Abstract
We report the first documented case of a mycetoma caused by Nocardia yamanashiensis after the initial description of this species. The 16S-rRNA gene sequence analysis was used to identify the novel species, which showed a similarity of 99.9% to the gene sequence of the type strain. The case showed both clinical non-response and reduced susceptibility in vitro to amoxicillin plus clavulanate, and it was treated successfully with trimethoprim-sulfamethoxazole and doxycycline. Given antibiotic resistance concerns, we suggest that antimicrobial susceptibility testing should be done for the majority of Nocardia species without well-established resistance patterns.
Introduction
Nocardia yamanashiensis was first isolated from the skin abscess of a 30-year-old female Japanese patient in 1987, but only in 2004 did Kageyama and others1 establish its taxonomic position and reliable criteria for its identification on the basis of phenotypic and phylogenetic characters. Their study of this actinomycete through a 16S ribosomal DNA (rDNA) technique revealed that N. yamanashiensis is closely affiliated to Nocardia pseudobrasiliensis and Nocardia otitidiscaviarum. To our knowledge, there have not been subsequent reports of human infection caused by N. yamanashiensis.
Treatment of primary cutaneous Nocardia infection is often challenging and depends mainly on the susceptibility pattern of the causative species and severity of disease. There are currently more than 30 species of nocardiae of human clinical significance, but mycetomas are mostly produced by Nocardia brasiliensis, which is isolated from about 80% of cases.2–4 Medical therapy with prolonged courses of antimicrobials is associated with substantial clinical improvement frequently observed within the initial 3 months of initiation of treatment. The most common antimicrobials used for this condition are sulfa drugs (sulfamethoxazole/trimethoprim and dapsone), aminoglycosides (streptomycin, amikacin), β-lactams (amoxicillin-clavulanate), and tetracyclines (minocycline). Combined drug therapy is always preferred to avoid drug resistance and to achieve microbiologic cure.
The study.
We report a case of N. yamanashiensis mycetoma in a 34-year-old immunocompetent male. In January 2011, he was admitted to Lihir Medical Center (Lihir Island, Papua New Guinea) with subcutaneous and bone involvement of his right lower extremity. He lived in an impoverished rural area and reported a history of local trauma associated with dirt contamination of the wound 6 months previously.
The condition started as a single, small subcutaneous nodule and histological examination of the former lesion revealed focal epidermal hyperplasia with a chronic inflammatory infiltrate comprising neutrophils and lymphohistiocytes. Special stains for infective organisms, including Gram-positive bacteria, mycobacteria (i.e., modified acid-fast stain), Leishmania, fungi, and spirochetes were all negative. Despite 3 months of empirically prescribed antimicrobial treatment with a combination of high dose oral amoxicillin and clavulanate and surgical debridement at a peripheral center the lesion progressively worsened. On admission, the man presented with a firm and nontender severe swelling and deformity on the anteromedial aspect of his foot with multiple sinus tracts that opened to the surface and drained purulent material with granules (Figure 1). Standard x-ray studies revealed periosteal erosion and osteoporosis. On ultrasonography the lesion showed multiple thick-walled cavities, without acoustic enhancement, with grains represented as fine echoes at the bottom of the cavities. The patient denied any associated systemic symptoms such as fever, weight loss, or malaise. As the swelling progressively increased, he developed difficulty in walking and was forced to cease his usual employment.
Figure 1.
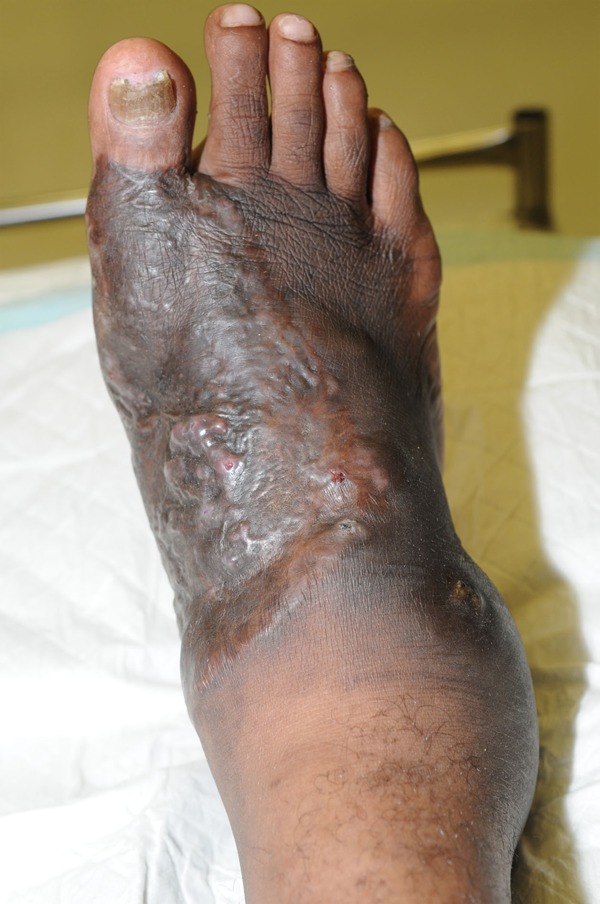
Primary cutaneous Nocardia yamanashiensis infection of a patient from Lihir Island, PNG, 2011. Note: A plaque of primary cutaneous N. yamanashiensis mycetoma on the anteromedial aspect of the foot and the right side swelling and sinus discharge. Source of photograph: Lihir Medical Center, Dr. Oriol Mitjà.
Gram stain of pus aspirated from a cystic lesion on the foot showed no bacteria and the direct acid-fast staining was also negative. The specimen was cultured on blood, McConkey, and anaerobic agar and held for 5 days with no growth but also inoculated after decontamination (3% NaOH) into liquid mycobacterial agar (BacT/ALERT, Biomerieux, France) at 32 and 37°C and a chocolate agar slope at 32°C. After 11 days growth of gram-positive branching organisms that were partially acid fast by the modified Kinyoun method (1% sulphuric acid decolorization) appeared on the chocolate slope and subsequently in the 32°C liquid mycobacterial medium. Species identification was done by means of 16S rRNA gene sequence analyses that were performed by the Mycobacteriology section, Queensland Health Pathology Services. Primer sets of 16S-F3 (5′-CAG GCC TAA CAC ATG CAA GT-3′)/16S-R3 (3′-GGG CGG WGT GTA CAA GGC-3′) were used. The DNA was amplified by polymerase chain reaction (PCR) and purification of the PCR product and sequencing performed. Sequences obtained were compared with the public database GenBank (http://www.ncbi.nlm.nih.gov/blast) using blast searches. The 16S rRNA gene sequence (1,414 bp) of the isolate showed a similarity of 99.9% to N. yamanashiensis sp. nov. IFM 0265T. Phenotypic characteristics were also consistent with this identification.
Susceptibility testing of reported antibiotics was performed by the broth microdilution method using the Clinical and Laboratory Standard Institute (CLSI) criteria with Sensititre microtiter trays (Sensititre; Treck Diagnostics Systems, West Sussex, England).5 Minimum inhibitory concentrations were recorded after 3 days incubation (Table 1). Amoxicillin-clavulanate in combination showed poor activity. The isolates were susceptible to trimethoprim-sulfamethoxazole and amikacin, but tobramycin showed a low level of activity. The strain was susceptible to minocycline.
Table 1.
Activities of antimicrobial agents against a clinical isolate of Nocardia yamanashiensis determined by the broth microdilution method (sensititre)
| Antimicrobials | Minimum inhibitory concentration (MIC) (mg/L) | Susceptibility |
|---|---|---|
| Amox/clav | 16/8 | I |
| Imipenem | < 2.00 | S |
| Trimethoprim/Sulfamethoxazole | 0.25/4.75 | S |
| Amikacin | < 1.00 | S |
| Tobramycin | > 16 | R |
| Ciprofloxacin | > 4.00 | R |
| Minocycline | < 1.00 | S |
| Clarithromycin | < 0.06 | S |
Note: CLSI breakpoints/interpretations were used.5I = intermediate; S = susceptible; R = resistant.
On the basis of the microbiological findings, our patient received a combination regimen of oral trimethoprim-sulfamethoxazole (TMP-SMX) (320 mg TMP and 1,600 mg SMX twice daily, respectively) combined with oral doxycycline (100 mg twice daily) for a period of 6 months, with a subsequent reduction in foot swelling, complete absence of discharge from sinuses, and healing of the papules and nodules.
Conclusions
This work describes the isolation of a bacteria identified as N. yamanashiensis as a cause of mycetoma in a patient in Papua New Guinea. This is a significant finding because there have been no reports of the isolation of this organism after the initial description of this species.1 We are confident with the adequacy of the identification of this isolate because of the high similarity (99.9%) of the 16S gene sequence to that of the type strain. Conville and others6 stated that nocardial isolates may not be identified correctly as N. yamanashiensis (and two other species) using the 16S rRNA gene when sequence analysis shows < 99.8% similarity, because the type strains of those species were found to have multiple differing copies of that gene. For clinical cases with a lower genetic similarity, an additional gene target could be tested to confirm the identification of an isolate as N. Yamanashiensis.
Managing Nocardia infections is often complicated by drug intolerance (e.g., manifested as cutaneous eruption following use of sulphonamides), treatment failure, recovery of primary drug-resistant strains, or development of resistance during therapy. Despite the described isolate was susceptible to agents that commonly cover Nocardia species (TMP-SMX and amikacin), the case was initially treated empirically for carbuncle with amoxicillin-clavulanate and progressed. This is not unexpected in mycetoma caused by Nocardia. Amoxicillin-clavulanate is an alternative treatment in Nocardia mycetoma in patients who cannot tolerate a sulphonamide.7 The case we report demonstrated both clinical non-response and reduced susceptibility in vitro to amoxicillin-clavulanate. This has also been reported for mycetomas caused by other Nocardia species, such Nocardia farcinica, N. otitidiscaviarum, Nocardia nova, N. pseudobrasiliensis, and, Nocardia mexicana.8–12 Therapeutic efficacy in individual patients may depend on species identity and on in vitro susceptibility studies. Therefore, for the majority of Nocardia species, that have not been studied for antimicrobial susceptibility, such as the newly described N. yamanashiensis, obtaining susceptibility testing would be prudent, especially when using an agent known to have variable activity against the genus.
ACKNOWLEDGMENTS
The authors would like to thank the patient for his collaboration, as well as the clinical and laboratorial personnel of the Lihir medical centre.
Footnotes
Financial support: This work was supported by InternationalSOS (Australasia) Pty Ltd., and Newcrest Mining (NML).
Disclosure: The patient provided signed consent authorizing publication of the case and images. Molecular identification was performed at the Mycobacterium Reference Laboratory, Queensland Health Scientific Services, Herston, Queensland, Australia.
Authors’ addresses: Oriol Mitjà, Russell Hays, Christian Van Straten, and Murray Koka, Department of Medicine, Lihir Medical Centre, Lihir Island, Papua New Guinea, E-mails: oriolmitja@hotmail.com, rhays@ozemail.com.au, cjvanstraten@hotmail.com, and MurrayJohn.koka@newcrest.com.au. Jenny Robson, Department of Microbiology, Sullivan Nicolaides Pathology, Brisbane, Australia, E-mail: jrobson@snp.com.au. Quique Bassat, Barcelona Centre for International Health Research (CRESIB), Hospital Clinic/University of Barcelona, Barcelona, Spain, E-mail: quique.bassat@cresib.cat.
References
- 1.Kageyama A, Yazawa K, Nishimura K, Mikami Y. Nocardia inohanensis sp. nov., Nocardia yamanashiensis sp. nov. and Nocardia niigatensis sp. nov., isolated from clinical specimens. Int J Syst Evol Microbiol. 2004;54:563–569. doi: 10.1099/ijs.0.02794-0. [DOI] [PubMed] [Google Scholar]
- 2.Satterwhite TK, Wallace RJ. Primary cutaneous nocardiosis. JAMA. 1979;242:333–336. [PubMed] [Google Scholar]
- 3.Maraki S, Chochlidakis S, Nioti E, Tselentis Y. Primary lymphocutaneous nocardiosis in an immunocompetent patient. Ann Clin Microbiol Antimicrob. 2004;15:24. doi: 10.1186/1476-0711-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tellez I, Franco-Paredes C. A woman with chronic subcutaneous swelling of the right foot associated with sinus tracts discharging yellow grains. PLoS Negl Trop Dis. 2010;4:e772. doi: 10.1371/journal.pntd.0000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CLSI . Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes: Approved Standard—Second Edition. Wayne, PA: Clinical and Laboratory Standards Institute; 2011. CLSI document M24-A2. [PubMed] [Google Scholar]
- 6.Conville P, Witebsky FG. Analysis of multiple differing copies of the 16S rRNA gene in five clinical isolates and three type strains of Nocardia species and implications for species assignment. J Clin Microbiol. 2007;45:1146–1151. doi: 10.1128/JCM.02482-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorrel TC, Mitchell DH, Iredell JR. Nocardia species. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. Seventh edition. Philadelphia, PA: Churchill Livingstone Elsevier; 2010. pp. 3199–3207. [Google Scholar]
- 8.Adhikari L, Dey S, Pal R. Mycetoma due to Nocardia farcinica. J Glob Infect Dis. 2010;2:194–195. doi: 10.4103/0974-777X.62868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark N, Braun D, Pasternak A, Chenoweth C. Primary cutaneous Nocardia otitidiscaviarum infection: case report and review. Clin Infect Dis. 1995;20:1266–1270. doi: 10.1093/clinids/20.5.1266. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu A, Ishikawa O, Nagai Y, Mikami Y, Nishimura K. Primary cutaneous nocardiosis due to Nocardia nova in a healthy woman. Br J Dermatol. 2001;145:154–156. doi: 10.1046/j.1365-2133.2001.04302.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Nava V, Couble A, Molinard C, Sandoval H, Boiron P, Laurent F. Nocardia mexicana sp. nov., a new pathogen isolated from human mycetomas. J Clin Microbiol. 2004;42:4530–4535. doi: 10.1128/JCM.42.10.4530-4535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steingrube VA, Wallace RJ, Jr, Brown BA, Pang Y, Zeluff B, Steele LC. Acquired resistance of Nocardia brasiliensis to clavulanic acid related to a change in beta-lactamase following therapy with amoxicillin-clavulanic acid. Antimicrob Agents Chemother. 1991;35:524–528. doi: 10.1128/aac.35.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]


