Abstract
We analyzed surveillance data of a dengue outbreak (2010) reported to the Hadramout Health Office (Yemen) and retrospectively analyzed dengue-related epidemiological and entomological events reported in Hadramout from 2005 to 2009. A total of 630 immunoglobulin M (IgM) -confirmed dengue cases of 982 febrile cases was reported during the period from February to June of 2010; 12 cases died, giving case fatality a rate of 1.9%. Among febrile cases, the highest proportion of dengue cases (37.3%) was reported in the 15- to 24-year-old age group. The overall attack rate was 0.89/1,000. The average number of cases reported by month over the preceding 5-year period compared with the 2010 data is consistent with endemicity of dengue in the region and supports epidemic designation for the dengue activity in 2010. Recognition of endemic dengue transmission and potential for substantial dengue epidemics highlight the need for consistent laboratory-based surveillance that can support prevention and control activities accordingly.
Introduction
Historically, dengue has been reported in Yemen as early as the 19th century, and imported dengue cases from East Africa (Zanzibar) were documented in 1872 and 1877.1,2 However, more frequent outbreaks of dengue have emerged since 2000, but some of these outbreaks were not well-documented or published, which was the case in Shabwah governorate in 2001, 2002, and 2005 and the outbreaks in Aden and Taiz (2010). Documented outbreaks were in Shabwah governorate (1994), Hadramout/Mukalla (2005), and Al-Hudidah governorate (1994, 2000, 2004, and 2005).3 Furthermore, travel-associated dengue has been reported among travelers from Yemen to the United States and Italy.4,5 These travel-associated cases were caused by dengue virus (DENV) 2 and 3. Dengue is an emerging disease in the Middle East, especially in the Arabian Peninsula; three major dengue outbreaks were reported from Saudi Arabia between 1993 and 2008.6
Most reported epidemics of dengue are based on symptomatic cases of dengue, and therefore, the true incidence of dengue infection may be underreported because of the large proportion of asymptomatic cases.7–10 Dengue is a tropical/subtropical disease with a seasonal variation that occurs particularly during the hot weather followed by rainy or monsoon seasons.7,8
Children and young adults have been most often affected,7–10 and high seroprevalence of dengue immunoglobulin G (IgG), particularly among younger age groups, along with frequently reported outbreaks suggest dengue endemicity within a country.11 In endemic countries, dengue virus transmission occurs annually but with cyclic variations largely caused by environmental and climatic factors and human and vector behavior.12 Underrecognition or underreporting because of limited resources or problems of accessibility limits the local description of dengue incidence and endemicity. Consistent epidemiological and disease surveillance measurements made on representative populations allow calculations of epidemic threshold, attack rate (AR), and case fatality rate (CFR). These observations and calculations are most meaningful and accurate when they are laboratory-based.6
Yemen lacks quality healthcare service and adequate infrastructure facilities. Dengue is a neglected disease, and concern is focused on frequent dengue epidemics and crisis management of the disease rather than strategic surveillance to define true disease burden, which could lead to design and implementation of effective control measures.
In 2010, several areas in Yemen experienced dengue outbreaks, the most devastating of which occurred in Hadramout coastal districts in southeastern Yemen (Figure 1). The population in the 15 coastal districts of Hadramout is around 0.7 million. This paper describes the epidemiology of the 2010 dengue outbreak in Hadramout districts and compares it with sporadic or aggregated dengue-related epidemiological and entomological events from previously reported dengue activity in Yemen. This work aims to better define epidemiology of the present outbreak of dengue in this geographically important area of the Middle East that is positioned only a short distance from the African horn.
Methods
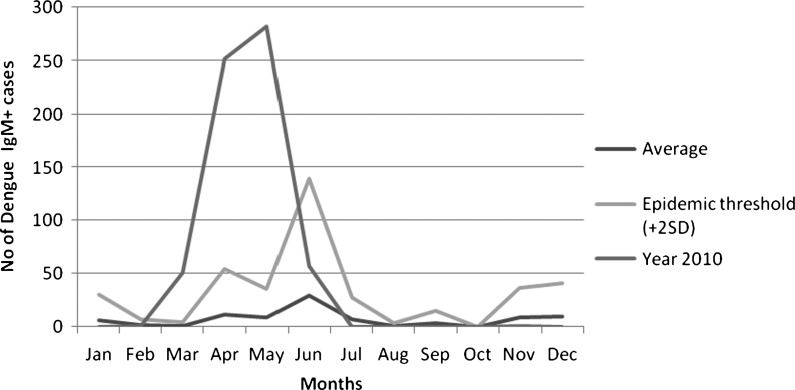
We analyzed surveillance and outbreak investigation data of dengue in Hadramout coastal districts (2010). In addition, we retrospectively analyzed other dengue-related data in Hadramout (2005–2009). Dengue surveillance in this region of Yemen is based on passive physician reports from both public and private sectors. All physicians in public and private hospitals, health centers, and clinics are trained in the use of the World Health Organization (WHO) case definition of dengue (1999) and expected to report to the Hadramout Governate Health Office Department of Surveillance on a standardized reporting form for dengue. Blood specimens were collected at the health facility and then sent to the public health laboratory for investigation for all cases suspected on the basis of the WHO dengue case definition. Only acute sera were available for laboratory testing, and laboratory diagnosis was made using Panbio Commercial kit for detection of IgM and IgG to dengue by capture enzyme-linked immunosorbent assay (ELIZA; Panbio Diagnostics, Brisbane, Queensland, Australia). These test kits have been validated in the past by the Yemen Central Public Health Laboratory in Sana, Yemen and East Mediterranean Regional Office/WHO. The PanbioIgM capture ELISA has a high sensitivity (99.0%) and specificity (84.4%) when tested against the Centers for Disease Control and Prevention (CDC) dengue IgM capture ELISA standard.13 In addition, for the outbreak in 2010, test results of IgM positives (N = 189) were validated with real-time reverse transcription polymerase chain reaction (RT-PCR) at King Abdulaziz University in Saudi Arabia using a Roche 2.0 light cycler. Dengue virus genome was amplified using oligonucleotide primers, and PCR products were detected using labeled hybridization probes (20 pmol/μL each primer; Madani T, personal communication). The following epidemiological variables were used in our analysis: demographic variables (age, sex, and residence), number of cases, number of deaths, and serological data (IgM and IgG). We measured age- and demographic-specific AR to describe the outbreak. We were able to establish average dengue case identification per month for a 5-year period of surveillance based on the reporting system described above (Figure 2). The dengue cases were reported based on the onset of the symptoms and specifically, the onset of fever. In addition, we used the proportion of IgG+ coinciding with IgM+ cases during the 2010 outbreak to estimate the likelihood of endemicity of the outbreak.
Figure 2.
Epidemic threshold and the historical incidence of IgM-positive dengue cases in 2005–2009 compared with the reported dengue cases in Hadramout, Yemen, in 2010.
To estimate the epidemic threshold using entomological data, vector control variables were used. (1) Container index (CI) is the percentage of water-holding containers infested with active immature mosquito larvae. (2) House index (HI) is the percentage of houses infested with larvae and/or pupae.14 Entomological investigation was conducted by the malaria control program (Hadramout office) during the period from February to July in 2010, and it targeted all of the affected districts in Hadramout and mainly Mukalla city (the capital of Hadramout). The mosquito species were collected from both outdoor and indoor water containers. At the beginning of the outbreak, 149 houses and 293 water containers were investigated entomologically for Aedes aegypti larvae. At the end of outbreak, a total of 15,665 houses and 20,441 water containers were investigated. There is no standard cutoff point for HI and CI, because critical thresholds of these indices have never been developed for dengue. HI of 15% and CI of 10% were used as thresholds for Ae. aegypti transmission of yellow fever in urban areas of central and northern South America. Practical use of these thresholds in Singapore was taken as additional justification for using HI at 15% and CI at 10% as predictive of potential dengue virus transmission to humans.14
Statistical analysis of proportions by χ2 test with a P < 0.05 level was deemed significant.
Results
Our results are based on 630 laboratory-confirmed IgM cases of 982 suspected and serologically tested cases reported between February and June of 2010 (Table 1). PCR testing by King Abdulaziz University confirmed 163 of 189 (86.2%) samples collected early in the 2010 outbreak. All of the PCR-confirmed specimens were DENV 3. A total of 172 of 630 cases in this outbreak had positive results for both IgM and IgG, indicating a moderate level (27.8%) of secondary infection among acutely infected patients. Among 352 IgM-negative tests from suspected cases, 147 tests were IgG+, indicating a previous infection rate of 42.8% in this subgroup of febrile patients.
Table 1.
Demographics, febrile cases, dengue cases, and age-, sex-, and residence-specific attack rates of dengue outbreak in Hadramout in 2010
| Demographic characteristics | Population | Total fever cases reported | IgM+ cases | IgG+ sera (%) | ||
|---|---|---|---|---|---|---|
| Dengue IgM-positive cases (%; N = 630) | Dengue positivity rate (%) among febrile cases by IgM | Attack rate/1,000 | ||||
| Age (years) | ||||||
| < 5 | 107,595 | 37 | 27 (4.3%) | 27/37 (73.0) | 0.25 | 14 (4.4%) |
| 5–14 | 213,773 | 271 | 172 (27.3%) | 172/271 (63.5) | 0.80 | 92 (28.8%) |
| 15–24 | 148,650 | 370 | 235 (37.3%) | 235/370 (63.5) | 1.58* | 114 (35.7%) |
| 25–34 | 89,190 | 199 | 134 (21.3%) | 134/199 (67.3) | 1.50 | 69 (21.6%) |
| 35–54 | 190,414 | 292 | 56 (8.8%) | 56/292 (65.1) | 0.55 | 28 (8.8%) |
| ≥55 | 47,427 | 12 | 6 (1%) | 6/12 (50.0) | 0.13 | 2 (0.6%) |
| Sex | ||||||
| Male | 364,547 | 640 | 408 (64.8%) | 408/640 (63.8) | 1.12* | 197 (61.7%) |
| Female | 343,412 | 342 | 222 (35.2%) | 222/342 (64.9) | 0.65 | 122 (38.3%) |
| Residence | ||||||
| Urban districts | 419,249 | 890 | 579 (91.9%) | 579/890 (65.1) | 1.38* | 273 (85.5%) |
| Rural districts | 288,610 | 92 | 51 (8.1%) | 51/92 (55.4) | 0.18 | 46 (14.5%) |
| Total | 707,859 | 982 | 630 | 630/982 (64.2) | 0.89 | 319 |
P < 0.001.
The time lapse between fever onset and drawing of blood specimens was a mean of 3.18 days and a median of 3 days after onset of fever.
Among the 630 IgM-confirmed cases, 201 (32%) cases were hospitalized, 15 cases had hemorrhagic manifestations (DHF), and 12 cases died; therefore, the CFR was 1.9%. Also, only two cases of DHF were also IgG-positive. Analysis by age, sex, and residence showed a significantly high proportion of cases in young adults (58.5% in the 15- to 34-year age group), males (65%), and urban residents (92%) (Table 1). Nearly one-third of cases (31.6%) occurred in children < 15 years old. The reported overall AR was 0.89/1,000. Significant variation was observed in AR (1.5/1,000) for ages 15–34 years, male gender (1.12/1,000), and urban residence (1.38/1,000). However, substantial proportions of dengue positivity were seen among females and rural residents with low calculated AR. Table 1 shows that the proportion of IgM+ febrile cases is over 60% in most demographic categories. The AR, based on confirmed cases, more accurately predicts population at risk than use of the proportion of IgM-positive tests derived from suspect cases who presented as febrile patients.
We were able to estimate the level of endemicity of dengue in Hadramout using surveillance data collected between 2005 and 2009 (Figure 2) that established a consistent occurrence over 5 years; during this time, only 1 month of the year reported no cases in the Hadramout region. Additionally, data presented in Table 2 using entomological thresholds to define the epidemic threshold in this outbreak show HI and CI of 50% and 40%, respectively, at the beginning of the outbreak, exceeding the previously reported epidemic threshold for vector parameters associated with outbreaks. By the end of the outbreak, these two indices had declined to HI and CI of 0% and 2%, respectively, after applying integrated vector control measures and health education activities to lessen public exposure to transmitting mosquitoes. IgG positivity proportion increases in an age-specific fashion to 21% or more of the population up to age 35 years (Table 1), and 27.3% of IgM-positive cases in this outbreak were also IgG-positive. These observations are consistent with a moderately high level of secondary infections and moderate endemic transmission between 2005 and 2009 followed by a DENV 3 epidemic in Hadramout in 2010.
Table 2.
Comparison of entomological and endemicity indicators (IgG proportions) between the 2010 outbreak and previously reported dengue outbreaks in Hadramout districts
| Dengue outbreaks in Yemen | House index (%) | Container index (%) | Total reported febrile cases | IgM positivity rate (%) | IgG positivity rate (%) | Entomological threshold level* |
|---|---|---|---|---|---|---|
| Hadramout (February to July 2010) | 50 | 40 | 982 | 64 | 31 | Exceeded |
| Hadramout (May 2005)† | 50 | 29 | 128 | 23 | 7 | Exceeded |
| Hadramout (July 2006)† | 31 | 29 | 562 | 24 | 21 | Exceeded |
Entomological threshold level was 15% for HI and 10% for CI.
The source of data is the Malaria Control Program of the Health Office in the Hadramout region.
Discussion
Here, we have described and substantiated the dengue fever outbreak in Hadramout (2010) that dramatically exceeded the epidemic threshold indicators shown in Figure 2. Endemicity of dengue fever in Yemen is further substantiated by the rising proportion of IgG to dengue as the population ages and a 27.3% secondary infection rate described in the current outbreak. We believe that this rate of IgG positivity is a fair indicator of secondary infection rate in this case, because the specimen gathering was so early in the febrile illness (i.e., at an average of 3.18 days). Frequency of outbreak occurrence in the past few years has increased compared with rare sporadic cases reported before the year 2000, supporting our speculation of dengue endemicity in the country. Most cases in Hadramout occurred in the spring and summer. This seasonal variation is similar to the pattern of dengue occurrence in Saudi Arabia.15 Previously unpublished data document presence of DENV 2 and 3 in Yemen before this outbreak. Using AR based on laboratory-based surveillance, we have identified risk groups as part of an early warning system that has been previously recommended.7 AR for male and urban residency is high, whereas proportion based on febrile cases failed to identify these variations. This finding indicates the necessity of the use of the area population as a denominator as opposed to institution-based numbers, such as reported fever cases, to maximize efficiency of public health interventions.
Figure 1.
Map of Yemen and location of Hadramout governorate in Yemen.
The work by Guha-Sapir and Schimmer16 reported that there is evidence for increasing infection rates among adults, which is contrary to the popular belief that dengue is a pediatric disease; they also report that three independent studies in India and Singapore found nearly two times the number of male patients compared with females,16 which is similar to the results reported in Saudi Arabia.15,17,18
The high proportion of infection in males and young adults in this study has two likely explanations. (1) The outbreak occurred in the hot season with high humidity, making people spend more time outside of their houses, especially at early morning and early evening. (2) Most females wear heavy clothes and cover most of their body, protecting them from mosquito bites, and they are culturally less likely to be outside the home compared with their male counterparts.
The work by Khan and others9 suggests that, during an epidemic period, increased CFR up to 5% can be expected. However, with improved healthcare services and clinical case management, it is expected that CFR will be less than 1%.3 The reported 1.9% CFR in the present outbreak is still high and is an indicator either of virulence of the infectious agent and/or poor case management and lack of healthcare services in the country.9
Entomological indicators such as HI and CI are used routinely for vector control activities in dengue12; increase of these indicators above the threshold (15% for HI and 10% for CI) is used for considering the start of vector control, but indicators above the threshold indicator levels are not always followed by dengue outbreaks. In South Vietnam, entomological indices were used as a predictor for pre-epidemic periods; however, dengue in Vietnam is endemoepidemic throughout the entire country.19 Neither research nor regular surveillance has been done in Yemen in the past to monitor the correlation between entomological indicators and trends of dengue outbreaks. High prevalence of dengue IgG in suspected cases during outbreaks suggests previous exposures and background endemicity preceding an outbreak.11,20 Here, we note that there was a marked rise in CI and HI that coincided with the outbreak. These indicators dropped dramatically in conjunction with increased vector control efforts. This finding suggests that vector control measures can be useful, even in the evolution of disease patterns from endemic to epidemic, if there is accurate and timely surveillance information. Most especially, where dengue virus transmission is endemic, there is a strong need to strengthen both laboratory-based surveillance and entomological surveillance.
The limitations of this study are that the data are passively reported suspect cases. However, the response of local providers was quite rapid, which was shown by the short time between onset of febrile illness and specimen collection, indicating a high level awareness of dengue and its importance in Hadramout. Our study was largely limited to single acute samples tested for IgM, which is partially detectable from the third day of fever, and IgM increases over the next 2 days. Hence, we have likely missed some cases by capturing disease in the early febrile period; this time is when viremia is high, but antibody response is not yet maximal.21
However, highly sensitive PCR testing on roughly one-third of our samples confirms the validity of the IgM positivity identified in the febrile period in which our serologic samples were drawn.22,23
Our serological assays were not all RT-PCR–confirmed, and therefore, we cannot rule out cross-reactions with other arboviruses circulating in the country. Cross-reactions could lead to an overestimate of dengue virus transmission. We did, however, validate the use of the Panbio kits at the Central Laboratory in Sana, and a subset of nearly one-third of IgM-positive cases was tested by RT-PCR at King Abdulaziz University in Saudi Arabia. The high reliability of IgM positivity and confirmation that IgM was reflective of dengue in 86.2% of the specimens retested by RT-PCR gives us confidence in the overall validity of the data reported here as a conservative estimate of the size of the outbreak.
Regarding entomological surveillance, a limitation is that the entomological indicators are simply indicators of vector activity that are indirectly predictive of potential dengue virus transmission, and there is no straightforward linkage between entomological indicators and occurrence of an outbreak. Indeed, increasing immunity to given serotypes in the population will perhaps make the indicators less reliable. There is no formula for adjusting for background endemicity, because there are newly unexposed individuals born into the population each year. The entomological surveillance that we report, however, seems to correlate well with this outbreak and its resolution.
Conclusions
Burden of dengue in Yemen is not as high as in dengue-endemic Asian or South American countries, but dengue seems to be emerging. The reported dengue outbreak in Hadramout (2010) indicates the potential threat of dengue as a public health issue. Data on entomological threshold trends and endemic disease status for a 5-year period as presented here support this prediction. Like in some other dengue-endemic countries, such as Singapore and Brazil, increased susceptibility among young adults in urban settings was observed. Learning lessons from Asia and South America should help to focus prevention and control activities based on epidemiological indicators, such as disease epidemic and endemic status and age-specific attack rates in the country. Furthermore, it is important to consider entomological indicators for vector control for dengue given our data on changing CI and HI in the context of an outbreak and the public health vector control response to that data. Use of good laboratory-based dengue surveillance in conjunction with vector control measures will lead to more effective dengue control activities, particularly in resource-scarce settings.
ACKNOWLEDGMENTS
The authors appreciate and thank the team of Professor Tareq Mudani at The King Abdulaziz University in Saudi Arabia for their valuable participation in validating the available sera for dengue virus by application of RT-PCR. Also, our great thanks is extended to all health professionals from the Central Public Health Laboratory, Epidemiology Unit, Health Office of Hadramout, and the Malaria Control Program in Hadramout governorate who participated in data and/or sample collection from individuals and the community.
Footnotes
Authors' addresses: Abdullah Salim Bin Ghouth, Department of Community Medicine, Hadramout University Malaria Control Program, Mukala 8892, Yemen, E-mails: abinghouth2007@yahoo.com. Ananda Amarasinghe, Epidemiology Unit, Ministry of Health, Sri Lanka, E-mail: ana_amarasinghe@yahoo.co.uk. G. William Letson, Global Public Health Consultant, Denver, CO, E-mail: bwletson@gmail.com.
References
- 1.Christie J. Dengue. BMJ. 1872;2:244. [Google Scholar]
- 2.Radcliffe JN. Dengue. BMJ. 1877;1:25–26. [Google Scholar]
- 3.World Health Organization . Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control, New Ed. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 4.Jimenez-Lucho VE, Fisher EJ, Saravolatz LD. Dengue with hemorrhagic manifestations: an imported case from the Middle East. Am J Trop Med Hyg. 1984;33:650–653. doi: 10.4269/ajtmh.1984.33.650. [DOI] [PubMed] [Google Scholar]
- 5.Ravanini P, Huhtamo E, Hasu E, Rosa F, Costantino S, Crobu MG, Ilaria V, Nicosia AM, Garavelli PL, Vapalahti O. Imported dengue virus serotype 3, Yemen to Italy, 2010. Emerg Infect Dis. 2011;17:929–931. doi: 10.3201/eid1705.101626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amarasinghe A, Letson GW. Dengue in the Middle East: a neglected and emerging disease of importance. Trans R Soc Trop Med Hyg. 2012;106:1–2. doi: 10.1016/j.trstmh.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Kabilan L, Velayutham T, Sundaram B, Tewari SC, Natarajan A, Rathnasamy R, Satyanarayana K. Field- and laboratory-based active dengue surveillance in Chennai, Tamil Nadu, India: observations before and during the 2001 dengue epidemic. Am J Infect Control. 2004;32:391–396. doi: 10.1016/j.ajic.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Ayyub M, Khazindar AM, Lubbad EH, Barlas S, Alfi AY, Al-Ukayli S. Characteristics of dengue fever in a large public hospital, Jeddah, Saudi Arabia. J Ayub Med Coll Abbottabad. 2006;18:9–13. [PubMed] [Google Scholar]
- 9.Khan E, Siddiqui J, Shakoor S, Mehraj V, Jamil B, Hasan R. Dengue outbreak in Karachi, Pakistan, 2006: experience at a tertiary care center. Trans R Soc Trop Med Hyg. 2007;101:1114–1119. doi: 10.1016/j.trstmh.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, Vaughn DW, Ennis FA. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 11.Brown MG, Vickers IE, Salas RA, Smikle MF. Seroprevalence of dengue virus antibodies in healthy Jamaicans. Hum Antibodies. 2009;18:123–126. doi: 10.3233/HAB-2009-0207. [DOI] [PubMed] [Google Scholar]
- 12.Vong S, Khieu V, Glass O, Ly S, Duong V, Huy R, Ngan C, Wichmann O, Letson GW, Margolis HS, Buchy P. Dengue incidence in urban and rural Cambodia: results from population-based active fever surveillance, 2006–2008. PLoS Negl Trop Dis. 2010;4:e903. doi: 10.1371/journal.pntd.0000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunsperger EA, Yoksan S, Buchy P, Nguyen VC, Sekaran SD, Enria DA, Pelegrino JL, Vazquez S, Artsob H, Drebot M, Gubler DJ, Halstead SB, Guzman MG, Margolis HS, Nathanson C-M, Rizzo Lic NR, Bessoff KE, Kliks S, Peeling RW. Evaluation of commercially available anti-dengue virus immunoglobulin M tests. Emerg Infect Dis. 2009;15:436–440. doi: 10.3201/eid1503.080923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott TW, Morrison AC. In: Vector dynamics and transmission of dengue virus: implications for dengue surveillance and prevention strategies: vector dynamics and dengue prevention. Current Topics in Microbiology And Immunology: Dengue Virus. Rothman A, editor. Berlin, Germany: Springer; 2010. pp. 115–126. [DOI] [PubMed] [Google Scholar]
- 15.Shahin W, Nassar A, Kalkattawi M, Bokhari H. Dengue fever in a tertiary hospital in Mukakah, Saudi Arabia. Dengue Bull. 2009;33:34–44. [Google Scholar]
- 16.Guha-Sapir D, Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerg Themes Epidemiol. 2005;2:1. doi: 10.1186/1742-7622-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Gilany A, Eldeib A, Hamman dS. Clinico-epidemiological features of dengue fever in Saudi Arabia. Asian Pac J Trop Med. 2010;3:220–223. [Google Scholar]
- 18.Fakeeh M, Zaki AM. Virologic and serologic surveillance for dengue fever in Jeddah, Saudi Arabia, 1994–1999. Am J Trop Med Hyg. 2001;65:764–767. doi: 10.4269/ajtmh.2001.65.764. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen TP, Luu LL, Vu TQ, Buisson Y. Increase of entomological indices during the pre-epidemic period of dengue in Ben Tre, South Vietnam. Bull Soc Pathol Exot. 2011;104:313–320. doi: 10.1007/s13149-011-0154-4. [DOI] [PubMed] [Google Scholar]
- 20.Mohammad Azami NA, Salleh SA, Neoh HM, Syed SZ, Jamal R. Dengue epidemic in Malaysia: not a predominately urban disease any more. BMC Res Notes. 2011;4:216. doi: 10.1186/1756-0500-4-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmanitiya S, Suntayakorn S, Rothman AL, Ennis FA, Nisalak A. Dengue in the early febrile phase: viremia and antibody responses. J Infect Dis. 1997;176:322–330. doi: 10.1086/514048. [DOI] [PubMed] [Google Scholar]
- 22.Kao CL, King CC, Chao DY, Wu HL, Chang GJ. Laboratory diagnosis of dengue virus infection: current and future perspectives in clinical diagnosis and public health. J Microbiol Immunol Infect. 2005;38:5–16. [PubMed] [Google Scholar]
- 23.Jarman RG, Nisalak A, Anderson BK, Klungthong C, Thaisomboonsuk B, Kaneechit W, Kalayanarooj S, Gibbons RV. Factors influencing dengue virus isolation by C6/36 cell culture and mosquito inoculation of nested PCR-positive clinical samples. Am J Trop Med Hyg. 2011;84:218–223. doi: 10.4269/ajtmh.2011.09-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]