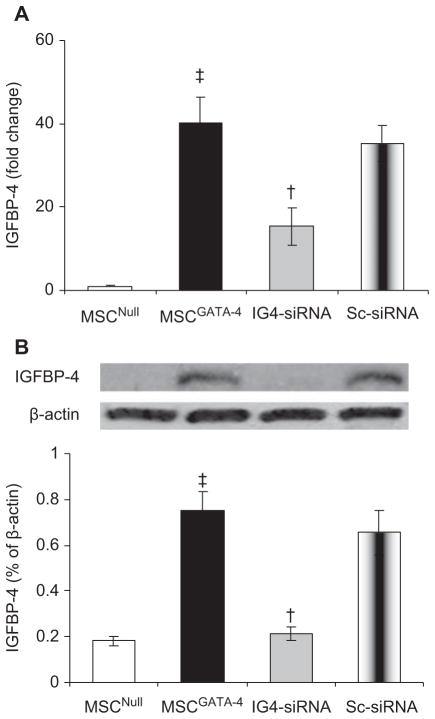
It was noted that IGFBP-4 was significantly up-regulated in MSCGATA-4 in our microarray study. The up-regulation of IGFBP-4 in MSCGATA-4 was further confirmed by Quantitative reverse transcription-PCR (qRT-PCR) and Western blot. IGFBP-4 expression was significantly up-regulated in MSCGATA-4 (compared with MSCNull) for mRNA (Figure 4A) and protein (Figure 4B) levels. To study the role of IGFBP-4 in GATA-4-mediated myocardial transdifferentiation, IGFBP-4 activity was knocked down in MSCGATA-4 using double-stranded smart pool small interfering RNA (siRNA) (Santa Cruz; sc-39590, CA, USA), designed to target IGFBP-4. Non-silencing, scrambled siRNA (Ambion; 4390843, TX, USA) was used as a negative control. Quantitative real-time PCR showed that the expression of IGFBP-4 in IGFBP-4-siRNA-MSCGATA-4 (IG4-siRNA) was only 38% of that in MSCGATA-4 (P < 0.05). IGFBP-4 protein was reduced in IG4-siRNA cells by 28% compared with MSCGATA-4 (P < 0.05). No significant change in expression of IGFBP-4 in scrambled siRNA-transfected MSCGATA-4 (Sc-siRNA) was detected.
Figure 4.
Expression of IGFBP-4 in MSC transduced with different genes. (A) Quantitative real-time PCR; (B) Western blot of IGFBP-4 and corresponding semi-quantitative data. ‡P < 0.05 versus MSCNull. †P < 0.05 versus MSCGATA-4. IG4-siRNA, IGFBP-4-siRNA-MSCGATA-4; Sc-siRNA, scrambled siRNA- MSCGATA-4.
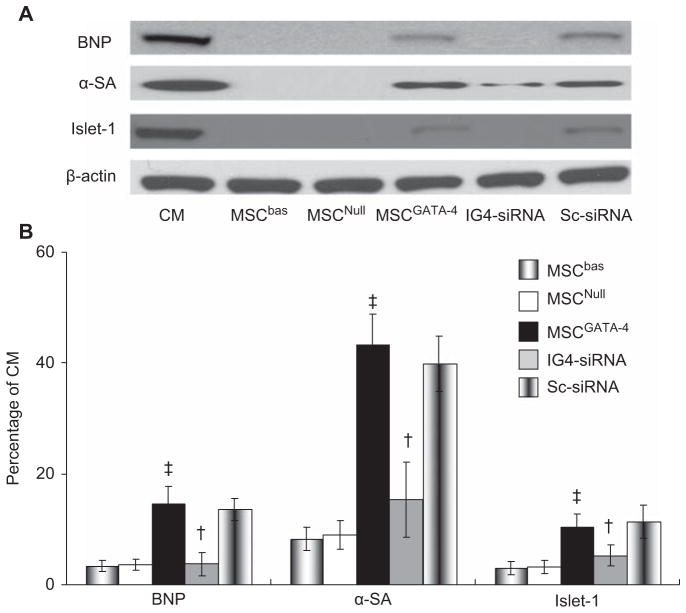
After culturing MSC with native CM in a dual-chamber system, no MSC beating was detected after 7 days. The cardiac proteins α-SA, BNP and Islet-1 in MSCNull were very low (α-SA 8.9 ± 2.5%, BNP 3.6 ± 1.0%, Islet-1 3.2 ± 1.2% in CM, respectively) compared with native CM. All of these proteins were significantly increased in MSCGATA-4 (α-SA 43.3 ± 5.4%, BNP 14.5 ± 3.1%, and Islet-1 10.4 ± 2.3%, respectively; P < 0.05 versus MSCNull). However, the effect of GATA-4 on expressing cardiac markers in MSC was suppressed when IGFBP-4-siRNA was transfected (Figure 5).
Figure 5.
Western blot analysis of MSC expressing cardiac markers when co-cultured with native CM in dual-chamber system for 7 days. (A) Representative Western blot of cardiac markers; (B) semi-quantitative data of cardiac markers expressed as a ratio to native CM. ‡P < 0.05 versus MSCNull; †P < 0.05 versus MSCGATA-4.
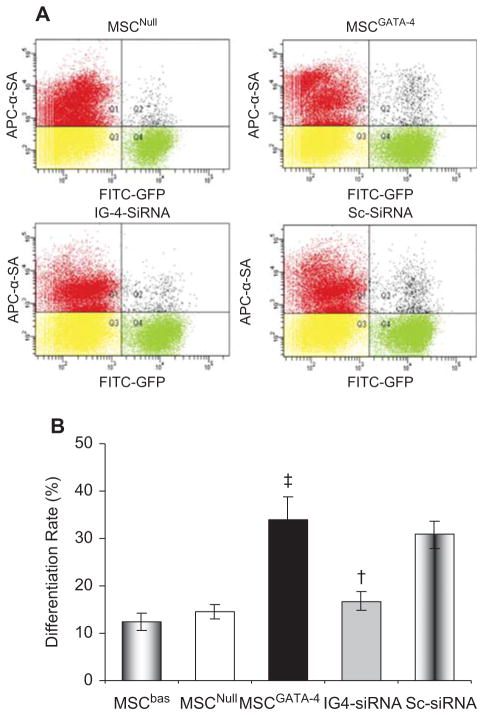
Furthermore, the transdifferentiation of various MSC was quantified using fluorescence-activated cell sorting (FACS) after MSC were co-cultured with native CM for 7 days. The co-cultured cells were stained with α-SA-conjugated with APC. Native CM were α-SA+, MSC were GFP+, and cardiac phenotypes that transdifferentiated from MSC were positive for both α-SA and GFP. The transdifferentiation rate, i.e. the percentage of α-SA+/GFP+ cells among total GFP+ cells, was higher in MSCGATA-4 (34.09 ± 4.65%) than in MSCNull (14.54 ± 1.62%; P < 0.05). However, this effect of MSCGATA-4 was abolished by transfecting IGFBP-4-siRNA (Figure 6). No significant difference was detected in Sc-siRNA-transfected MSCGATA-4 compared with MSCGATA-4. These results indicated that the up-regulation of IGFBP-4 might play an important role in myocardial transdifferentiation of MSC mediated by GATA-4.
Figure 6.
Transdifferentiation rate of various MSC assessed by flow cytometery following co-culture with native CM for 7 days. (A) Representative flow cytometery data of α-SA+/GFP+ cells. (B) Quantitative assay in various groups. ‡P < 0.05 versus MSCNull; †P < 0.05 versus MSCGATA-4.
Discussion
The potential of MSCGATA-4 differentiation into a cardiac phenotype was investigated in vitro not only by analyzing the expression of cardiac markers but also by recording cell beating and AP. Our study indicated that MSC engineering with GATA-4 achieved superior functional and structural benefits in myocardial transdifferentiation, which might be associated with up-regulation of IGFBP-4.
The studies in our laboratory and others have shown that implanted stem cells can repair in part the infarcted heart both structurally and functionally (1,29–31). The contribution of implanted cells to cardiac repair and/or recovery of function can occur through transdifferentiation of implanted cells into myocytes and endothelial cells, and subsequent myocardial regeneration. Rota et al. (29) have reported how transplanted BM cells (BMC) regenerated myocardium from engraftment in the infarcted myocardium to form a functional myocyte. Within 12–48 h after infarction and cell implantation, donor cells were integrated structurally with resident cells. Junctional and adhesion complexes were detected between transplanted BMC, and between BMC and adjacent resident myocytes and fibroblasts. Engraftment of BMC shortly after their delivery was followed by myocardial regeneration, which expanded from 5 to 10 and 30 days after infarction. Donor cell-derived myocytes exhibited electrical characteristics similar to spared myocytes but showed a prolongation of the AP and enhanced cell shortening at 15–30 days post-transplantation (29).
In this study, the transdifferentiation of MSC into a cardiac phenotype was evidenced by the expression of cardiac markers, cell contraction and CM-like AP in GFP+ MSC after co-culture with native CM for 7 days. All of these results indicated that MSC had the potential to transdifferentiate and behave as a functional myocardial phenotype.
An alternative possibility for cardiac transdifferentiation involves fusion of MSC with native CM. Some studies have suggested that the phenotypic changes of stem cells are the result of cell fusion (32, 33). It has been reported that cell fusion occurs in approximately 30–40% of cells that acquire a myogenic phenotype (34). We observed directly the contraction of GFP+ MSC following CM beating (supplementary video A–D). The MSC were connected with native CM. The beating MSC showed both green and red fluorescence signals under a fluorescence microscope. We have investigated the possibility of cell fusion and cell communication between MSC and CM in a previous study (26). Approximately 40% MSC were PKH26+ after being co-cultured with PKH26 pre-labeled CM for 7 days. MSC showing double positivity for both PKH26 and GFP were connected with CM by gap junctions expressing Connexin43, or fused with native CM showing multinucleated cells. To investigate further the role of cell connection and fusion in MSC transdifferentiation into cardiac phenotypes, we cultured MSC with CM in a dual-chamber system. The results showed that the expression of cardiac-associated genes was up-regulated (Figure 5), but no beating cells were detected in MSC after 7 days of culture. We did not find α-SA+/GFP+ cells in MSC cultures with native CM in a dual-chamber system. This suggests that specific communication between stem cells and native myocytes is required in the transdifferentiation of stem cells into matured CM. These results indicate that cell connection or fusion is necessary for MSC transdifferentiation into CM-like cells.
The results obtained from quantitative real-time PCR, Western blotting and FACS demonstrated that MSCGATA-4 had a higher differentiating potential compared with vector-transduced control MSC (MSC-Null). Our study is consistent with a previous report that GATA-4 enhances MSC transdifferentiation into a cardiac phenotype (14,15,35). It is well known that GATA-4 is a member of the GATA family of zinc finger transcription factors and is an early cardiomyocyte marker. GATA-4 plays an important role in transducing nuclear events that modulate cell lineage differentiation (9,11,12).
We have shown in this study that the expression of IGFBP-4 is up-regulated in MSCGATA-4 and transfection of IGFBP-4-siRNA into MSCGATA-4 significantly reduces transdifferentiation of MSCGATA-4. Recently, it has been reported that IGFBP-4 enhances P19CL6 cell differentiation into a cardiac phenotype. Treatment with IGF-I and IGF-II or neutralizing antibodies against IGF-I and IGF-II had no effect on the IGFBP-4-induced CM differentiation of P19CL6 cells (23). These observations indicate that IGFBP-4 induces CM differentiation in an IGF-independent manner. Reporter gene assays and β-catenin stabilization assays revealed that IGFBP-4 was the most potent canonical signaling pathway (Wnt) inhibitor (23). Canonical Wnt proteins bind to Frizzled receptors, which then complex with the co-receptor LRP (Low-density lipoprotein (LDL) receptor-related protein). The signal is transduced by β-catenin, which enters the nucleus to modulate expression of target genes (36). It has been shown that canonical Wnt signals inhibit cardiogenesis. Activation of Wnt/β-catenin signaling in the late phase after embryoid body (EB) formation inhibits CM differentiation (37). Inhibition of canonical Wnt signaling promotes cardiomyocyte differentiation in embryonic stem cells and in chick, Xenopus and zebrafish embryos (37–39). To investigate whether IGFBP-4 promotes the differentiation of P19CL6 cells into CM by inhibition of the canonical Wnt pathway, Zhu et al. (23) showed that the expression of dominant-negative LRP6 in P19CL6 enhanced CM differentiation of these cells and reversed the inhibitory effect of IGFBP-4 knockdown on cardiomyogenesis. These results therefore collectively suggest that IGFBP-4 promotes cardiogenesis by antagonizing the Wnt/β-catenin pathway through direct interactions with Frizzled and LDL receptor-related proteins LRP-5 or LRP-6 (LRP5/6). Further study is needed to explore how GATA-4 up-regulates IGFBP-4 resulting in MSC myocardial transdifferentiation.
GATA-4 promotes MSC differentiation into myocardial phenotypes after co-culture with native CM. The augmentation of GATA-4 on MSC-mediated myogenesis can be abrogated by transfecting IGFBP-4-siRNA into MSCGATA-4. This suggests that GATA-4 in MSC transdifferentiation into a cardiac phenotype is, at least partially, associated with up-regulating IGFBP-4.
Acknowledgments
We gratefully acknowledge Dr Hongsheng Wang (Department of Pharmacology and Cell Biophysics, University of Cincinnati Medical Center, Cincinnati, OH, USA) for his help in analysis of AP in cultured cells, and Dr Vien Khach Lai for his technical help in FACS. This work was supported by National Institutes of Health grants HL083236, HL105176 (MX) and HL087246 (MA).
Footnotes
Author disclosure: None.
References
- 1.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–26. doi: 10.1016/s0003-4975(02)03517-8. [DOI] [PubMed] [Google Scholar]
- 2.Makkar RR, Price MJ, Lill M, Frantzen M, Takizawa K, Kleisli T, et al. Intramyocardial injection of allogenic bone marrow-derived mesenchymal stem cells without immunosuppression preserves cardiac function in a porcine model of myocardial infarction. J Cardiovasc Pharmacol Ther. 2005;10:225–33. doi: 10.1177/107424840501000403. [DOI] [PubMed] [Google Scholar]
- 3.Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–7. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 4.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–35. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 5.Gruh I, Beilner J, Blomer U, Schmiedl A, Schmidt-Richter I, Kruse ML, et al. No evidence of transdifferentiation of human endothelial progenitor cells into cardiomyocytes after coculture with neonatal rat cardiomyocytes. Circulation. 2006;113:1326–34. doi: 10.1161/CIRCULATIONAHA.105.559005. [DOI] [PubMed] [Google Scholar]
- 6.Koninckx R, Hensen K, Daniels A, Moreels M, Lambrichts I, Jongen H, et al. Human bone marrow stem cells co-cultured with neonatal rat cardiomyocytes display limited cardiomyogenic plasticity. Cytotherapy. 2009;11:778–92. doi: 10.3109/14653240902988818. [DOI] [PubMed] [Google Scholar]
- 7.Lin H, Shabbir A, Molnar M, Yang J, Marion S, Canty JM, Jr, et al. Adenoviral expression of vascular endothelial growth factor splice variants differentially regulate bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2008;216:458–68. doi: 10.1002/jcp.21414. [DOI] [PubMed] [Google Scholar]
- 8.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA. 2007;104:1643–8. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oka T, Xu J, Molkentin JD. Re-employment of developmental transcription factors in adult heart disease. Sem Cell Develop Biol. 2007;18:117–31. doi: 10.1016/j.semcdb.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charron F, Paradis P, Bronchain O, Nemer G, Nemer M. Cooperative interaction between GATA-4 and GATA-6 regulates myocardial gene expression. Mol Cell Biol. 1999;19:4355–65. doi: 10.1128/mcb.19.6.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pikkarainen S, Tokola H, Majalahti-Palviainen T, Kerkela R, Hautala N, Bhalla SS, et al. GATA-4 is a nuclear mediator of mechanical stretch-activated hypertrophic program. J Biol Chem. 2003;278:23807–16. doi: 10.1074/jbc.M302719200. [DOI] [PubMed] [Google Scholar]
- 12.Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, et al. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circu Res. 2006;98:837–45. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi S, Volden P, Timm D, Mao K, Xu X, Liang Q. Transcription factor GATA4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. J Biol Chem. 2010;285:793–804. doi: 10.1074/jbc.M109.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grepin C, Robitaille L, Antakly T, Nemer M. Inhibition of transcription factor GATA-4 expression blocks in vitro cardiac muscle differentiation. Mol Cell Biol. 1995;15:4095–102. doi: 10.1128/mcb.15.8.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grepin C, Nemer G, Nemer M. Enhanced cardiogenesis in embryonic stem cells overexpressing the GATA-4 transcription factor. Development. 1997;124:2387–95. doi: 10.1242/dev.124.12.2387. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Zuo S, He Z, Yang Y, Pasha Z, Wang Y, et al. Paracrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. Am J Physiol. 2010;299:H1772–81. doi: 10.1152/ajpheart.00557.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rysa J, Tenhunen O, Serpi R, Soini Y, Nemer M, Leskinen H, et al. GATA-4 is an angiogenic survival factor of the infarcted heart. Circ Heart Fail. 2010;3:440–50. doi: 10.1161/CIRCHEARTFAILURE.109.889642. [DOI] [PubMed] [Google Scholar]
- 18.Ning Y, Schuller AG, Conover CA, Pintar JE. Insulin-like growth factor (IGF) binding protein-4 is both a positive and negative regulator of IGF activity in vivo. Mol Endocrinol. 2008;22:1213–25. doi: 10.1210/me.2007-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyakoshi N, Richman C, Qin X, Baylink DJ, Mohan S. Effects of recombinant insulin-like growth factor-binding protein-4 on bone formation parameters in mice. Endocrinology. 1999;140:5719–28. doi: 10.1210/endo.140.12.7175. [DOI] [PubMed] [Google Scholar]
- 20.Edmondson SR, Russo VC, McFarlane AC, Wraight CJ, Werther GA. Interactions between growth hormone, insulin-like growth factor I, and basic fibroblast growth factor in melanocyte growth. J Clin Endocrinol Metab. 1999;84:1638–44. doi: 10.1210/jcem.84.5.5692. [DOI] [PubMed] [Google Scholar]
- 21.Bartling B, Koch A, Simm A, Scheubel R, Silber RE, Santos AN. Insulin-like growth factor binding proteins-2 and -4 enhance the migration of human CD34−/CD133+ hematopoietic stem and progenitor cells. Int J Mol Med. 2010;25:89–96. [PubMed] [Google Scholar]
- 22.Ueno K, Hirata H, Majid S, Tabatabai Z, Hinoda Y, Dahiya R. IGFBP-4 activates the Wnt/beta-catenin signaling pathway and induces M-CAM expression in human renal cell carcinoma. Int J Cancer. 2011 doi: 10.1002/ijc.25899. in press. [DOI] [PubMed] [Google Scholar]
- 23.Zhu W, Shiojima I, Ito Y, Li Z, Ikeda H, Yoshida M, et al. IGFBP-4 is an inhibitor of canonical Wnt signalling required for cardiogenesis. Nature. 2008;454:345–9. doi: 10.1038/nature07027. [DOI] [PubMed] [Google Scholar]
- 24.Xu M, Wani M, Dai Y-S, Wang J, Yan M, Ayub A, et al. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation. 2004;110:2658–65. doi: 10.1161/01.CIR.0000145609.20435.36. [DOI] [PubMed] [Google Scholar]
- 25.Dai YS, Markham BE. p300 functions as a coactivator of transcription factor GATA-4. J Biol Chem. 2001;276:37178–85. doi: 10.1074/jbc.M103731200. [DOI] [PubMed] [Google Scholar]
- 26.He Z, Li H, Zuo S, Pasha Z, Wang Y, Yang Y, et al. Transduction of Wnt11 promotes mesenchymal stem cells transdifferentiation into cardiac phenotypes. Stem Cells Devel. 2011 doi: 10.1089/scd.2010.0380. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang HS, Cohen IS. Calcium channel heterogeneity in canine left ventricular myocytes. J Physiol. 2003;547:825–33. doi: 10.1113/jphysiol.2002.035410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao W, Yuan Q, Qian J, Waggoner JR, Pathak A, Chu G, et al. The presence of Lys27 instead of Asn27 in human phospholamban promotes sarcoplasmic reticulum Ca2+-ATPase superinhibition and cardiac remodeling. Circulation. 2006;113:995–1004. doi: 10.1161/CIRCULATIONAHA.105.583351. [DOI] [PubMed] [Google Scholar]
- 29.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA. 2007;104:17783–8. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108:2070–3. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 31.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circulation Res. 2006;98:1414–21. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 32.Metzele R, Alt C, Bai X, Yan Y, Zhang Z, Pan Z, et al. Human adipose tissue-derived stem cells exhibit proliferation potential and spontaneous rhythmic contraction after fusion with neonatal rat cardiomyocytes. FASEB J. 2011;25:830–9. doi: 10.1096/fj.09-153221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonde S, Pedram M, Stultz R, Zavazava N. Cell fusion of bone marrow cells and somatic cell reprogramming by embryonic stem cells. FASEB J. 24:364–73. doi: 10.1096/fj.09-137141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He XQ, Chen MS, Li SH, Liu SM, Zhong Y, McDonald Kinkaid HY, et al. Co-culture with cardiomyocytes enhanced the myogenic conversion of mesenchymal stromal cells in a dose-dependent manner. Mol Cell Biochem. 2010;339:89–98. doi: 10.1007/s11010-009-0372-2. [DOI] [PubMed] [Google Scholar]
- 35.Hu DL, Chen FK, Liu YQ, Sheng YH, Yang R, Kong XQ, et al. GATA-4 promotes the differentiation of P19 cells into cardiac myocytes. Int J Mol Med. 2010;26:365–72. [PubMed] [Google Scholar]
- 36.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nature Rev. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 37.Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, et al. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci USA. 2006;103:19812–7. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, et al. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:9685–90. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foley A, Mercola M. Heart induction: embryology to cardiomyocyte regeneration. Trends Cardiovasc Med. 2004;14:121–5. doi: 10.1016/j.tcm.2004.01.003. [DOI] [PubMed] [Google Scholar]