Abstract
Haemophilus influenzae type b (Hib) conjugate vaccine for infants (6, 10, and 14 weeks of age) was introduced into the Malian Expanded Program on Immunization in July 2005, to diminish invasive Hib disease in young children. Antibodies to Hib capsular polysaccharide (PRP) were measured in infants and toddlers from an area already served by the Hib immunization program (Bamako) and in unimmunized children of the same age in a district (Kangaba) where Hib immunization had not yet begun. Among vaccinated Bamako children 6–23 months of age, 77–93% exhibited PRP titers ≥ 1.0 μg/mL, indicating long-term protection, versus only 10–23% of Kangaba children of that age. High PRP antibody titers in immunized children persisted through 2 years of age. Moreover, ∼50% of Bamako children exhibited anti-PRP titers ≥ 5.0 μg/mL; a level that impedes Hib upper respiratory carriage, and may thereby diminish the Hib transmission to the unimmunized susceptible population (i.e., providing indirect protection).
Introduction
In the first years of the millennium, before the widespread introduction of conjugate vaccine to prevent invasive disease caused by Haemophilus influenzae type b (Hib) in developing countries, the World Health Organization (WHO) estimated that more than 3 million cases of invasive Hib disease, such as meningitis, pneumonia, and septicemia, and 386,000 deaths occurred annually in children < 5 years of age worldwide.1 Circa 2000, Africa had one of the highest regional burdens of Hib meningitis, with an incidence rate of ∼60–70/100,000 in children < 5 years of age2,3 and a case-fatality rate of ∼29%.2
The burden is highest in infants and toddlers, 4–18 months of age; Hib uncommonly affects children < 1 month or over 5 years of age.1 In the absence of immunization, the period of highest susceptibility commences as maternal antibodies begin to wane at ∼4 months of age and before children naturally acquire bactericidal antibodies against Hib. Serum bactericidal antibodies are overwhelmingly mediated by serum immunoglobulin G (IgG) directed against polyribosylribitol phosphate (PRP), the Hib capsular polysaccharide. Typically, natural bactericidal antibodies acquired consequent to either upper respiratory colonization with Hib or with bacteria such as Escherichia coli K100 that express cross-reacting surface molecules that do not appear until the second year of life.4,5
Hib polysaccharide-protein conjugate vaccines developed in the 1980s stimulate a T cell-dependent immune response, which leads to immunologic memory, and an immunoglobulin class switch with resultant increased antibody affinity and avidity.6–10 Accordingly, Hib conjugate vaccines are highly immunogenic, even in young infants.11–13 Introduction of Hib conjugate vaccines into the routine immunization schedule has led to near eradication of invasive Hib disease in many industrialized and transitional countries, and some developing countries.11,14–17
A serum anti-PRP titer ≥ 1.0 μg/mL, originally proposed by Kayhty and others18 is now widely accepted in vaccinology and public health as a titer that is associated with long-term protection against invasive Hib disease. Accordingly, this is the most frequently used measure to assess the immunogenicity of Hib conjugate immunization schedules and to predict protection that will endure throughout the period of risk for infants, toddlers, and pre-school children.10,19–34 Moreover, a study in the Dominican Republic has indicated that even higher serum PRP antibody levels, ≥ 5.0 μg/mL, can be correlated with protection against upper respiratory tract colonization with Hib.17
Since 2002, the Center for Vaccine Development - Mali (CVD-Mali), in Bamako (a collaborative enterprise maintained jointly by the Ministry of Health of Mali and the Center for Vaccine Development of the University of Maryland School of Medicine), has been conducting systematic surveillance studies of invasive pediatric bacterial infections among infants and children admitted to l'Hôpital Gabriel Touré, the one government hospital where severely ill children are admitted.35 In the period June 2002 through May 2005, a high incidence of invasive Hib disease was documented—45.2/100,000 in children < 5 years of age, with a peak incidence rate of 370/100,000 in infants 6–7 months of age.15 A baseline serosurvey undertaken in Bamako before the introduction of Hib vaccine revealed that only 1.5% of 6- to 7-month-old infants had PRP antibody concentrations ≥ 0.15 μg/mL and only 0.5% had titers ≥ 1.0 μg/mL.15 Thus, in the absence of Hib immunization, Malian infants were serologically highly susceptible at the age of peak Hib disease incidence.
Hib conjugate was introduced into the Malian Expanded Program on Immunization (EPI) in a three-step program, beginning with Bamako in July 2005, followed by other urban areas in July 2006, and finally expanding to all infants countrywide in July 2007. In Mali, Hib vaccine (as a component of a pentavalent combination vaccine) is targeted to be administered to infants at 6 weeks, 10 weeks, and 14 weeks of age. In contrast with the immunization schedules used widely in North and South America and most European countries, Malian toddlers do not receive a reinforcing booster dose of Hib conjugate in the second year of life. Although serosurveys were performed to document the susceptibility of infants 6–7 months of age in Bamako,15 no data were available about the kinetics and persistence of the PRP antibody response following administration of pentavalent vaccine in Malian infants, nor were Hib serosurvey data available from infants in rural areas. To fill these knowledge gaps, we conducted a cross-sectional survey of two populations, one Hib-vaccinated (urban Bamako) and the other Hib-unvaccinated (rural Kangaba). In each population, serum specimens were collected for testing from an age cross section of children representing subjects 6 weeks, 6 months, 12 months, 15 months, 18 months, and 23 months of age. The PRP titers in Bamako children were used to model the antibody response to the 3-dose Hib vaccination schedule and were compared with PRP titers in Kangaba children, which revealed the rate of acquisition of natural immunity to Hib (or lack thereof) in unvaccinated children.
Materials and Methods
Study site.
Mali is a land-locked country in Western Africa ranked among the six least developed countries, with an under 5 mortality rate of 191/1000 live births.36 Bamako, the capital city of 1.5 million inhabitants located on the Niger River in the southwestern part of the country, is the most populous city of Mali and is divided into six communes. Bamako's population incorporates a diverse cultural and ethnic mix, including Bambara, Malinke, Dongo, Sarakole, Peuhl, and Sonrai. For the Hib-vaccinated study population (urban Bamako), a sample of children who live in Djikoroni-para and Banconi, two low socioeconomic level quartiers within Commune 4 and Commune 1, respectively, were randomly selected from a demographic surveillance system database. Children for the Hib-unvaccinated study population represent a sample from rural Kangaba cercle, which lies in the Koulikoro region of Mali, ∼100 km from the capital. The cercle has a population of 80,923 distributed over 60 villages. The population of Kangaba is mainly of Malinke ethnic background. At the time of our sample collection (2008) the national stepwise Hib vaccine implementation program had saturated Bamako but had not yet started in Kangaba. Because a demographic surveillance system is not maintained in rural Kangaba, CVD-Mali field staff used WHO immunization coverage survey methods to randomly select villages and to identify start points for the house-to-house searches for age-eligible children. At each household the staff inquired if there was an age-eligible child. If so, informed consent procedures were initiated to ascertain whether the parents or caretakers were willing to have their child participate. Twelve of the 60 villages were sampled, and the refusal rate was 3%.
Sample size calculations.
On the basis of the data of Sow and others15 and Campagne and others,13 we assume that at least 75% of Hib-vaccinated children 6 months and older in Bamako would have a PRP antibody titer > 1 μg/mL,13,15 however no more than 20% of children in rural Kangaba who had not received Hib conjugate would likely have such titers.13,15 Thus, by comparing a sample of 30 subjects per specified age group from each site, we would have 84% power to detect a significant difference (α = 0.025, one-tail, Fisher's exact test) if the prevalence of such titers of PRP antibody was no more than 35% for Hib-unvaccinated (Kangaba) versus 75% for Hib-vaccinated children (Bamako), respectively. Power calculations were done using PASS 2008 (Number Cruncher Statistical Systems, Kaysville, UT).
Study design.
The study was conducted as a cross-sectional survey in which demographic data and immunization status were collected on each child and a serum specimen was collected and tested for PRP antibodies. Eligible participants were healthy children at 6 weeks and 6, 12, 15, 18, and 23 months of age in urban Bamako and rural Kangaba (each age group had a 2 week ± enrollment window). To be eligible, children in Bamako had to have evidence of immunization with three doses of pentavalent vaccine at 6 weeks of age (eligible range, age 6–12 weeks), 10 weeks (eligible range, age 10–18 weeks), and 14 weeks (eligible range, age 14–22 weeks); whereas children in Kangaba had to be vaccinated with diphtheria-tetanus-whole cell pertussis (DTwP) vaccine at 6 weeks of age (eligible range, age 6–12 weeks), 10 weeks (eligible range, age 10–18 weeks), and 14 weeks (eligible range, age 14–22 weeks), but not with Hib (i.e., pentavalent) vaccine. The interval between immunizations had to be at least 24 days.
The extended upper range for eligibility with respect to vaccination ages was necessary because in Mali immunization of infants often occurs several weeks beyond the recommended EPI target ages. The Hib vaccine administered, consisting of 10 μg of PRP covalently bound to 20–40 μg of tetanus toxoid (PRP-T), was a component of Tritanrix-HBV-TM pentavalent vaccine (GSK Biologicals, Rixensart, Belgium) that included DTwP and hepatitis B vaccines in a single syringe. The immunization history was confirmed by immunization card review; no oral reports or other medical records were accepted. Children were excluded from the study if they had received any blood products within the last 90 days; or if they had been vaccinated with Hib and were in the Kangaba study population.
Study review and informed consent.
The study was approved by the Ethics Committee of the Malian Medical University (Faculté de Médecine, Pharmacologie et Odonto-Stomatologie) and the Institutional Review Board of the University of Maryland, Baltimore, MD. The consent process involved obtaining initial concurrence from community leaders to work in the communities, followed by individual consent from parents or guardians with communication aids in local dialects and audio tapes for illiterate community members.
Specimen and data collection and laboratory methods.
Initial interview, immunization card review, and sample collection all took place on the same day for participants who were included in the study. Blood was collected and processed at the respective local health facilities (CVD-Mali Clinical Unit in Djikoroni-para, the community health center in Banconi, or the community health center in Kangaba village), according to standard operating procedures. The blood samples were centrifuged to separate serum, which was then transported under cold chain to the CVD-Mali Immunology Laboratory. An aliquot of each serum specimen was shipped frozen to CVD-Baltimore and analyzed for anti-PRP antibodies using indirect enzyme-linked immunosorbent assay (ELISA). Briefly, Immunolon II plates (Thermo Labsystem, Franklin, MA) were coated with purified PRP (HbO-HA antigen, Lederle-Praxis Biologicals, Inc., Rochester, NY, Lot #17) at 1 μg/mL in phosphate buffered saline for 3 hours at 37°C and blocked with 10% dried milk (Nestle USA, Inc., Glendale, CA) overnight at 4°C. Sera were added in serial 2-fold dilutions along with the standard (CBER/FDA lot 1983; Center for Biologics and Evaluation Research, Food and Drug Administration, Bethesda, MD) and incubated for 1 hour at 37°C. Peroxidase-labeled goat anti-human IgG was used as conjugate (Jackson Immuno Research Laboratories, Inc., West Grove, PA, Cat #76255) and tetramethylbenzidine (TMB, Kirkegaard and Perry Laboratories, Inc., Gaithersburg, MD) as substrate. The reaction was stopped with H2PO4 and Absorbance values450nm were measured with an ELISA reader (Multiskan Ascent, Thermo Labsystem, Helsinki, Finland). Antibody titers were calculated by interpolating regression corrected Absorbance values of serum samples in the curve of the FDA Hib standard (concentration: 70 μg/mL) and results were expressed as μg/mL.
Data analysis.
Percentages of infants with protective antibody levels were calculated at the 1 μg/mL (long-term protection) and 5 μg/mL (protection against colonization) concentrations. Differences in percentages of protected infants for unvaccinated and vaccinated populations per age group were calculated using Fisher's exact test. The geometric mean titer (GMT) and the corresponding 95% confidence interval (CI) of Hib antibodies in each age group and site were calculated. The Wilcoxon rank sum test was used to compare unvaccinated and vaccinated populations per age group, because the data were not distributed normally. One-sided P < 0.025 was considered statistically significant in comparisons of unvaccinated and vaccinated groups at a particular age; there was no correction for multiple comparisons. Analysis of variance on log (base 10) titers was used to examine the overall differences in titers over age groups in each population separately; P < 0.05 was considered significant. Calculations were performed using Intercooled Stata 9.0 (StataCorp LP, College Station, TX) and NCSS 2007 (Number Cruncher Statistical Systems, Kaysville, UT).
Results
Immunization history data and blood specimens in Kangaba (Hib-unvaccinated) were collected from May 17, 2007 to January 11, 2008, and in Bamako (Hib-vaccinated) from February 26, 2008 to June 11, 2008. A total of 360 children were enrolled, with a single blood specimen being collected from 30 children per age group (6 weeks and 6, 12, 15, 18, and 23 months of age), per site. The volume of serum from each specimen was sufficient so that the PRP titer could be measured in all 360 specimens. Males and females were almost evenly distributed in both groups: 90 males (50%), 90 females (50%) in urban Bamako; and 97 males (54%), 83 males (46%) in rural Kangaba. Review of the children's vaccination cards revealed that infants in Bamako and Kangaba received their first dose of pentavalent (with Hib) or quadrivalent (without Hib) vaccine at an average of 6.9 weeks of age (range 6–11.1), the second dose at 12.1 weeks (range 10.1–16.3), and their third dose at 17.3 weeks (range 14.4–22.0), as shown in Table 1. The interval between each sequential vaccination for every child was always greater than 24 days. Thus, the timing of actual Hib vaccination (6.9, 12.1, and 17.3 weeks of age) was later than the ages officially intended by the EPI schedule (6, 10, and 14 weeks of age).
Table 1.
Timing of vaccination in Malian children*
| Administration of DTP/HepB/+Hib | Administration of DTP/HepB/+Hib | Administration of DTP/HepB/+Hib | |
|---|---|---|---|
| EPI schedule in Mali (study enrollment eligibility) | 6 weeks (6–12 weeks) | 10 weeks (10–18 weeks) | 14 weeks (14–22 weeks) |
| Average timing of vaccination in study subjects (range) | 6.9 weeks (6–11.1 weeks) | 12.1 weeks (10.1–16.3 weeks) | 17.3 weeks (14.4–22.0 weeks) |
EPI = Malian Expanded Program on Immunization.
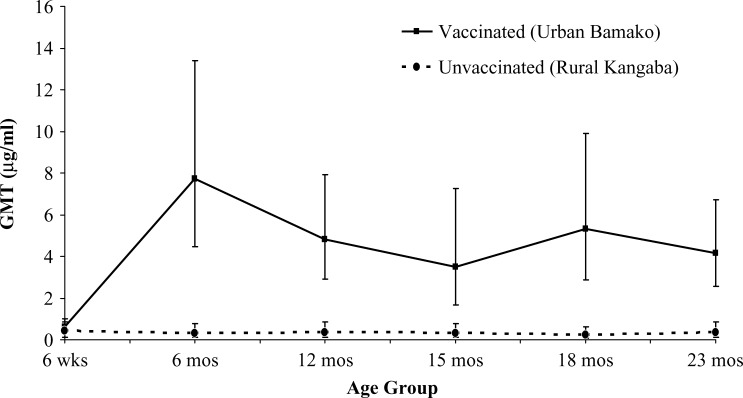
Figure 1 depicts the GMTs with 95% CI for Hib-unvaccinated and Hib-vaccinated children, by age group. The PRP antibody titers were significantly higher in the Bamako children for all age groups except for infants 6 weeks of age (Wilcoxon rank sum test). No child in the vaccinated population received Hib vaccine before the sample collection in the 6-week age group, as this was meant as a baseline measurement. The differences in titers among the different age groups were not significant for the unvaccinated, whereas the differences over age in the vaccinated group were significant (analysis of variance of log10 [titer]), with all post-vaccination GMTs being higher than the GMT at baseline.
Figure 1.
Geometric mean titer (GMT) and 95% confidence interval of serum IgG anticapsular polysaccharide antibody in children from rural Kangaba (Hib unvaccinated) and urban Bamako (Hib vaccinated) by age group. The GMTs for the 6-week age groups represent pre-vaccination immune status and were not significantly different. The GMTs for all other age groups were significantly higher in Bamako than in Kangaba (one-sided P < 0.025, Wilcoxon rank sum test). The number of children per age group per site was 30.
Table 2 shows the percentage of children from 6 months of age (by which time maternal PRP antibodies have typically disappeared) through 23 months of age who exhibit PRP antibody titers ≥ 1 μg/mL (predictive of long-term protection) or ≥ 5 μg/mL (correlated with prevention of Hib colonization of the upper respiratory tract). The first 2 years of life represent the age period of greatest risk for invasive Hib disease. At each age point, the percentage of children who manifest a PRP titer ≥ 1 μg/mL or ≥ 5 μg/mL was significantly higher in the vaccinated population compared with the unvaccinated. Particularly striking is the difference in the percentage of children with titers of PRP antibodies ≥ 5 μg/mL; 40.0–66.7% of the Hib-vaccinated children exhibited such high titers versus 0.0% of Hib-unvaccinated children.
Table 2.
Titers of antibodies against Haemophilus influenzae type b capsular polysaccharide (PRP) associated with long-term protection against invasive disease (≥ 1 μg/mL) and against upper respiratory tract colonization by Hib in unvaccinated (Kangaba) and vaccinated (Bamako) populations, by age group
| Age Group | Antibody titers ≥ 1 μg/mL | P* | Antibody titers ≥ 5 μg/mL | P* | ||
|---|---|---|---|---|---|---|
| Kangaba | Bamako | Kangaba | Bamako | |||
| 6 mos. (N = 30) | 13.3% | 90.0% | < 0.0001 | 0.0% | 66.7% | < 0.0001 |
| 12 mos. (N = 30) | 16.7% | 93.3% | < 0.0001 | 0.0% | 46.7% | < 0.0001 |
| 15 mos. (N = 30) | 16.7% | 76.7% | < 0.0001 | 0.0% | 50.0% | < 0.0001 |
| 18 mos. (N = 30) | 10.0% | 83.3% | < 0.0001 | 0.0% | 56.7% | < 0.0001 |
| 23 mos. (N = 30) | 23.3% | 93.3% | < 0.0001 | 0.0% | 40.0% | < 0.0001 |
One-sided P value from Fisher's exact test.
Discussion
This field study shows that children in urban Bamako, Mali exhibited a robust serologic response to the recently introduced Hib conjugate vaccine given as a component of a pentavalent formulation, in combination with DTwP/HepB, according to the EPI schedule with scheduled immunizations at 6, 10, and 14 weeks of age. Of importance, the immunologic response was sustained throughout the first 2 years of life, which is the highest risk period for invasive Hib disease, without the administration of a reinforcing booster immunization in the second year of life. Including a reinforcing booster dose for toddlers is typical of Hib immunization schedules in most industrialized and transitional countries. An exception was the original Hib conjugate immunization schedule used in the United Kingdom, which did not include a booster dose for toddlers.37,38 The original immunization regimen in the United Kingdom, which involved administering PRP-T combined with DTwP at 2, 3, and 4 months of age, initially led to an impressive decrease of invasive Hib disease.37–39 However, a resurgence of cases occurred in the late 1990s.40,41 This was brought under control by a catch-up campaign and eventual introduction of a Hib booster dose at 12 months of age.42
With the primary United Kingdom immunization schedule, the PRP antibody GMT for 5-month olds was 4.6 μg/mL (95% CI = 3.51–6.03).43 In comparison, the GMT for 6-month olds in Mali was higher, reaching 7.73 μg/mL (95% CI = 4.46–13.41). Interestingly, before a reinforcing booster dose, titers in many United Kingdom infants dropped below the long-term protective level (1 μg/mL), as a GMT of 0.88 μg/mL (95% CI = 0.66–1.17) was reported for 12-month olds. By contrast, the GMT for vaccinated Malian 12-month olds in our study remained high at 4.8 μg/mL (95% CI = 2.91–7.92); moreover, the GMT remained close to this level throughout the second year of life (see Figure 1). A similar pattern can be seen in other developing world populations. Among infants in Niger who received DTwP/PRP-T according to the EPI schedule (with immunizations at 6, 10, and 14 weeks of age), a GMT of 6.09 μg/mL was observed at 4.5 months of age and the GMT at 9 months of age was still 2.14 μg/mL.13 Overall, reports of serologic data following immunization with Hib conjugate have been sparse for children in sub-Saharan Africa. Nevertheless, insights can be gleaned from data from other non-industrialized country populations. A 1998 study that directly compared industrialized country (Belgium) infants with developing country (Chile) infants showed significantly higher titers in the latter infants for all components of a quadrivalent combination (DTwP/PRP-T).44
What accounts for the stronger and more enduring anti-PRP responses in infants in developing countries and do the persisting high titers protect against disease? The enhanced anti-PRP responses in developing country infants could derive from immunologic priming consequent to more extensive colonization with Hib45,46 or with other bacteria expressing polysaccharides that cross-react with PRP (e.g., E. coli expressing K100)4,47–50; such immunologic priming may occur even though the infant does not exhibit serum anti-PRP antibodies.
The vaccines co-administered with Hib conjugate can also influence the anti-PRP response. Although industrialized countries have transitioned to acellular pertussis vaccine in the last decade, developing countries still use whole cell pertussis vaccine. The lipopolysaccharide in whole cell pertussis has well-known adjuvant properties mediated by stimulation of TLR4. Consequently, infants immunized with wP/PRP-T compared with aP/PRP-T have stronger responses, better persistence of anti-PRP antibodies, and less risk of vaccine failure.51
These boosting factors may decrease in the future as widespread Hib vaccination and improved socioeconomic conditions (decreased crowding) progressively diminish upper respiratory carriage of Hib.11,52 They would also be lower if in the future acellular pertussis vaccine came to replace whole cell pertussis in the infant combination vaccine. Indeed, rising rates of Hib disease were observed both in the United Kingdom and in The Gambia, 7–9 years after the introduction of Hib vaccination in infancy,53,54 without a booster dose in the second year of life. However, it is unclear what roles in the rise of Hib disease were played respectively by vaccine failure, fall in Hib immunization coverage, change of Hib vaccine, switch to acellular pertussis vaccine, or diminished natural boosting consequent to decreased carriage.53,54 Therefore, both continued epidemiologic surveillance and serosurvey monitoring are advisable to detect the re-emergence of Hib disease. Moreover, if costs and logistics preclude the administration of a fourth booster dose of Hib in the second year of life, serious consideration must be given to modify the three-dose immunization schedules in developing countries to a regimen of two doses in early infancy followed by the third dose being given at ∼9–12 months of age.
Our serologic data suggest that Malian children are protected throughout the critical first 2 years of life. The seroepidemiologic data presented herein corroborate systematic surveillance data from Mali that showed a drastic reduction in the incidence of invasive Hib disease after the introduction of the Hib conjugate vaccine.15 Thirty months after implementation of the Hib conjugate immunization program in Bamako, when the vaccine coverage rate in infants 6–7 months of age was documented to be 81.1% by survey and when 81.5% of infants that age had PRP titers ≥ 1 μg/mL, Hib incidence had decreased by 86%.15 This effect carried over into the 12–23 months age group after the vaccinated infants reached toddler age.15 These data support the hypothesis that the high PRP antibody titers found in the vaccinated Malian pediatric population protect against Hib disease. Thus, the sustained immune response persists through the second year of life, despite lack of a booster dose in the second year of life, and epidemiologic data show that protection also persists for the 12–23 months age group. Moreover, indirect protection may also be provided to unvaccinated infants and non-target age groups caused by decreased transmission of Hib organisms, because ∼50% of vaccinated children in Bamako had serum anti-PRP antibody above a level that is thought to inhibit upper respiratory tract carriage (≥ 5 μg/mL).17 Our study highlights the widespread serological evidence immunity in Malian infants following programmatic introduction of Hib conjugate vaccine and evidence that seroprotection endures through at least the second year of life. The results also show the susceptibility of infants and toddlers in areas where the Hib vaccine program had not yet reached. These population-based seroprotection data complement and corroborate the earlier demonstration of the drop in invasive Hib disease burden in Malian infants and toddlers where Hib vaccine had been introduced and provide further justification for including Hib conjugate in the routine EPI schedule for all Malian infants.
ACKNOWLEDGMENTS
We thank Mardi Reymann, Lilian Cuberos, and Inna Ruslanova from the CVD Applied Immunology Section for technical assistance measuring Hib antibodies. We also thank William Blackwelder, the senior biostatistician of the CVD, for his valuable input in the statistics section.
Footnotes
Financial Support: Grant from the Bill and Melinda Gates Foundation to Myron M. Levine.
Authors' addresses: Julia Hutter, Marcela F. Pasetti, Milagritos D. Tapia, and Myron M. Levine, University of Maryland School of Medicine, Center for Vaccine Development, Baltimore, MD, E-mails: jthutter@gmail.com, mpasetti@medicine.umaryland.edu, mtapia@medicine.umaryland.edu, and mlevine@medicine.umaryland.edu. Doh Sanogo and Samba O. Sow, Ministry of Health, Centre pour le Développement des Vaccins – Mali, Ex-Institut Marchoux, Bamako, Mali, E-mails: sanogodoh@yahoo.fr and ssow@medicine.umaryland.edu.
References
- 1.World Health Organization WHO position paper on Haemophilus influenzae type b conjugate vaccines. Wkly Epidemiol Rec. 2006;81:445–452. [PubMed] [Google Scholar]
- 2.Peltola H. Burden of meningitis and other severe bacterial infections of children in Africa: implications for prevention. Clin Infect Dis. 2001;32:64–75. doi: 10.1086/317534. [DOI] [PubMed] [Google Scholar]
- 3.Levine OS, Lagos R, Munoz A, Villaroel J, Alvarez AM, Abrego P, Levine MM. Defining the burden of pneumonia in children preventable by vaccination against Haemophilus influenzae type b. Pediatr Infect Dis J. 1999;18:1060–1064. doi: 10.1097/00006454-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Bradshaw MW, Schneerson R, Parke JC, Jr, Robbins JB. Bacterial antigens cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b. Lancet. 1971;1:1095–1096. doi: 10.1016/s0140-6736(71)91837-x. [DOI] [PubMed] [Google Scholar]
- 5.Robbins JB, Schneerson R, Argaman M, Handzel ZT. Haemophilus influenzae type b: disease and immunity in humans. Ann Intern Med. 1973;78:259–269. doi: 10.7326/0003-4819-78-2-259. [DOI] [PubMed] [Google Scholar]
- 6.Kelly DF, Moxon ER, Yu LM, Pollard AJ. Anti-polyribosylribitol phosphate antibody concentrations and avidities in children since the start of Haemophilus influenzae type b immunization of infants in the United Kingdom. Clin Vaccine Immunol. 2009;16:246–252. doi: 10.1128/CVI.00023-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9:213–220. doi: 10.1038/nri2494. [DOI] [PubMed] [Google Scholar]
- 8.Schlesinger Y, Granoff DM. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. The Vaccine Study Group. JAMA. 1992;267:1489–1494. [PubMed] [Google Scholar]
- 9.Campbell JD, Lagos R, Levine MM, Losonsky GA. Standard and alternative regimens of Haemophilus influenzae type b conjugate vaccine (polyribosylribitol phosphate-tetanus toxoid conjugate vaccine) elicit comparable antibody avidities in infants. Pediatr Infect Dis J. 2002;21:822–826. doi: 10.1097/00006454-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Goldblatt D, Vaz AR, Miller E. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J Infect Dis. 1998;177:1112–1115. doi: 10.1086/517407. [DOI] [PubMed] [Google Scholar]
- 11.Watt JP, Levine OS, Santosham M. Global reduction of Hib disease: what are the next steps? Proceedings of the meeting Scottsdale, Arizona, September 22–25, 2002. J Pediatr. 2003;143:S163–S187. doi: 10.1067/s0022-3476(03)00576-6. [DOI] [PubMed] [Google Scholar]
- 12.Granoff DM, Anderson EL, Osterholm MT, Holmes SJ, McHugh JE, Belshe RB, Medley F, Murphy TV. Differences in the immunogenicity of three Haemophilus influenzae type b conjugate vaccines in infants. J Pediatr. 1992;121:187–194. doi: 10.1016/s0022-3476(05)81186-2. [DOI] [PubMed] [Google Scholar]
- 13.Campagne G, Garba A, Schuchat A, Boulanger D, Plikaytis BD, Ousseini M, Chippaux JP. Response to conjugate Haemophilus influenzae B vaccine among infants in Niamey, Niger. Am J Trop Med Hyg. 1998;59:837–842. doi: 10.4269/ajtmh.1998.59.837. [DOI] [PubMed] [Google Scholar]
- 14.Adegbola RA, Secka O, Lahai G, Lloyd-Evans N, Njie A, Usen S, Oluwalana C, Obaro S, Weber M, Corrah T, Mulholland K, McAdam K, Greenwood B, Milligan PJ. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: a prospective study. Lancet. 2005;366:144–150. doi: 10.1016/S0140-6736(05)66788-8. [DOI] [PubMed] [Google Scholar]
- 15.Sow SO, Tapia MD, Diallo S, Keita MM, Sylla M, Onwuchekwa U, Pasetti MF, Kotloff KL, Levine MM. Haemophilus influenzae type b conjugate vaccine introduction in Mali: impact on disease burden and serologic correlate of protection. Am J Trop Med Hyg. 2009;80:1033–1038. [PubMed] [Google Scholar]
- 16.Cisse MF, Breugelmans JG, Ba M, Diop MB, Faye PC, Mhlanga B, Mueller JE, Koffi D, Gessner BD. The elimination of Haemophilus influenzae type b meningitis following conjugate vaccine introduction in Senegal. Pediatr Infect Dis J. 2010;29:499–503. doi: 10.1097/INF.0b013e3181ccb0a0. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez J, Levine OS, Sanchez J, Balter S, LaClaire L, Feris J, Romero-Steiner S. Prevention of Haemophilus influenzae type b colonization by vaccination: correlation with serum anti-capsular IgG concentration. J Infect Dis. 2000;182:1553–1556. doi: 10.1086/315870. [DOI] [PubMed] [Google Scholar]
- 18.Kayhty H, Peltola H, Karanko V, Makela PH. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983;147:1100. doi: 10.1093/infdis/147.6.1100. [DOI] [PubMed] [Google Scholar]
- 19.Khatami A, Snape MD, John T, Westcar S, Klinger C, Rollinson L, Boutriau D, Mesaros N, Wysocki J, Galaj A, Yu LM, Pollard AJ. Persistence of immunity following a booster dose of Haemophilus influenzae type B-Meningococcal serogroup C glycoconjugate vaccine: follow-up of a randomized controlled trial. Pediatr Infect Dis J. 2011;30:197–202. doi: 10.1097/INF.0b013e3181f728fd. [DOI] [PubMed] [Google Scholar]
- 20.Eskola J, Kayhty H, Takala AK, Peltola H, Ronnberg PR, Kela E, Pekkanen E, McVerry PH, Makela PH. A randomized, prospective field trial of a conjugate vaccine in the protection of infants and young children against invasive Haemophilus influenzae type b disease. N Engl J Med. 1990;323:1381–1387. doi: 10.1056/NEJM199011153232004. [DOI] [PubMed] [Google Scholar]
- 21.Slack MH, Schapira D, Thwaites RJ, Burrage M, Southern J, Goldblatt D, Miller E. Responses to a fourth dose of Haemophilus influenzae type B conjugate vaccine in early life. Arch Dis Child Fetal Neonatal Ed. 2004;89:F269–F271. doi: 10.1136/adc.2003.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ladhani S, Heath PT, Ramsay ME, Slack MP, Kibwana E, Pollard AJ, Booy R. Long-term immunological follow-up of children with Haemophilus influenzae serotype b vaccine failure in the United Kingdom. Clin Infect Dis. 2009;49:372–380. doi: 10.1086/600292. [DOI] [PubMed] [Google Scholar]
- 23.Habermehl P, Leroux-Roels G, Sanger R, Machler G, Boutriau D. Combined Haemophilus influenzae type b and Neisseria meningitidis serogroup C (HibMenC) or serogroup C and Y-tetanus toxoid conjugate (and HibMenCY) vaccines are well-tolerated and immunogenic when administered according to the 2,3,4 months schedule with a fourth dose at 12–18 months of age. Hum Vaccin. 2010;6:640–651. doi: 10.4161/hv.6.8.12154. [DOI] [PubMed] [Google Scholar]
- 24.Ladhani S, Heath PT, Slack MP, McIntyre PB, Diez-Domingo J, Campos J, Dagan R, Ramsay ME. Haemophilus influenzae serotype b conjugate vaccine failure in twelve countries with established national childhood immunization programmes. Clin Microbiol Infect. 2010;16:948–954. doi: 10.1111/j.1469-0691.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 25.Madhi SA, Kuwanda L, Saarinen L, Cutland C, Mothupi R, Kayhty H, Klugman KP. Immunogenicity and effectiveness of Haemophilus influenzae type b conjugate vaccine in HIV infected and uninfected African children. Vaccine. 2005;23:5517–5525. doi: 10.1016/j.vaccine.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 26.Mulholland EK, Hoestermann A, Ward JI, Maine N, Ethevenaux C, Greenwood BM. The use of Haemophilus influenzae type b-tetanus toxoid conjugate vaccine mixed with diphtheria-tetanus-pertussis vaccine in Gambian infants. Vaccine. 1996;14:905–909. doi: 10.1016/0264-410x(95)00260-8. [DOI] [PubMed] [Google Scholar]
- 27.Madore DV, Johnson CL, Phipps DC, Myers MG, Eby R, Smith DH. Safety and immunogenicity of Haemophilus influenzae type b oligosaccharide-CRM197 conjugate vaccine in infants aged 15 to 23 months. Pediatrics. 1990;86:527–534. [PubMed] [Google Scholar]
- 28.Mulholland EK, Byass P, Campbell H, Fritzell B, Greenwood AM, Todd J, Greenwood BM. The immunogenicity and safety of Haemophilus influenzae type b-tetanus toxoid conjugate vaccine in Gambian infants. Ann Trop Paediatr. 1994;14:183–188. doi: 10.1080/02724936.1994.11747715. [DOI] [PubMed] [Google Scholar]
- 29.Obaro SK, Enwere GC, Deloria M, Jaffar S, Goldblatt D, Brainsby K, Hallander H, McInnes P, Greenwood BM, McAdam KP. Safety and immunogenicity of pneumococcal conjugate vaccine in combination with diphtheria, tetanus toxoid, pertussis and Haemophilus influenzae type b conjugate vaccine. Pediatr Infect Dis J. 2002;21:940–947. doi: 10.1097/00006454-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Punjabi NH, Richie EL, Simanjuntak CH, Harjanto SJ, Wangsasaputra F, Arjoso S, Rofiq A, Prijanto M, Julitasari, Yela U, Herzog C, Cryz SJ. Immunogenicity and safety of four different doses of Haemophilus influenzae type b-tetanus toxoid conjugated vaccine, combined with diphtheria-tetanus-pertussis vaccine (DTP-Hib), in Indonesian infants. Vaccine. 2006;24:1776–1785. doi: 10.1016/j.vaccine.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 31.Hirve S, Bavdekar A, Juvekar S, Agarwal D, Barde P, Mangrule S, Patwardhan M, Pandit A, Kulkarni PS. A comparative study to evaluate the safety and immunogenicity of two lots of Haemophilus influenzae type-B conjugate vaccine manufactured at different scales. Vaccine. 2011;29:5363–5367. doi: 10.1016/j.vaccine.2011.05.081. [DOI] [PubMed] [Google Scholar]
- 32.Panpitpat C, Thisyakorn U, Chotpitayasunondh T, Furer E, Que JU, Hasler T, Cryz SJ., Jr Elevated levels of maternal anti-tetanus toxin antibodies do not suppress the immune response to a Haemophilus influenzae type b polyribosylphosphate-tetanus toxoid conjugate vaccine. Bull World Health Organ. 2000;78:364–371. [PMC free article] [PubMed] [Google Scholar]
- 33.Espinoza F, Tregnaghi M, Gentile A, Abarca K, Casellas J, Collard A, Lefevre I, Jacquet JM. Primary and booster vaccination in Latin American children with a DTPw-HBV/Hib combination: a randomized controlled trial. BMC Infect Dis. 2010;10:297. doi: 10.1186/1471-2334-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CY, Thipphawong J, Huang LM, Lee PI, Chiu HH, Lin W, Debois H, Harrison D, Xie F, Barreto L. An evaluation of the safety and immunogenicity of a five-component acellular pertussis, diphtheria, and tetanus toxoid vaccine (DTaP) when combined with a Haemophilus influenzae type b-tetanus toxoid conjugate vaccine (PRP-T) in Taiwanese infants. Pediatrics. 1999;103:25–30. doi: 10.1542/peds.103.1.25. [DOI] [PubMed] [Google Scholar]
- 35.Sow SO, Diallo S, Campbell JD, Tapia MD, Keita T, Keita MM, Murray P, Kotloff KL, Levine MM. Burden of invasive disease caused by Haemophilus influenzae type b in Bamako, Mali: impetus for routine infant immunization with conjugate vaccine. Pediatr Infect Dis J. 2005;24:533–537. doi: 10.1097/01.inf.0000164768.28135.0d. [DOI] [PubMed] [Google Scholar]
- 36.United Nations Children's Fund . State of the World's Children: Adolescence, an Age of Opportunity. Tinton Falls, NJ: Hatteras Press; 2011. pp. 1–128. [Google Scholar]
- 37.Booy R, Hodgson S, Carpenter L, Mayon-White RT, Slack MP, Macfarlane JA, Haworth EA, Kiddle M, Shribman S, Roberts JS. Efficacy of Haemophilus influenzae type b conjugate vaccine PRP-T. Lancet. 1994;344:362–366. doi: 10.1016/s0140-6736(94)91400-1. [DOI] [PubMed] [Google Scholar]
- 38.Hargreaves RM, Slack MP, Howard AJ, Anderson E, Ramsay ME. Changing patterns of invasive Haemophilus influenzae disease in England and Wales after introduction of the Hib vaccination programme. BMJ. 1996;312:160–161. doi: 10.1136/bmj.312.7024.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slack MP, Azzopardi HJ, Hargreaves RM, Ramsay ME. Enhanced surveillance of invasive Haemophilus influenzae disease in England, 1990 to 1996: impact of conjugate vaccines. Pediatr Infect Dis J. 1998;17:S204–S207. doi: 10.1097/00006454-199809001-00026. [DOI] [PubMed] [Google Scholar]
- 40.Booy R, Heath PT, Slack MP, Begg N, Moxon ER. Vaccine failures after primary immunization with Haemophilus influenzae type-b conjugate vaccine without booster [see comments] Lancet. 1997;349:1197–1202. doi: 10.1016/s0140-6736(96)06392-1. [DOI] [PubMed] [Google Scholar]
- 41.Trotter CL, Ramsay ME, Slack MP. Rising incidence of Haemophilus influenzae type b disease in England and Wales indicates a need for a second catch-up vaccination campaign. Commun Dis Public Health. 2003;6:55–58. [PubMed] [Google Scholar]
- 42.Ladhani S, Slack MP, Heys M, White J, Ramsay ME. Fall in Haemophilus influenzae serotype b (Hib) disease following implementation of a booster campaign. Arch Dis Child. 2008;93:665–669. doi: 10.1136/adc.2007.126888. [DOI] [PubMed] [Google Scholar]
- 43.Heath PT, Booy R, Azzopardi HJ, Slack MP, Bowen-Morris J, Griffiths H, Ramsay ME, Deeks JJ, Moxon ER. Antibody concentration and clinical protection after Hib conjugate vaccination in the United Kingdom. JAMA. 2000;284:2334–2340. doi: 10.1001/jama.284.18.2334. [DOI] [PubMed] [Google Scholar]
- 44.Hoppenbrouwers K, Lagos R, Swennen B, Ethevenaux C, Knops J, Levine MM, Desmyter J. Safety and immunogenicity of an Haemophilus influenzae type b-tetanus toxoid conjugate (PRP-T) and diphtheria-tetanus-pertussis (DTP) combination vaccine administered in a dual-chamber syringe to infants in Belgium and Chile. Vaccine. 1998;16:921–927. doi: 10.1016/s0264-410x(97)00303-4. [DOI] [PubMed] [Google Scholar]
- 45.Michaels RH, Norden CW. Pharyngeal colonization with Haemophilus influenzae type b: a longitudinal study of families with a child with meningitis or epiglottitis due to H. influenzae type b. J Infect Dis. 1977;136:222–228. doi: 10.1093/infdis/136.2.222. [DOI] [PubMed] [Google Scholar]
- 46.Hall DB, Lum MK, Knutson LR, Heyward WL, Ward JI. Pharyngeal carriage and acquisition of anticapsular antibody to Haemophilus influenzae type b in a high-risk population in southwestern Alaska. Am J Epidemiol. 1987;126:1190–1197. doi: 10.1093/oxfordjournals.aje.a114758. [DOI] [PubMed] [Google Scholar]
- 47.Schneerson R, Bradshaw M, Whisnant JK, Myerowitz RL, Parke JC, Jr, Robbins JB. An Escherichia coli antigen cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b: occurrence among known serotypes, and immunochemical and biologic properties of E. coli antisera toward H. influenzae type b. J Immunol. 1972;108:1551–1562. [PubMed] [Google Scholar]
- 48.Myerowitz RL, Gordon RE, Robbins JB. Polysaccharides of the genus Bacillus cross-reactive with the capsular polysaccharides of Diplococcus pneumoniae type 3, Haemophilus influenzae type b, and Neisseria meningitidis group A. Infect Immun. 1973;8:896–900. doi: 10.1128/iai.8.6.896-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Handzel ZT, Argaman M, Parke JC, Jr, Schneerson R, Robbins JB. Heteroimmunization to the capsular polysaccharide of Haemophilus influenzae type b induced by enteric cross-reacting bacteria. Infect Immun. 1975;11:1045–1052. doi: 10.1128/iai.11.5.1045-1052.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneerson R, Robbins JB. Induction of serum Haemophilus influenzae type b capsular antibodies in adult volunteers fed cross-reacting Escherichia coli 075:K100:H5. N Engl J Med. 1975;292:1093–1096. doi: 10.1056/NEJM197505222922103. [DOI] [PubMed] [Google Scholar]
- 51.Southern J, McVernon J, Gelb D, Andrews N, Morris R, Crowley-Luke A, Goldblatt D, Miller E. Immunogenicity of a fourth dose of Haemophilus influenzae type b (Hib) conjugate vaccine and antibody persistence in young children from the United Kingdom who were primed with acellular or whole-cell pertussis component-containing Hib combinations in infancy. Clin Vaccine Immunol. 2007;14:1328–1333. doi: 10.1128/CVI.00191-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adegbola RA, Mulholland EK, Secka O, Jaffar S, Greenwood BM. Vaccination with a Haemophilus influenzae type b conjugate vaccine reduces oropharyngeal carriage of H. influenzae type b among Gambian children. J Infect Dis. 1998;177:1758–1761. doi: 10.1086/517440. [DOI] [PubMed] [Google Scholar]
- 53.Heath PT, McVernon J. The UK Hib vaccine experience. Arch Dis Child. 2002;86:396–399. doi: 10.1136/adc.86.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howie SR, Antonio M, Akisanya A, Sambou S, Hakeem I, Secka O, Adegbola RA. Re-emergence of Haemophilus influenzae type b (Hib) disease in The Gambia following successful elimination with conjugate Hib vaccine. Vaccine. 2007;25:6305–6309. doi: 10.1016/j.vaccine.2007.06.023. [DOI] [PubMed] [Google Scholar]