Abstract
We investigated infection of rodents and shrews by Leptospira spp. in two localities of Cambodia (Veal Renh, Kaev Seima) and in four types of habitat (forests, non-flooded lands, lowland rain-fed paddy fields, houses) during the wet and the dry seasons. Habitat preference was common, and rodent and shrew species were found only in houses or in rain-fed paddy fields or in forests. Among 649 small mammals trapped belonging to 12 rodent species and 1 shrew species, 71 of 642 animals tested were carriers of Leptospira according to the 16S ribosomal RNA marker used. Rodent infection was higher in low-slope locations, corresponding to rain-fed paddy fields, especially in the rainy season and in Kaev Seima. Rodents (Rattus exulans) and shrews (Suncus murinus) inhabiting households showed significantly low levels of infections, whereas rodents living in and near to forests (shrubby wasteland, orchards) showed high levels of infection.
Introduction
Leptospirosis is a global re-emergent zoonotic disease caused by pathogenic spirochetes of the genus Leptospira.1–4 This genus comprises approximately 20 species and more than 300 serovars, with about half of the pathogenic serovars belonging to L. interrogans or L. borgpetersenii.5 The disease shows seasonal peaks of incidence during the rainy season in tropical regions and most outbreaks are related to flooding events.1,5 Southeast Asia is a region of particularly high incidence.6 Thailand, Cambodia, Laos, and Vietnam are considered areas to which leptospirosis is endemic.7 Seroprevalence in humans can reach 24% in flood-prone areas in Laos8 and 12.8% in Vietnam,9 and countries such as Thailand have experienced repeated severe outbreaks throughout the past decade.5 Although few studies have been conducted, Cambodia appears to be a country of high incidence (with more than 10 cases/100,000 persons). Of the patients hospitalized with clinical symptoms compatible with leptospirosis in the province of Takeo, 29.9% were positive for Leptospira biological markers, IgM, and/or DNA.10
Rodents are recognized as important mammal reservoirs of Leptospira spp.11,12 Infection may occur early in the lifespan of the animal and the chances of infection increases with age.1 After infection, the spirochetes are localized in the kidneys and excreted by in urine discontinuously.1 Once excreted, the bacteria can survive in a favorable environment for months or years before infecting new hosts, including humans.11 Human infection results from direct or indirect exposure to the urine of carrier animals. Sample collection of rodent species and documentation of their infestation levels are repeatedly mentioned in surveillance and rodent control.5
Most research on the presence of leptospires in rodents has been conducted in urban areas or in rural areas in the vicinity of households,13,14 but few studies have investigated rodents within their various environments. In Southeast Asia, a region of high murine diversity, only the few rodent species that live near humans in households or in rain-fed paddy rice fields were investigated, whereas the numerous species inhabiting other plantations or forests were not surveyed. However, rodent species show habitat preference in Southeast Asia15–17 and this finding may have some consequences in term of disease transmission ecology in relation to habitat as emphasized for other rodent-borne diseases.18
Whereas several studies in Thailand have investigated the presence of Leptospira spp. in rodents,19,20 few studies have been performed in neighboring countries despite the occurrence of leptospirosis. Rodent infection has been investigated by using serologic methods in Vietnam and Laos,21 but no molecular surveys have been performed. We focused our study in Cambodia and, to our knowledge, this study is the first conducted on the infection of rodents by Leptospira spp. in this country.
Our aims were to identify the rodent species that may act as reservoirs of Leptospira spp. and the environmental characteristics favorable for transmission. Analyses were conducted in two sites in Cambodia over two seasons. Rodent infections, assessed by the detection of spirochetes in kidneys by using a polymerase chain reaction (PCR)–based method were analyzed in relation to rodent species and several environmental factors: season (wet or dry) types of habitats (forest, flooded or non-flooded agricultural areas and human settlements), vegetation index and slope. More precisely, we aimed to test 1) the role of climate for the hypothesis that the wet season should favor higher infection rate in rodents because of better bacterial survival in humid environments and enhancement of transmission;1 2) the effect of vegetation for the hypothesis that dense vegetation, by maintaining higher humidity than areas of sparse vegetation, may increase the survival of the bacteria and the likelihood of transmission; and 3) the effect of habitat for the hypothesis that rain-fed paddy rice fields or low sloped fields will offer better bacterial survival and higher transmission particularly during the rainy season.
Methods
Trapping protocol.
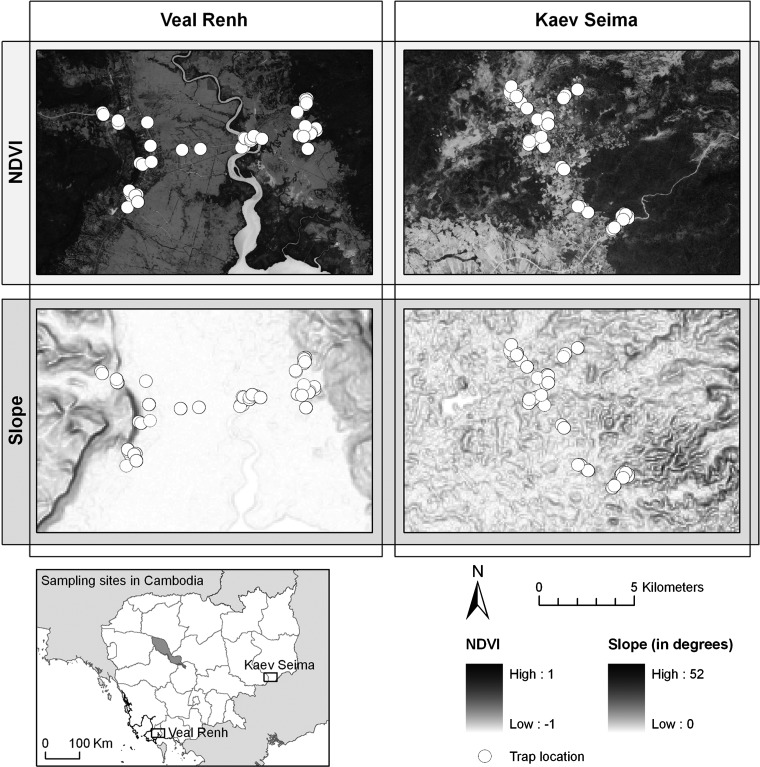
Rodents were trapped in the Cambodian Provinces of Preah Sihanouk and Mondulkiri, respectively, in Veal Renh (10°71′ N, 103°82′ E) and Kaev Seima (12°12′ N, 106°89′ E) districts (Figure 1). Two trapping sessions were conducted per locality during different seasons. Each session was characterized as wet or dry, according to the average rainfall recorded during the month of trapping and the former month, and provided by the Global Precipitation Climatology Center (http://gpcc.dwd.de).22 The trapping protocol was originally established to test the effect of season on the infection of rodents. Trapping dates were selected within the usual dry and wet season dates. However, the climate was more variable in 2008–2009 during a La Niña event of 2008, which dramatically affected the monsoon regimen in Cambodia and other countries in Southeast Asia, while El Niño was active in 2009. In Veal Renh, the first session was at the beginning of the dry season (November 2008 with 192 mm average monthly rainfall) and the second session was during the wet season (July 2009 with 383 mm). In Kaev Seima, the first session was also during the dry season (March 2009 with 28 mm) and the second session was during the wet season (November 2009 with 196 mm).
Figure 1.
Normalized Difference Vegetation Index (NDVI) and slope maps with location of trap lines in the two study sites, Veal Renh and Kaev Seima, Cambodia.
These locations represent a variety of habitats in relation to human pressures and land use. Habitats were ranked as 1) forests and mature plantations; 2) non-flooded lands or fields (shrubby waste land, young plantations, orchards); 3) rain-fed lowland paddy rice fields (cultivated floodplain); and 4) households (in villages or city). Each natural and agricultural habitat was sampled with an equal pressure by using a stratified trapping protocol. For each trapping session, 30 trap lines of 10 locally made cage traps (separated by five-meter intervals) were deployed during four nights. The trapping pressure could be estimated at 1,200 trap nights for each locality at each season. Villages and isolated houses, which correspond to a fourth habitat category, the human settlement, were also sampled by using cage traps distributed to residents.
Geographic coordinates of trap line devices and households were systematically recorded, and landscape details were described by field observation with a three-level classification: low resolution for the main landscape categories; medium resolution for a more detailed category (e.g., village, rice field, corn field, dry evergreen); and high resolution to give more precision (harvested, flooded). Pictures, habitat description, and coordinates of trap lines are available in the research/study areas and research/protocols sections of the Ceropath project web site (www.ceropath.org).
Rodent identification and tissue sampling.
Rodents were identified on the basis of their morphology or by using species primer specific and/or barcoding assignment.23,24 Complete data for animals used as reference for barcoding assignment are consultable on the Barcoding Tool/RodentSEA section of the Ceropath project (http://www.ceropath.org/).
Rodents were humanely killed and dissected to collect organs including kidneys according to CERoPath protocols25 (www.ceropath.org), which follow animal care, health security for field parasitologists and quality data handlings. Animal care and manipulation followed the international rules (American Veterinary Medical Association Council on Research). Tissue samples were stored in liquid nitrogen in the field and conserved in a –80°C freezer at the Institut Pasteur in Cambodia (Phnom Penh, Cambodia) before molecular investigation.
DNA extraction and PCR.
Kidney was used to identify rodents as carriers of leptospires. Kidney samples were homogenized and DNA was extracted by using the Qiagen Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions for tissue extraction.
The protocol by Mérien and others26 was used to amplify a 290-basepair fragment of the 16S ribosomal RNA gene. Briefly, the first step of the nested PCR containing 1× Taq buffer, 2 mM MgCl2, 200 μM of each dNTP, 1 μM of each primer (LeptoA: 5′-GGCGGCGCGTCTTAAACATG-3′ and LeptoB: 5′-TTCCCCCCATTGAGCAAGATT-3′), 2 units of Taq polymerase, and 3 μL of undiluted DNA template in a final volume 25 μL. The PCR mixtures were subjected to an initial denaturation at 94°C for 3 minutes, followed by 40 cycles (94°C for 30 seconds, 63°C for 1.5 minutes, and 72°C for 1.5 minutes), and final extension at 72°C for 10 minutes. The second step of the nested PCR contained 1× Taq buffer, 2.5 mM MgCl2, 200 μM of each dNTP, 1 μM of each primer (LeptoC: 5′-CAAGTCAAGCGGAGTAGCAA-3′ and LeptoD: 5′-CTTAACTGCTGCCTCCCGTA-3′), 2 units of Taq polymerase, and 3 μL of undiluted PCR product in a final volume of 25 μL. The amplification program was initial denaturation at 94°C for 3 minutes, followed by 25 cycles (94°C for 30 seconds, 61°C for 1.5 minutes, and 72°C for 1.5 minutes), and final extension at 72°C for 10 minutes.
Spatial analysis.
To estimate environmental variables involved in Leptospira infection, we used a geographic information system to characterize trapping habitats. We first obtained a Digital Elevation Model at a spatial resolution of 3 arc-seconds (approximately 90 meters) from the Shuttle Radar Topography Mission program (http://srtm.usgs.gov/). We used spatial analysis tools implemented in ArcGIS 9.3® to calculate the slope of each trapping site. We also acquired images from the SPOT 5 satellite high resolution geometric sensor, with a spatial resolution of 10 meters and spectral ranges in the green (0.50–0.59 μm), red (0.61–0.68 μm), near infrared (0.78–0.89 μm), and mean infrared (1.58–1.75 μm) wavelengths. These images were subsidized by the CERoPath project and Centre National d'Etudes Spatiales–Incentive for the Scientific Use of Images from the SPOT system program (project 220). Acquisition of the images corresponds to dates of March 16, 2008 for Veal Renh and March 22, 2007 for Kaev Seima.
In the present study, we calculated the Normalized Difference Vegetation Index (NDVI) to estimate the vegetation density by using Erdas version 9.3®. The NDVI is a ratio derived by dividing the difference between near-infrared (NIR) and red (R) reflectance measurements by their sum: NDVI = (NIR – R)/(NIR + R).27 This index is one of the most commonly used vegetation index. It ranges from –1.0 to +1.0, with higher positive values indicating vigor and quantity of vegetation and negative values indicating non-vegetated surfaces such as bare soils, inundated fields, or lakes. The variability of NDVI has been shown to relate to the heterogeneity of habitats and consequently with species richness.28
We estimated that the variables based on the value of only one pixel may induce some bias, and may not represent the point of rodent trapping site in a perfect way. We decided to take into account the neighbor pixels and to choose a buffer of 1.5 times the spatial resolution of the Digital Elevation Model, which corresponds to a buffer zone of 135 meters.
Statistical analysis.
We performed principal components analysis on individual number of rodent species trapped in each the four types of habitat to illustrate their habitat distributions. We performed generalized linear models (GLM) by using binomial distribution of individual host infection and logit function to identify likely variables that may explain infection of rodents by leptospires in R software (R Development Core Team, 2010). Selection of the best model was based on Akaike information criterion (AIC) using host species, sex and age, season, NDVI, slope, habitat, and district as independent variables.
Results
Trapped hosts and infection.
A total of 649 small mammals belonging to 12 rodent species and 1 shrew species were trapped (Table 1). Some species (two species of Bandicota, Leopoldamys edwardsi, Mus caroli, and Rhizomys pruinosus) were found only in Kaev Seima, and some species (Rattus argentiventer, Rattus norvegicus, and the shrew Suncus murinus) were found only in Veal Renh.
Table 1.
Rodent and shrew species trapped in Kaev Seima and Veal Renh, Cambodia, with their main and eventually secondary habitats
| Mammal | Species | Locality | Main habitat* | Secondary habitat* | Sample size |
|---|---|---|---|---|---|
| Rodents | Bandicota indica | Kaev Seima | 2† | 3 | 3 |
| Bandicota savilei | Kaev Seima | 2 | 3 | 77 | |
| Berylmys berdmorei | Kaev Seima, Veal Renh | 2 | 1, 3 | 12 | |
| Leopoldamys edwardsi | Kaev Seima | 1 | 2 | ||
| Maxomys surifer | Kaev Seima, Veal Renh | 1 | 2 | 107 | |
| Mus caroli | Kaev Seima | 3 | 1 | ||
| Niviventer fulvescens | Kaev Seima, Veal Renh | 1 | 2 | 16 | |
| Rattus argentiventer | Veal Renh | 3 | 2 | 44 | |
| Rattus exulans | Kaev Seima, Veal Renh | 4 | 177 | ||
| Rattus norvegicus | Veal Renh | 4 | 3 | 25 | |
| Rattus tanezumi | Kaev Seima, Veal Renh | 4 | 1, 2, 3 | 128 | |
| Rhizomys pruinosus | Kaev Seima | NA | 1 | ||
| Shrews | Suncus murinus | Veal Renh | 4 | 57 |
2 = shrubby wasteland and non-flooded cultivated fields (plantations, orchards); 1 = forests; 3 = lowland rain-fed rice fields; 4 = houses (villages).
This result is biased by the small size of the sample collected during this study. Bandicota indica usually mainly inhabits floodplains but can occur in dry lands during the dry season.
NA = not available.
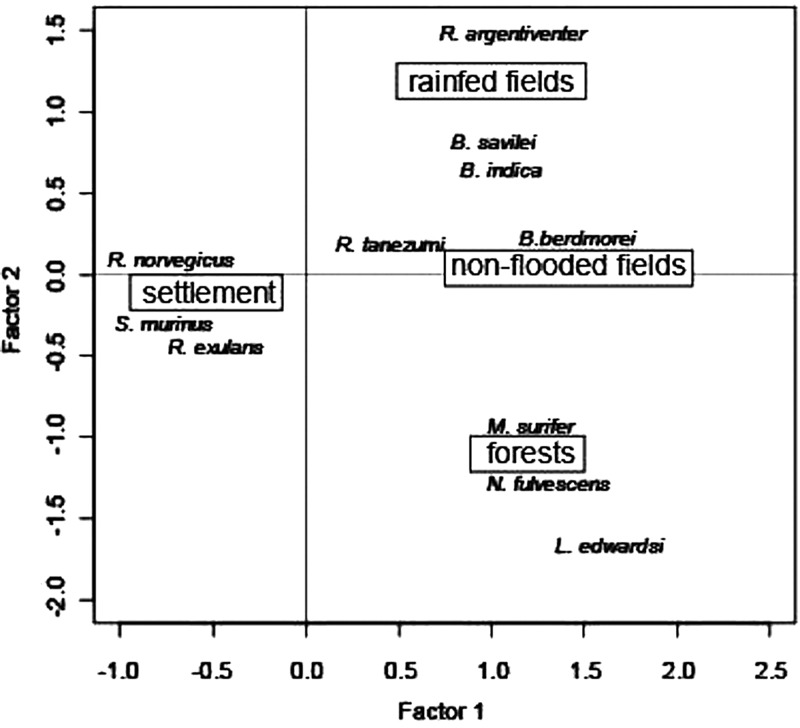
Principal components analysis showed that the first two axes accounted for most of the total variability in the data set. The first axis explained 49% of the variability, and the second axis explained 35% of the variability. Habitat preference is generally the rule in these rodent and shrew species. Some species were found only in households (Rattus exulans, R. norvegicus, S. murinus) or showed a strong preference for rain-fed paddy fields (Bandicota indica, R. argentiventer) or forests (L. edwardsi, Maxomys surifer, Niviventer fulvescens) (Figure 2 and Table 1). However, some species showed low habitat specificity, particularly Rattus tanezumi, which was found in all types of habitat (Table 1 and Figure 2) or Berylmys berdmorei, which was found in forests and non-flooded land.
Figure 2.
Distribution of rodent species in Cambodia according to habitat types: paddy fields (lowland rain-fed), non-flooded lands, forests, houses on the two first axes of principal component analysis. The factor 1 and 2 accounted for 85% of the variance.
Seventy-one (11.1%) of the 642 animals tested were found to be carriers of Leptospira according to the 16S ribosomal RNA marker used (Table 2). With the exception of very low trapped species (number < 3) (L. edwardsi, M. caroli, and R. pruinosus), all species were PCR positive for Leptospira, with the notable exception of R. norvegicus (27 negative animals). Four species (L. edwardsi, M. caroli, B. indica, and R. pruinosus) were not used in the statistical analyses because of their low numbers (< 5).
Table 2.
Species of rodents and shrew from Kaev Seima and Veal Renh, Cambodia, investigated for Leptospira spp. by using nested PCR for the 16S rRNA gene
| Locality | Rodent species | No. positive/total (%) in dry season | No. positive/total (%) in rainy season |
|---|---|---|---|
| March 2009 | November 2009 | ||
| Kaev Seima | Bandicota indica | 0/1 (0) | 2/2 (100) |
| Bandicota savilei | 3/51 (5.9) | 14/29 (48.3) | |
| Berylmys berdmorei | 0/1 (0) | 2/3 (66.7) | |
| Leopoldamys edwardsi | 0/2 (0) | – | |
| Maxomys surifer | 2/14 (14.3) | 5/23 (21.7) | |
| Mus caroli | 0/1 (0) | – | |
| Niviventer fulvescens | 0/6 (0) | 3/6 (50) | |
| Rattus exulans | 0/25 (0) | 4/35 (11.4) | |
| Rattus tanezumi | 3/25 (8.3) | 2/21 (9.5) | |
| Rhizomys pruinosus | – | 0/1 (1) | |
| Veal Renh | November 2008 | July 2009 | |
| Berylmys berdmorei | 1/7 (14.3) | 1/2 (50) | |
| Maxomys surifer | 2/43 (4.7) | 0/24 (0) | |
| Niviventer fulvescens | 0/2 (0) | – | |
| Rattus argentiventer | 7/12 (58.3) | 7/36 (19.4) | |
| Rattus exulans | 0/44 (0) | 2/51 (3.9) | |
| Rattus norvegicus | 0/17 (0) | 0/10 (0) | |
| Rattus tanezumi | 5/37 (13.5) | 5/51 (9.8) | |
| Suncus murinus | 0/41 (0) | 1/19 (5.3) |
PCR = polymerase chain reaction; rRNA = ribosomal RNA.
Environment, mammal characteristics, and infection.
We conducted a GLM analysis to investigate the effects of host species and their characteristics (age and maturity) and environmental-linked variables (season, slope, NDVI, and locality). The best model obtained by using AIC criterion showed significant effects of locality, host species and maturity, and slope on the level of individual infection by Leptospira spp. (Table 3). Significantly higher infection was observed in the wet season and was particularly noticeable in Kaev Seima. Two species living in households showed significantly lower infection compared with others, i.e., Rattus exulans and S. murinus. Adult hosts were significantly more likely to be infected than juveniles. Slope of the trapping location was significant but the NDVI was not. However, low slope values were observed in rice field areas (estimate = –2.14, SD = 0.25, P < 0.0001) and human settlements (estimate = –1.83, SD = 0.19, P < 0.0001), where high NDVI values were obtained in forests (estimate = 0.11, SD = 0.01, P < 0.0001).
Table 3.
General linear model of rodent infection, Cambodia, with binomial distribution and logit link function (log-likelihood type 1 test)
| Variable | Estimate (SD) | P | Deviance | Degrees of freedom | AIC |
|---|---|---|---|---|---|
| Kaev Seima | 0.53 (0.57) | 0.35 | |||
| Wet season | 0.94 (0.30) | < 0.001* | |||
| Slope | −0.21 (0.10) | 0.040† | |||
| Berylmus berdmorei | 0.76 (0.77) | 0.32 | |||
| Maxomys surifer | −0.87 (0.55) | 0.11 | |||
| Niviventer fulvescens | −0.32 (0.81) | 0.69 | |||
| Rattus argentiventer | 0.45 (0.63) | 0.48 | |||
| Rattus exulans | −2.39 (0.58) | < 0.001* | |||
| Rattus norvegicus | −15.98 (781.6) | 0.98 | |||
| Rattus tanezumi | −0.891 (0.50) | 0.07 | |||
| Suncus murinus | −2.87 (1.14) | 0.01† | |||
| Adult | 1.26 (0.46) | 0.004‡ | |||
| Kaev Seima§ and wet season | 2.18 (0.46) | < 0.001* | |||
| Intercept | −1.40 (0.50) | 0.005‡ | 472.8 | 611 | 360.2 |
P = 0.001.
P = 0.05.
P = 0.01.
Selection of the best model using Akaike information criterion (AIC) with an initial model with locality, season, host species, habitat, slope, Normalized Difference Vegetation Index, sex, and maturity of rodents as explicative variables.
There was potential non-independence between the distribution of rodent species among localities and the environmental variables (i.e., habitat) (Table 1). Two species were strictly restricted to a unique habitat, i.e., R. exulans and S. murinus, in households. We removed data concerning these two species and conducted a second GLM analysis with the same potential explicative variables (host species, age and maturity, season, slope, NDVI, and locality). We found similar results with the selection of the best model using AIC criterion. Only R. tanuzami showed significantly lower infection (estimate = –1.20, SD = 0.62, P = 0.05).
Finally, because of potential and confounding influence of the household habitat, we conducted a GLM analysis removing all host individuals obtained from this habitat. We again found similar results, with slope of trapping location, host maturity, and the locality of Kaev Seima more significant at the wet season. However, there was no effect of host species on individual infection, which indicated that species living in forests and in non-flooded habitats, such as B. berdmorei and N. fulvescens, have similar level of infection to species inhabiting rice fields (i.e., with low slope values).
Discussion
Our results confirm the importance of the rainy season for leptospire infections of rodent species in these two sites in Cambodia. Rodent infection was higher in rain-fed fields, especially in the wet season. Rodents living in and near to forests (shrubby wasteland, plantations, orchards), such as B. berdmorei and N. fulvescens, also showed significantly higher levels of infection. However, shrew and rodent species living in households showed remarkably stable and low levels of infection whatever the locality and the season.
Our study provides new data on rodent species as carriers of Leptospira in countries in Southeast Asia. An exhaustive survey of the published literature concerning Thailand (the most investigated country in the region) showed that only B. indica, B. savilei, R. argentiventer, R. exulans, R. losea, R. norvegicus, and R. tanezumi were infected, whereas investigated species of the genus Mus appeared not to be infected, i.e., M. caroli, M. cervicolor, M. musculus, M. cookii, or B. berdmorei.
In the present study, new rodent species, such as M. surifer and N. fulvescens, were investigated. Although our results confirmed the importance of Bandicota spp. and Rattus spp. as hosts of leptospires of human health importance, high prevalence was observed in rarely investigated species such as B. berdmorei and N. fulvescens.
High prevalence was also observed in R. argentiventer, a species found in rain-fed cultivated areas, which was only trapped in the district of Veal Renh. Bandicota savilei and B. berdmorei, which also showed high prevalence, are present in paddy fields, B. savilei is present in non-flooded fields, and B. berdmorei is present in forests and dry crops in Kaev Seima. This high prevalence in rodents trapped in newly cultivated areas and in degraded forests (shrubby wasteland) in Kaev Seima suggests that these habitats may present a high risk of leptospirosis for humans.
An intriguing result concerns the lack of infection in the brown rat R. norvegicus. Most studies conducted in predominantly urban areas have demonstrated high prevalence of bacteria in R. norvegicus: 80.3% in Brazil,13 45.8% in Argentina, and 23% in Colombia.14 The predominance of human leptospirosis in Pacific coastal regions of Asia is hypothesized to be correlated with the presence of semi-domestic brown rats. However, the presence of this rat in small villages of the Veal Renh district was unexpected because it usually inhabits large harbors (such as Sihanouk City) and large cities (such as Phnom Penh). Its unusual presence is likely linked to the nearby port of Sihanouk city (distance = 46 km). However, it remains difficult to explain the lack of infection in these trapped rats. Our results confirmed the findings of Levett,1 who emphasized that the prevalence of infection increases with age because infection is chronic and not lethal for rodents.
Season, locality, and slope significantly explained the risk of rodent infection by leptospires. The conditions experienced during the wet season obviously increase the prevalence of rodent infection, confirming in rodents the observed seasonality of the disease in humans.1 However, the effect of the wet season was more pronounced among rodents in Kaev Seima. One explanation may be the difference in rainfall between the two localities. Veal Renh was investigated in July 2009 during the wet season, and Mondolkiri was investigated in November 2009 during the end of the wet season.
The NDVI, which describes the intensity of vegetation, is a common index used in several epidemiologic studies because of its sensitivity to vegetation change.29 This index does not seem to explain rodent infection in areas with dense vegetation. However, different vegetation types, corresponding to different habitats for rodents, may have similar values as for rice fields and forests. A major bias lies in the periods of image acquisition (March 2007 and March 2008), which does not correspond to the dates of capture of rodents (2008 and 2009).
The delimitation of risky habitats is highly pathogen dependent.18–31 In the case of leptospirosis, the slope of the trapping location was significantly correlated with increasing rodent infection with decreasing slope. These trapping locations correspond to areas such as paddy rice fields or other flooded lands. Low slope locations, where water and leptospires can accumulate, may likely explain why rodents inhabiting these places are more likely to be infected.
We used the 16S ribosomal RNA gene and the protocol of Mérien,26 which was developed to amplify Leptospira spp. Although used routinely for clinical diagnostics, the major disadvantage of this gene is its low discriminating power in identifying species of Leptospira32 because of the short sequences obtained. Several other markers have been developed.33,34 These markers need to be tested on leptospire isolates from rodents for precise identification of leptospires. Accurate markers are essential to better characterize the Leptospira–rodent association.20
Our data should also encourage public health authorities to improve public awareness through media communication and health education by using preventative measures, such as wearing protective clothing and boots, especially during the rainy season and floods; covering up cuts and abrasions on the skin during outdoor activities; and implementing rodent control strategies. Rat control is difficult and use of chemicals has proven its non-sustainability and potential impacts on human health and domestic animals.34 The trap barrier system has been recommended for use by rice farmers in several countries in Southeast Asia,35 where its introduction and adoption led to a significant decrease in threats from rats. Unfortunately, implementing anti-leptospirosis vaccination in low-income countries such as Cambodia is difficult, and the local variability in serovars complicates development of an accessible vaccine that could be used worldwide.
We have shown that the wet season is favorable for transmission of leptospires in rodents, particularly in rain-fed fields. This study suggests that not only rice fields but forests, secondary forests, and their interface with agricultural fields are also areas of potential risk for leptospirosis infection in humans. Habitat fragmentation and new land uses may favor increasing contacts of rodent species, which may increase the risk of spread and emergence leptospirosis. This preliminary study with its contributions from molecular markers and more efficient and fine environmental data should identify areas of high-risk transmission and help in development of effective disease surveillance.
ACKNOWLEDGMENTS
We thank Hul Vibol and Kim Aun for help in rodent trapping and all other members of CERoPath project for assistance.
Footnotes
Authors' addresses: Svilena Ivanova, Institut des Sciences de l'Evolution Centre National de la Recherche Scientifique, Université Montpellier 2, Institut de Recherche pour le Developpement, F-34095 Montpellier, France, E-mail: s.ivanova@hotmail.fr. Vincent Herbreteau, Centre de Coopération Internationale en Recherche Agronomique pour le Développement, Unité Mixte de Recherche, Territoires, Environnement, Télédétection et Information Spatiale, Montpellier, France, E-mail: vincent.herbreteau@ird.fr. Kim Blasdell, Philippe Buchy, and Bertrand Guillard, Institut Pasteur in Cambodia, Phnom Penh, Cambodia, E-mails: kblasdell@hotmail.co.uk, pbuchy@pasteur-kh.org, and bguillard@pasteur-kh.org. Yannick Chaval, Institut National de la Recherche Agronomique, Unité Mixte de Recherche, Centre de Biologie et de Gestion des Populations (Institut National de la Recherche Agronomique, Institut de Recherche pour le Developpement, Centre de Coopération Internationale en Recherche Agronomique pour le Développement, Montpellier SupAgro), Campus International de Baillarguet, Montferrier-sur-Lez, France, E-mail: chaval@supagro.inra.fr. Serge Morand, Institut des Sciences de l'Evolution Centre National de la Recherche Scientifique, Université Montpellier 2, Institut de Recherche pour le Developpement, F-34095 Montpellier, France; and Centre de Coopération Internationale en Recherche Agronomique pour le Développement, Unité Propre de Recherche, Animal et Gestion Intégrée des Risques, Montpellier, France, E-mail: serge.morand@univ-montp2.fr.
References
- 1.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 3.McBride AJ, Athanazio DA, Reis MG, Ko AI. Leptospirosis. Curr Opin Infect Dis. 2005;18:376–386. doi: 10.1097/01.qco.0000178824.05715.2c. [DOI] [PubMed] [Google Scholar]
- 4.Adler B, de la Peña Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2010;140:287–296. doi: 10.1016/j.vetmic.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Victoriano AF, Smythe LD, Gloriani-Barzaga N, Cavinta LL, Kasai T, Limpakarnjanarat K, Ong BL, Gongal G, Hall J, Coulombe CA, Yanagihara Y, Yoshida SI, Adler B. Leptospirosis in the Asia Pacific region. BMC Infect Dis. 2009;9:147. doi: 10.1186/1471-2334-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pappas G, Papadimitriou P, Siozopoulou V, Christou L, Akritidis N. The globalization of leptospirosis: worldwide incidence trends. Int J Infect Dis. 2008;12:351–357. doi: 10.1016/j.ijid.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Laras K, Cao BV, Bounlu K, Nguyen TK, Olson JG, Thongchanh S, Tran NV, Hoang KL, Punjabi N, Ha BK, Ungsa SA, Insisiengmay S, Watts DM, Beecham HJ, Corwin AL. The importance of leptospirosis in Southeast Asia. Am J Trop Med Hyg. 2002;67:278–286. doi: 10.4269/ajtmh.2002.67.278. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi L, Sengkeopraseuth B, Tsuyuoka R, Koizumi N, Akashi H, Vongphrachanh P, Watanabe H, Aoyama A. Seroprevalence of leptospirosis and risk factor analysis in flood-prone rural areas in Lao PDR. Am J Trop Med Hyg. 2008;78:957–961. [PubMed] [Google Scholar]
- 9.Thai KT, Binh TQ, Giao PT, Phuong HL, Hung LQ, Van Nam N, Nga TT, Goris MG, de Vries PJ. Seroepidemiology of leptospirosis in southern Vietnamese children. Trop Med Int Health. 2006;11:738–745. doi: 10.1111/j.1365-3156.2006.01619.x. [DOI] [PubMed] [Google Scholar]
- 10.Berlioz-Arthaud A, Guillard B, Goarant C, Hem H. Surveillance active de la leptospirose humaine en milieu hospitalier au Cambodge. Bull Soc Pathol Exot. 2010;103:111–118. doi: 10.1007/s13149-010-0043-2. [DOI] [PubMed] [Google Scholar]
- 11.Guerra MA. Leptospirosis. J Am Vet Med Assoc. 234. 2009:472–478. doi: 10.2460/javma.234.4.472. [DOI] [PubMed] [Google Scholar]
- 12.Meerburg BG, Singleton GR, Kijlstra A. Rodent-borne diseases and their risks for public health. Crit Rev Microbiol. 2009;35:221–270. doi: 10.1080/10408410902989837. [DOI] [PubMed] [Google Scholar]
- 13.de Faria MT, Calderwood MS, Athanazio DA, McBride AJ, Hartskeerl RA, Pereira MM, Ko AI, Reis MG. Carriage of Leptospira interrogans among domestic rats from an urban setting highly endemic for leptospirosis in Brazil. Acta Trop. 2008;108:1–5. doi: 10.1016/j.actatropica.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agudelo-Florez P, Londono AF, Quiroz VH, Angel JC, Moreno N, Loaiza ET, Munoz LF, Rodas JD. Prevalence of Leptospira spp. in urban rodents from a groceries trade center of Medellin, Colombia. Am J Trop Med Hyg. 2009;81:906–910. doi: 10.4269/ajtmh.2009.09-0195. [DOI] [PubMed] [Google Scholar]
- 15.Adler GH, Mangan SA, Suntsov V. Richness, abundance, and habitat relations of rodents in the Lang Bian Mountains of southern Viet Nam. J Mammal. 1999;80:891–898. [Google Scholar]
- 16.Adler GH. Habitat relations within lowland grassland rodent communities in Taiwan. J Zool (Lond) 2009;237:563–576. [Google Scholar]
- 17.Suntsov VV, Ly TV, Adler GH. Distribution of rodents along a gradient of disturbance on the Tay Nguyen Plateau of southern Vietnam. Mammalia. 2003;67:379–383. [Google Scholar]
- 18.Chaisiri K, Chaeychomsri W, Siruntawineti J, Bordes F, Herbreteau V, Morand S. Human-dominated habitats and helminth parasitism in southeast Asian murids. Parasitol Res. 2010;107:931–937. doi: 10.1007/s00436-010-1955-2. [DOI] [PubMed] [Google Scholar]
- 19.Thaipadungpanit J, Wuthiekanun V, Chierakul W, Smythe LD, Petkanchanapong W, Limpaiboon R, Apiwatanaporn A, Slack AT, Suputtamongkol Y, White NJ, Feil EJ, Day NP, Peacock SJ. A dominant clone of Leptospira interrogans associated with an outbreak of human leptospirosis in Thailand. PLoS Negl Trop Dis. 2007;1:e56. doi: 10.1371/journal.pntd.0000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wangroongsarb P, Petkanchanapong W, Yasaeng S, Imvithaya A, Naigowit P. Survey of leptospirosis among rodents in epidemic areas of Thailand. J Trop Med Parasitol. 2002;25:55–58. [Google Scholar]
- 21.Singleton GR, Smythe L, Smith G, Spratt DM, Aplin K, Smith AL. Rodent diseases in southeast Asia and Australia: inventory of recent surveys. In: Singleton GR, Hinds LA, Krebs CJ, Spratt DM, editors. Rats, Mice and People: Rodent Biology and Management. Canberra: Australian Centre for International Agricultural Research; 2003. pp. 26–30. [Google Scholar]
- 22.Rudolf B, Schneider U. Calculation of gridded precipitation data for the global land-surface using in-situ gauge observations. Monterey, CA; EUMETSAT: 2004. pp. 231–247. Second Workshop of the International Precipitation Working Group IPWG. [Google Scholar]
- 23.Pagès M, Chaval Y, Herbreteau V, Waengsothorn S, Cosson JF, Hugot JP, Morand S, Michaux J. Revisiting the taxonomy of the Rattini tribe: a phylogeny-based delimitation of species boundaries. BMC Evol Biol. 2010;10:e184. doi: 10.1186/1471-2148-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaval Y, Dobigny G, Michaux J, Pages M, Corbisier C, Cosson JF, Herbreteau V. A multi-approach survey as the most reliable tool to accurately assess biodiversity: an example of Thai murine rodents. Kasetsart J Nat Sc. 2010;44:590–603. [Google Scholar]
- 25.Herbreteau V, Jittapalapong S, Rerkamnuaychoke W, Chaval Y, Cosson JF, Morand S. Protocols for Field and Laboratory Rodent Studies. Bangkok: Kasetsart University Press; 2011. [Google Scholar]
- 26.Mérien F, Amouriaux P, Perolat P, Baranton G, Saint Girons I. Polymerase chain reaction for detection of Leptospira spp. in clinical samples. J Clin Microbiol. 1992;30:2219–2224. doi: 10.1128/jcm.30.9.2219-2224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouse JW, Haas RH, Schell JA, Deering DW. Monitoring vegetation systems in the great plains with ERTS. 1973;1:309–317. Third ERTS Symposium, NASA SP-351. [Google Scholar]
- 28.Oindo BO, Skidmore AK. Interannual variability of NDVI and species richness in Kenya. Int J Remote Sens. 2002;23:285–298. [Google Scholar]
- 29.Herbreteau V, Salem G, Souris M, Hugot JP, Gonzalez JP. Sizing up human health through remote sensing: uses and misuses. Parassitologia. 2005;47:63–79. [PubMed] [Google Scholar]
- 30.Meerburg BG, Jacobs-Reitsma WF, Wagenaar JA, Kijlstra A. Presence of Salmonella and Campylobacter spp. in wild small mammals on organic farms. Appl Environ Microbiol. 2006;72:960–962. doi: 10.1128/AEM.72.1.960-962.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jittapalapong S, Sarataphan N, Maruyama S, Hugot JP, Morand S, Herbreteau V. Seroprevalence of Toxoplasma gondii infections of rodents in Thailand. Vector Borne Zoonotic Dis. 2011;11:231–237. doi: 10.1089/vbz.2009.0238. [DOI] [PubMed] [Google Scholar]
- 32.La Scola B, Bui LT, Baranton G, Khamis A, Raoult D. Partial rpoB gene sequencing for identification of Leptospira species. FEMS Microbiol Lett. 2006;263:142–147. doi: 10.1111/j.1574-6968.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed A, Engelberts MF, Boer KR, Ahmed N, Hartskeerl RA. Development and validation of a real-time PCR for detection of pathogenic Leptospira species in clinical materials. PLoS ONE. 2009;4:e7093. doi: 10.1371/journal.pone.0007093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerqueira GM, Picardeau M. A century of Leptospira strain typing. Infect Genet Evol. 2009;9:760–768. doi: 10.1016/j.meegid.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Meerburg BG, Bonde M, Brom FW, Endepols S, Jensen AN, Leirs H, Lodal J, Singleton GR, Pelz H-J, Rodenburg TB, Kijlstra A. Towards sustainable management of rodents in organic animal husbandry. NJAS-Wag J Life Sci. 2004;52:195–205. [Google Scholar]