Abstract
Burkholderia pseudomallei was quickly identified from blood cultures collected from septicemic patients by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis using an in-house reference library. This procedure reduced the time to definitive identification by more than 24 hours. This analysis is a useful addition to laboratory methods for early recognition of septicemic melioidosis in non-endemic settings.
The introduction of proteome-based identification of bacteria has changed the tempo of medical bacteriology. Application of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis to machine-positive blood culture fluids has significantly shortened the time to definitive identification of bacteria causing bloodstream infection.1 It has been inferred from work on commoner bacteremic agents that faster identification of bacteria from blood cultures will result in faster defervescence and other benefits to septicemic patients.2
In the case of melioidosis, an uncommon cause of septicemia in most centers, progression to multiple organ systems failure and death can be rapid.3 Some patients who do not immediately die of septicemia can remain bacteremic for several days despite appropriate intravenous antimicrobial chemotherapy.4 Melioidosis has a variety of clinical presentations and a broad differential diagnosis.5 In septicemic melioidosis, the diagnosis depends on detection of Burkholderia pseudomallei by blood culture, followed by a series of confirmatory tests. A long-held clinical laboratory aim has been to substantially reduce the time to definitive identification of B. pseudomallei from the first positive blood culture. Currently, this identification relies on the expertise and prior experience of clinical laboratory staff.
Large centers in the melioidosis-endemic region use a battery of additional confirmatory tests such as substrate utilization panels,6 singleplex polymerase chain reaction (PCR) assays,7 or cell wall fatty acid methyl ester analysis.8 In centers in which melioidosis is rare, discretionary tests for unusual pathogens are unlikely to be used until other identification methods have been tried, adding further delays to definitive identification. We describe the construction of an in-house MALDI-TOF MS identification library to support further rapid Burkholderia species identification and its use in rapid identification of B. pseudomallei from blood cultures in the clinical setting of septicemic melioidosis.
The MALDI Biotyper Reference Library (version 3.1.2.0; Bruker Daltonics, Bremen, Germany) contains several Burkholderia species, including B. cepacia and B. thailandensis but does not include B. pseudomallei. Burkholderia mallei and B. pseudomallei can be reliably identified to species level with a dedicated MALDI Biotyper Security Reference Library once this has been installed. The Security Library was not available in our laboratory at the time of the two cases reported. We therefore recovered a collection of 43 distinct strains from the Western Australian Burkholderia Collection reference, historical, and clinical series.9 This collection included two B. pseudomallei reference strains from the National Collection of Type Cultures (Porton Down, United Kingdom), five Burkholderia species that have been fully sequenced,10 and 10 B. cepacia complex reference strains from the Central Public Health Laboratory (Colindale, United Kingdom).
Each of these strains and species were subcultured onto 5% horse blood agar (PathWest Media Laboratory Products, Nedlands, Western Australia, Australia) and incubated at 37°C in air for 24 hours, then subjected to definitive ethanol/formic acid extraction before undergoing the MALDI-TOF MS identification process by using the manufacturer's protocol. Six replicates were analyzed per strain. The library was tested by growing the reference Burkholderia species isolates on different agar media and for varying time periods relevant to clinical laboratory practice.
In brief, strains were grown on horse blood agar for 1, 2 and 3 days in air at 37°C; on horse blood agar for 48 hours in air at 37°C, then at ambient room temperature (22°C) for seven days; and on Ashdown's selective agar and on B. pseudomallei selective agar in air for 24 hours at 37°C. Within- and between-strain variation in mass spectra patterns was measured by using the proprietary software dendrogram-generating function (MALDI Biotyper 3.0; Bruker Daltonics).
In a direct extraction from blood culture, MALDI-TOF MS–based identification of bacteria from blood cultures was conducted according to the manufacturer's recommended protocol (MALDI Sepsityper Kit; Bruker Daltonics) on machine-positive blood cultures containing one morphologic type of bacteria by gram stain. In brief, 1.0 of mL blood culture fluid was mixed with 200 μL of lysis buffer, centrifuged, and the pellet was suspended in 1.0 mL of washing buffer. This suspension was centrifuged, the pellet was resuspended in 300 μL of deionized water, and 900 μL of ethanol added. An ethanol/formic acid extraction was then performed according to the manufacturer's instructions (Bruker Daltonics). A 1-μL aliquot of each blood culture extract was then placed on the MALDI target, overlaid with α–cyano-4-hydroxycinnamic acid, and the automated identification process was conducted.
In a short extraction from subculture on agar media, the rapid extraction method was performed according to the manufacturer's instructions (Bruker Daltonics) with a colony transferred to the MALDI target, overlaid with formic acid, air-dried, and then overlaid with α–cyano-4-hydroxycinnamic acid. The automated identification procedure was performed as in the previous paragraph.
A local resident, non-indigenous, 60-year-old man from Australia (international traveler) was hospitalized because of abdominal pain after an overseas vacation. A blood culture was obtained as a part of his routine work-up before he was discharged. Bacterial growth was detected in his blood culture by using an autoanalyzer (BACTEC 9260; Becton Dickinson, Cockeysville, MD) and shown to contain gram-negative bacilli. After obtaining a preliminary identification of Burkholderia species by using the standard MALDI Biotyper Reference Library, the patient was hospitalized. Further blood cultures were collected and additional bacterial identification procedures were conducted, including alkaline lysis extraction followed by LpxO PCR assay,11,12 gas-liquid chromatography analysis of bacterial fatty acid methyl esters, a substrate utilization panel, and sequencing of PCR-amplified recA gene product. After hospitalization, the patient's recent travel to Cambodia was established and preliminary results from the initial blood culture were obtained. He was then given intravenous meropenem therapy.
A non-indigenous, 63-year-old man from Australia (international traveler) came to a nearby hospital because of septicemia. A suspected Burkholderia species was isolated from blood culture and referred to our center for prompt identification. The short extraction method was used to identify the species directly from the primary isolation medium by using the newly created in-house Burkholderia library. The blood culture bottle (BactiLert; bioMerieux, Marcy L'Etoile, France) was also referred for direct extraction. Additional bacterial identification procedures, as in the first patient, were used.
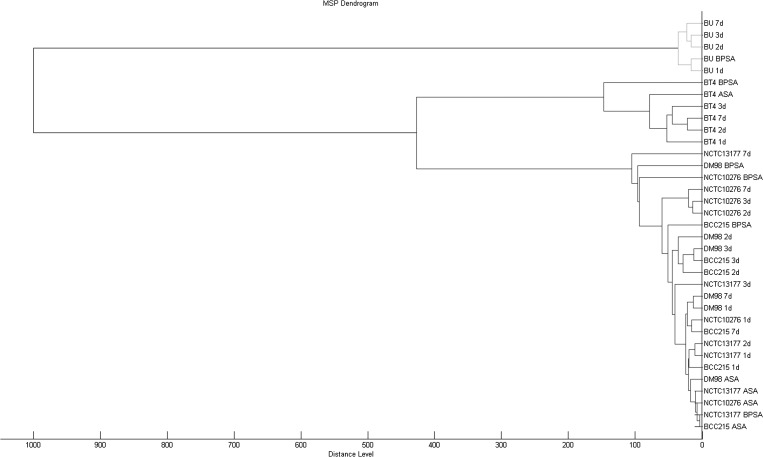
The Burkholderia MALDI-TOF MS library was constructed in two days from mass spectra generated by analysis of external reference strains, fully sequenced local reference strains, referred reference strains, historical strains, and clinical strains. The expanded Burkholderia library correctly identified all historic and Western Australia clinical B. pseudomallei isolates previously confirmed by polyphasic identification methods.7 All but one Burkholderia reference strains analyzed were correctly identified irrespective of agar type or incubation duration. The exception was B. ubonensis, which did not grow on Ashdown's selective agar. The dendrogram generated from the Burkholderia library separated B. pseudomallei and its near neighbors into distinct clades (Figure 1), but within-species variation was not consistent when extracts from different media or incubation duration were used. Similar to other phenotypic methods such as gas-liquid chromatography fatty acid methyl ester analysis,8 subtyping Burkholderia spp. appears to be dependent on consistent growth conditions for all isolates analyzed and is thus less reliable than genotyping methods.
Figure 1.
Burkholderia reference strains analyzed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and displayed in dendrogram format by using proprietary software (MALDI Biotyper; Bruker Daltonics, Bremen, Germany). Burkholderia pseudomallei NCTC 13177, DM 98, and BCC 215, B. thailandensis Bt4 (BT4), and B. ubonensis Bu (BU) have been fully sequenced.11 These and B. pseudomallei NCTC 10276 are archived in the Western Australian Burkholderia Collection reference collection (WABCr). 1d, 2d, 3d, and 7d indicate isolates grown on 5% horse blood agar for 1, 2, 3, days and for 24 hours, then held at ambient room temperature for 6 days (7d) ASA = Ashdown's selective agar; BPSA = B. pseudomallei selective agar; MSP = mass spectrophotometer pattern simi.
The initial MALDI-TOF MS result from the blood culture extract from the first patient generated an identification score of 1.7–2.0 (genus level identification) for Burkholderia species (two replicates, 1.851, 1.729) from the standard MALDI Biotyper Reference Library (version 3.1.2.0; Bruker Daltonics) (Table1). The MALDI Biotyper software provided interpretive advice that B. thailandensis and B. pseudomallei are closely related, which was sufficient to prompt further discretionary investigation, but not enough evidence to confirm septicemic melioidosis. When analyzed against the newly created Burkholderia library, the previous MALDI Sepsityper blood culture extract was identified as B. pseudomallei (log score = 2.635), indicating reliable species-level identification. The Burkholderia library was then linked to the MALDI Biotyper Reference Library so that any future identification would incorporate our collection of Burkholderia-specific reference spectra.
Table 1.
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry identification of reference Burkholderia species grown under a range of conditions with varying duration of incubation and different agar media*
| Species | Designation | HBA 24 hours | HBA 2 days | HBA 3 days | HBA 7 days | ASA 24 hours | BPSA 24 hours |
|---|---|---|---|---|---|---|---|
| B. pseudomallei | NCTC10276 | B. pseudomallei 2.625 | B. pseudomallei 2.619 | B. pseudomallei 2.514 | B. pseudomallei 2.48 | B. pseudomallei 2.578 | B. pseudomallei 2.486 |
| B. pseudomallei | NCTC13177 | B. pseudomallei 2.586 | B. pseudomallei 2.438 | B. pseudomallei 2.416 | B. pseudomallei 2.43 | B. pseudomallei 2.647 | B. pseudomallei 2.627 |
| B. pseudomallei | DM98 | B. pseudomallei 2.604 | B. pseudomallei 2.495 | B. pseudomallei 2.565 | B. pseudomallei 2.548 | B. pseudomallei 2.564 | B. pseudomallei 2.626 |
| B. pseudomallei | BCC 215 | B. pseudomallei 2.647 | B. pseudomallei 2.499 | B. pseudomallei 2.495 | B. pseudomallei 2.544 | B. pseudomallei 2.588 | B. pseudomallei 2.561 |
| B. thailandensis | Bt4 | B. thailandensis 2.546 | B. thailandensis 2.379 | B. thailandensis 2.198 | B. thailandensis 2.498 | B. thailandensis 2.54 | B. thailandensis 2.541 |
| B. ubonensis | Bu | B. ubonensis 2.728 | B. ubonensis 2.593 | B. ubonensis 2.627 | B. ubonensis 2.446 | No growth | B. ubonensis 2.588 |
HBA = 5% horse blood agar; ASA = Ashdown's selective agar; BPSA = Burkholderia pseudomallei selective agar. Highest identification score from four repeats is shown.
As a further test of reliability, the later blood cultures from the first septicemic patient were extracted de novo by using the MALDI Sepsityper Kit (Bruker Daltonics), and the initial B. pseudomallei isolate was extracted on the steel target (no formic acid) from the 24 hour subculture of B. pseudomallei colonies, and identified by using the composite library. All but one individual analysis identified B. pseudomallei with a high score (> 2.5), indicating a reliable identification to species level. The exception was one duplicate colony smear, which failed to generate sufficient mass spectrum peaks for identification. The blood culture of the second septicemic patient contained B. pseudomallei and had an identification score of 2.695 when the newly created in-house library, directly from primary culture media, was used.
For the first patient, the LpxO PCR assay12 confirmed the identity of the blood culture isolate as B. pseudomallei just less than 24 hours after the initial MALDI-TOF MS result. Subsequent sequencing of the recA PCR product, gas-liquid chromatography fatty acid methyl ester analysis, and the substrate use panel confirmed this identification. The repeat blood culture and further sets of blood cultures obtained at three and five days after hospitalization grew B. pseudomallei in the aerobic bottles. Defervescence occurred slowly over five days until the patient's previously poorly controlled maturity-onset diabetes had been brought under full control. He was then discharged and continued receiving intravenous antibiotic therapy, but was hospitalized again because of chest pain and a pleural effusion, which was tapped and yielded growth of B. pseudomallei confirmed by MALDI-TOF MS.
For the second patient, identity of B. pseudomallei was confirmed on the day the primary subculture was received (identification score = 2.695). No mass spectrum pattern was obtained on direct extraction from the blood culture bottle, most probably because of the charcoal contained in the blood culture bottle. Additional identification procedures confirmed the MALDI-TOF MS result.
Until we are able to source additional independently verified B. thailandensis and other Burkholderia species strains, our preference is to confirm an initial B. pseudomallei or B. thailandensis identification on the basis of MALDI-TOF MS with a confirmatory method such as gas-liquid chromatography fatty acid methyl ester analysis and specific PCR assay.7,8,12 Nevertheless, accurate identification of B. pseudomallei less than one hour after machine-positive blood culture detection of a gram-negative bacillus from a septicemic patient with no specific indication of melioidosis will shorten the time to definitive identification and enable choice of a suitable antibiotic choice at least 24 hours earlier. We note that the atypical mucoid phenotype represented by B. pseudomallei strain DM98 was reliably identified as B. pseudomallei by MALDI-TOF analysis using the extended Burkholderia library. The incorporation of additional mass spectra into the bacterial identification library removed the discretionary decision-making process on which early recognition of this rare isolate has previously depended.8,12 We believe that the ability to rapidly screen suspect colonies from a mixed growth of bacteria in samples such as sputa will increase detection of cases when blood cultures are negative. Despite optimum therapy with meropenem and co-trimoxazole the first patient showed relapse with a pleural effusion. Burkholderia pseudomallei was isolated from pleural fluid and rapidly confirmed by the simplest rapid procedure available: direct MALDI-TOF MS analysis from colonies growing on blood agar. The development of an in-house Burkholderia mass spectrophotometer pattern library has enabled us to identify B. pseudomallei directly from blood culture or from primary isolates faster than any other method currently in use in our laboratory. In our experience, MALDI-TOF MS represents the most useful laboratory development in rapid laboratory confirmation of septicemic melioidosis in more than a decade.6,9Additional Burkholderia species isolates will be added to the library to expand its geographic coverage. Enquiries about access to the Burkholderia library, the current status of geographic coverage, or addition of isolates from underrepresented regions should be addressed to Timothy J. J. Inglis.
ACKNOWLEDGMENTS
We thank Dr. Meredith Hodge for providing the referred B. pseudomallei isolate and corresponding blood culture used in this study.
Footnotes
Disclosure: Leith J. Fremlin is an employee of Bruker Biosciences, the manufacturers of the MALDI-TOF MS we used. His role in this work was to assist in the bioinformatic software analysis of MS results and reporting of that process as a scientist. This statement is made in the interest of full disclosure and not because the authors consider this to be a conflict of interest.
Authors' addresses: Timothy J. J. Inglis, School of Pathology and Laboratory Medicine, Faculty of Medicine, Dentistry, and Health Sciences, University of Western Australia, Nedlands 6009, Western Australia, Australia and Division of Microbiology and Infectious Diseases, PathWest Laboratory Medicine, Queen Elizabeth II Medical Centre, Nedlands 6009, Western Australia, Australia, E-mail: tim.inglis@health.wa.gov.au. Paul E. Healy and Clayton L. Golledge, Division of Microbiology and Infectious Diseases, PathWest Laboratory Medicine, Queen Elizabeth II Medical Centre, Nedlands 6009, Western Australia, Australia, E-mails: paul.healy@health.wa.gov.au and clay.gollege@health.wa.gov.au. Leith J. Fremlin, Bruker Daltonics Division, Bruker Biosciences, Melbourne, Victoria, Australia, E-mail: leith.fremlin@bruker-daltonics.com.au.
References
- 1.Stevenson LG, Drake SK, Murray PR. Rapid identification of bacteria in positive blood culture broths by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2010;48:444–447. doi: 10.1128/JCM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inglis TJ, Hodge M, Ketharanathan S. A hospital-wide study of the impact of introducing a personal data assistant-augmented blood culture round. J Med Microbiol. 2008;57:43–49. doi: 10.1099/jmm.0.47385-0. [DOI] [PubMed] [Google Scholar]
- 3.Hore C. Important unusual infections in Australia: a critical care perspective. Crit Care Resusc. 2001;3:262–272. [PubMed] [Google Scholar]
- 4.Inglis TJ, Golledge CL, Clair A, Harvey J. Case report: recovery from persistent septicemic melioidosis. Am J Trop Med Hyg. 2001;65:76–82. doi: 10.4269/ajtmh.2001.65.76. [DOI] [PubMed] [Google Scholar]
- 5.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inglis TJ, Chiang D, Lee GS, Chor-Kiang L. Potential misidentification of Burkholderia pseudomallei by API 20NE. Pathology. 1998;30:62–64. doi: 10.1080/00313029800169685. [DOI] [PubMed] [Google Scholar]
- 7.Inglis TJ, Merritt A, Chidlow A, Aravena-Roman M, Harnett G. Comparison of diagnostic laboratory methods for identification of Burkholderia pseudomallei. J Clin Microbiol. 2005;43:2201–2206. doi: 10.1128/JCM.43.5.2201-2206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inglis TJ, Aravena-Roman M, Ching S, Croft K, Wuthiekanun V, Mee BJ. Cellular fatty acid profile distinguishes Burkholderia pseudomallei from avirulent Burkholderia thailandensis. J Clin Microbiol. 2003;41:4812–4814. doi: 10.1128/JCM.41.10.4812-4814.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inglis TJ, O'Reilly L, Merritt AJ, Levy A, Heath C. The aftermath of the Western Australian melioidosis outbreak. Am J Trop Med Hyg. 2011;84:851–857. doi: 10.4269/ajtmh.2011.10-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukhopadhyay S, Thomason MK, Lentz S, Nolan N, Willner K, Gee J, Glass MB, Inglis TJ, Merritt A, Levy A, Sozhamannan S, Mateczun A, Read T. High-redundancy draft sequencing of 15 clinical and environmental Burkholderia strains. J Bacteriol. 2010;192:6313–6314. doi: 10.1128/JB.00991-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Soud WA, Lantz P-G, Bäckman A, Olcén P, Rådström P. A sample preparation method which facilitates detection of bacteria in blood cultures by the polymerase chain reaction. J Microbiol Methods. 1998;32:217–224. [Google Scholar]
- 12.Merritt A, Inglis TJ, Chidlow G, Harnett G. PCR-based identification of Burkholderia pseudomallei. Rev Inst Med Trop Sao Paulo. 2006;48:239–244. doi: 10.1590/s0036-46652006000500001. [DOI] [PubMed] [Google Scholar]