Abstract
The causative factors for severe anemia incidence in sub-Saharan Africa are multifactorial. In an observational, longitudinal study of two cohorts of about 300 infants followed-up for six months in a malaria hyperendemic area, the risk factors for severe anemia incidence were clinical malaria and pneumonia, which outweighed nutritional and sociodemographic factors. Severe anemia incidence was 1–2/year at age 2 months, peaked around 6–7/year at age 7–12 months, and decreased back to 1–2/year at age 16–22 months. The age-dependent increase of severe anemia incidence was shown to be parallel to the age-dependent increase of clinical malaria. Previous clinical malaria episodes increased the severe anemia risk by 80%, and gametocyte carriage and pneumonia at prior visit was associated with a six-fold increase and a > 10-fold increase, respectively. The role of pneumonia and malaria as risk factors, and areas for interventions for severe anemia, should not be underestimated.
Introduction
Anemia is one of the biggest public health problems in sub-Saharan Africa. The roots of the problem are multifactorial, but iron deficiency is a major contributor.1 Nutritional factors and malaria infection are major reasons for iron deficiency in children in sub-Saharan Africa. A strong association between anemia measured either by hemoglobin (Hb) levels or by packed cell volume and Plasmodium falciparum parasitemia was seen in several studies on mainland Tanzania.2–5 Severe anemia has been identified as the most frequent life-threatening complication of malaria in high transmission areas in small children.3 Beyond being a reason for increased mortality, iron deficiency anemia also causes a delay in the achievement of developmental milestones measured by cognitive or motor skills.6
Malaria is an important contributor to anemia and impaired development on Pemba Island, Tanzania.7,8 At the time when the study was carried out, P. falciparum malaria was the leading cause of morbidity and mortality in children on the island.8,9 Parasite prevalence varied between 30% and 50% in the age group under study; mean = 39%.10 Malaria transmission has decreased substantially in recent years on Zanzibar and Pemba Island.10,11
In this study, we estimated the incidence and prevalence of anemia and identified common risk factors for severe anemia prospectively. A focus of our analysis relies on malaria as an important contributing factor, including subclinical parasitemia and parallel infections with multiple malaria genotypes.
Materials and Methods
Study population and study site.
Two cohorts of 537 children total were followed-up for six months each on a biweekly visitation schedule on Pemba Island, which is located on the east coast of Africa, north of Zanzibar Island. The basic demographics of the children are shown in Table 1. The second cohort of children had a higher average family-income (73% versus 51% in the middle income bracket; P < 0.001). Use of bed nets at enrollment was more prevalent in the second cohort (48% versus 23%; P < 0.001). There were no age or sex differences between the two cohorts. Overall compliance was good; in general, 10–16 visits were recorded per child (mean ± SD = 12.4 ± 2.6, range = 2–19). Malaria treatment was given according to the guidelines of the Ministry of Health in Zanzibar and according to World Health Organization recommendations. Supplemental oral iron substitution was prescribed (and provided for free) for children with Hb levels < 7 mg/dL. For children with life-threatening anemia (Hb level < 5 mg/dL) hospitalization was advised. The study is described elsewhere in greater detail.10 Ethical clearance was obtained by the Johns Hopkins Institutional Review Board and the Zanzibar Health Research Council.
Table 1.
Demographic characteristics of two cohorts of children on Pemba Island, Zanzibar*
| Characteristic | First cohort (n = 291) | Second cohort (n = 246) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age at enrollment, months | ||||
| 1–6 | 109 | 37 | 97 | 39 |
| 7–12 | 130 | 45 | 75 | 31 |
| > 13 | 52 | 18 | 74 | 30 |
| Sex | ||||
| M | 142 | 49 | 111 | 45 |
| F | 149 | 51 | 135 | 55 |
| Household income ($/month) | ||||
| < 10 | 132 | 45 | 33 | 13 |
| 10–40 | 147 | 51 | 179 | 73 |
| > 40 | 12 | 4 | 34 | 14 |
| Father's education | ||||
| Illiterate | 197 | 68 | 76 | 31 |
| Primary | 65 | 22 | 83 | 34 |
| Secondary | 29 | 10 | 87 | 35 |
| Mother's education | ||||
| Illiterate | 139 | 48 | 52 | 21 |
| Primary | 79 | 27 | 73 | 30 |
| Secondary | 73 | 25 | 121 | 49 |
| Bed net use by child | ||||
| No | 191 | 66 | 111 | 45 |
| Yes | 67 | 23 | 119 | 48 |
| Do not know | 33 | 11 | 16 | 7 |
The study periods were December 15, 2003–June 15, 2004 for the first cohort and December 15, 2003–June 15, 2004 for the second cohort.
The cohort was nested into a large ongoing micronutrient trial that had changed from a four-factorial design (zinc, iron, zinc plus iron, and placebo) to a two-factorial design. Iron supplementation was stopped three months before the beginning of the cohort study after increased morbidity and a trend towards increased mortality was seen in the iron group.12 During the study period, children in the intervention group received zinc supplementation with vitamin A and children in the placebo group received only vitamin A. Children were enrolled without knowledge to which study arm they belonged. The study took place during December 2003–January 2005 in Wete and Michweni Districts, approximately halfway between Chake Chake and Wete Towns. The main income-generating sources in this region are fishing, farming, and clove production.
DNA extraction and microsatellite genotyping.
Blood samples were obtained to standard procedures by a finger prick. Blood slides for malaria parasites were prepared according to standard protocols and read against 200 leukoyctes.13 Blood was also collected on dried No. 1 filter paper (Whatman, Maidstone, United Kingdom), placed into individual envelopes, and stored at room temperature. The protocol for DNA extraction was modified from that of Plowe and others.14 A 0.5% saponin solution in PBS was used for lysis of erythrocytes. One milliliter of the mixed solution was used to soak each dried filter paper, and then incubated for 15–30 minutes in 37°C. The soaked filter papers were stored overnight at 4°C. Approximately 1 mL of PBS from each tube was aspirated and discarded; new PBS was inserted and incubated for 15–30 minutes at 4°C. Again, the aspirated 1 mL of PBS from each tube was discarded. For metal chelation, 10 g of chelex were mixed in 50 mL of distilled water to prepare a 20% chelex-solution. A total of 200 μL of the 20% chelex solution were added to each tube and heated at 95°C for 10 minutes and vortexed. Tubes were then centrifuged at 10,000 × g for 2 minutes and supernatant were collected into new autoclaved tubes.
A heminested microsatellite polymerase chain reaction (PCR) protocol was adapted from Anderson and others.15 The total reaction volume was 15 μL containing 2 μL of template, 1.5 μL of 10× buffer, 0.2 units of Taq polymerase (Mg++ included), and 0.75 μL of each primer. The thermocycling was initiated with denaturation at 94°C for 2 minutes; followed by 25 cycles at 30 seconds at 94°C, 30 seconds at 42°C, 30 seconds at 40°C, and 40 seconds at 65°C; and 2 minutes at 65°C. Ninety-six well plates (Fisher Scientific, Pittsburgh, PA) were used for the first two rounds of PCR and for genotyping (ABI 96-well plates; Applied Biosystems, Foster City, CA).
After amplification, microsatellites were genotyped by using an ABI 3100 Avant capillary sequencer (Applied Biosystems). Analysis of microsatellite alleles was performed by using the Genemapper 4.0 program (Applied Biosystems). Peaks were automatically scored with minimum height of 500 and > 25% of the respective highest peak in every category/run. All microsatellite peaks were checked by individual visual inspection in the in the Genemapper program. Typical artifacts (e.g., stuttering or bleed-over) were filtered out manually by visual inspection. Because of the amplification properties of the heminested PCR protocol, peak height as a limiting parameter is only a semiquantitative measure; thus, it could fall below a threshold with a peak still clearly visible. Peaks below the height of 500 that were clearly visible were manually added to the existing peaks.
Three selection-neutral microsatellite markers were used for genotyping (Supplemental Table 1). They were chosen as they are located on different chromosomes, are trinucleotide markers (which are known to be more robust), and performed best among the markers we tested before. Multiplicity of infection (MOI) was calculated in PCR-positive samples as the maximum number of alleles present per sample measured by up to 3 microsatellite markers.
Malaria and pneumonia definitions.
Pneumonia was defined according to Integrated Management of Childhood Illness (IMCI) criteria (radiographic evidence was not available). We evaluated four definitions of a clinical malaria episode as previously described10 as possible risk factors for severe anemia incidence. The first definition was a child with 5,000 parasites/μL plus a history of fever (past 48 ours) or fever (≥ 37.5°C) at presentation.16 The second definition was a child with either 1) documented fever (≥ 37.5°C) and a parasite count > 1,000/μL; 2) history of fever (past 48 hours) and a parasite count > 3,000/μL; or 3) a parasite count > 8,000/μL. This definition was developed and used in the zinc/iron trial12 in which this study was nested. The third definition was a child with any parasites per microliter plus history of fever (past 48 hour) or fever ≥ 37.5°C) at presentation. This definition represents the performance of most malaria rapid diagnostic tests or malaria dipstick test with a detection threshold of approximately 50–100 parasites/μL. The fourth definition was child with a history of fever (past 48 hours or fever (≥ 37.5°C) at presentation and no sign of invasive bacterial disease (e.g., bacterial pneumonia) by judgment of the clinician (IMCI definition).
Statistical analysis.
Risk factors for incident severe anemia cases (Hb level ≤ 7 mg/dL) were evaluated by using a nested case–control approach based on incidence density sampling. Candidate risk factors included malaria episodes, bed net use, nutrition, family income, parents' education, acute respiratory infections, and MOI. Predictors or candidate risk factors were either obtained at enrollment, were generated from previous visits, or from the history (2 weeks/3 days before current visits) of the current visit. Predictors from the previous visit were generated for those visits in the time window 5–21 days before the current visit. In 88% of all visits, the previous visit was within the time window of 5–21 days before the current visit.
An incident case of anemia was defined as a patient with a diagnosis of severe anemia (Hb level < 7 mg/dL) at any given visit provided that information on the Hb level from the previous visit (up to 20 days before the current visit) was available and that the Hb level was still above the respective threshold (7 mg/dL). Among the controls available, a rank was created and controls were selected based on their degree of non-missing data on genotyping and other covariates. In 120 cases of severe anemia, up to 5 controls were matched to one case depending on availability of controls.
A conditional (fixed effects) logistic regression was fitted (clogit command in STATA version 9.2; StataCorp LP, College Station, TX) with robust standard error and grouped on the case–control match by date of presentation (Logit P(anemia)/1 – P(anemia) = b0 + b1×1 + b2×2 + b3×3, grouped by identification (which is the case-to-control-match). The robust option of the standard error was chosen because of the internal correlation of some of the cases (multiple cases of anemia per person). In case of several similar predictors (e.g., socioeconomic status: family income, father's education, mother's education) the best predictor was selected using the correlation between those variables, comparing univariate, bivariate, and multivariate regression and variance component estimation. Potential collinearity was assessed by using variance inflation factors (vif command in STATA).
Results
Hemoglobin level at enrollment was available for 480 (89.4%) of 537 children. Data for Hb level at enrollment was missing for 57 children. Mean ± SD Hb level at enrollment was 9.3 ± 1.7 mg/dL (range = 4.8–17.3 mg/dL). At enrollment 34 (7.0%) of 480 children were severely anemic and had an Hb level < 7 mg/dL and 296 children (61.7%) had an Hb level < 10 mg/dL. Life-threatening anemia (Hb level < mg/dL) was recorded for two children at enrollment and subsequently 18 times during the follow-up. These children were treated immediately with oral iron supplementation and/or hospital admission. Most of the anemia encountered in children on Pemba Island was microcytic anemia (mean corpuscular volume = 62 fl) within the whole cohort. Follow-up values for Hb levels were available for 5,635 of 6,125 visits.
Anemia prevalence and incidence.
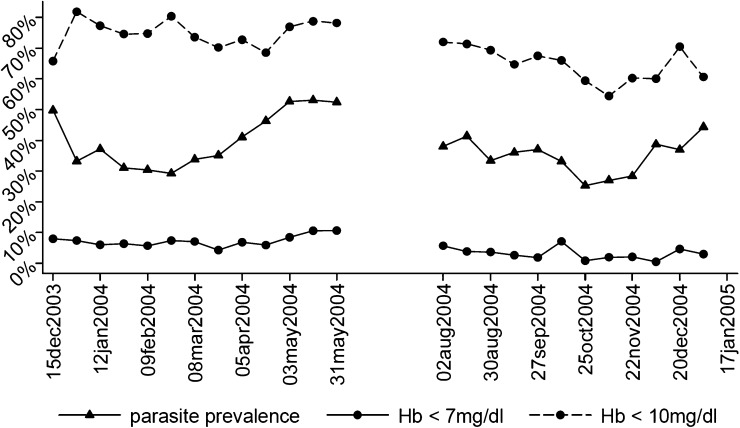
Anemia period prevalence per two-week period is shown in Figure 1. Anemia with an Hb level < 10 mg/dL was highly prevalent. Approximately 60–80% of the children were moderately anemic at any point in time. Severe anemia with an Hb level < 7 mg/dL was present in approximately 5–10% of the children. The Hb values were higher in the second cohort (9.5 mg/dL; 95% confidence interval = 9.45–9.55 mg/dL) compared with the first cohort (9.1 mg/dL; 95% confidence interval = 9.03–9.12 mg/dL), which may have been caused by higher family income average in the second cohort (Table 1).
Figure 1.
Anemia prevalence (< 7 mg/dL; < 10 mg/dL) and malaria parasite prevalence per two-week period in 537 children on Pemba Island, Zanzibar, Tanzania. Hb = hemoglobin.
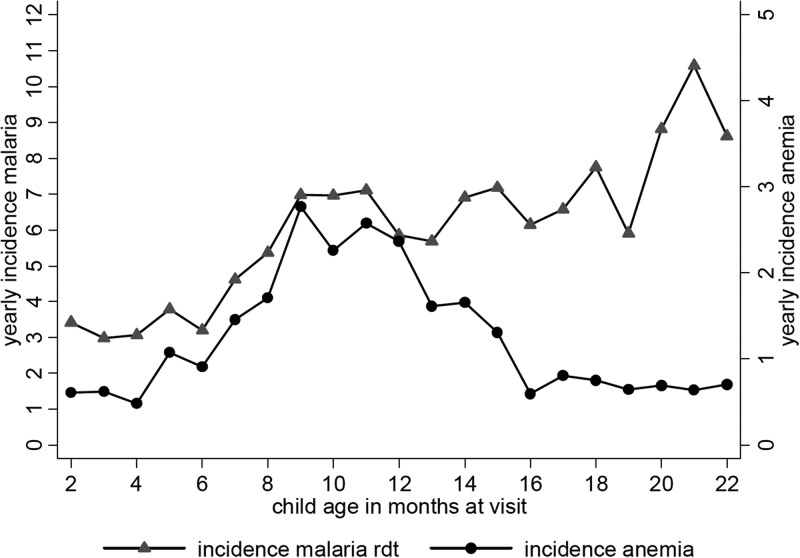
Incidence of severe anemia (Hb level < 7 mg/dL) peaked between the age at visitation of 8 and 13 months. The increase in severe anemia incidence occurred synchronously with the increase in malaria incidence, pointing towards the important role of malaria in the causation of severe anemia (Figure 2). After 13 months, severe anemia incidence markedly decreases and clinical malaria incidence continued to show a slow increase.
Figure 2.
Incidence of severe anemia (hemoglobim < 7 mg/dL) and incidence of malaria (as by any parasites plus fever/history of fever) per age in months in 537 children on Pemba Island, Zanzibar, Tanzania, December 2003–January 2005. rdt = rapid diagnostic test.
Multiplicity of infection.
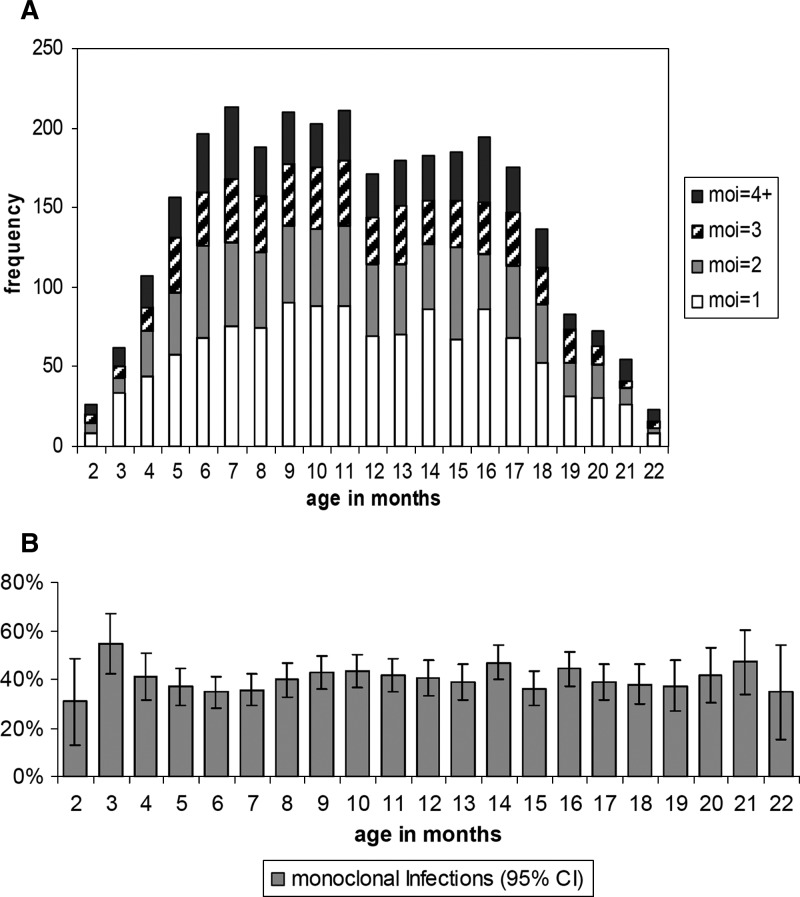
In 3,066 PCR-positive visits (of 6125 total visits), MOI was 1 in approximately 40% of the cases, 2 in 25%, and ≥ 3 in 35% (Table 2). The MOI did not differ by bed net use reported at enrollment (P = 0.397, by chi-square test) as unlike previously reported.17 Within the age range of 5–18 months (where most observations were made) (Figure 3A), MOI was constant with age. An MOI of 1 showed a maximum of 55% at three months of age and decreased to approximately 40% by month 5 to month 6 (Figure 3B). We also found a non-significant trend towards lower MOI in gametocyte-positive malaria infections (P = 0.244, by chi-square test), which needs to be further explored (gametocytes were found in 78 of 6,125 visits among 537 children).
Table 2.
MOI in 3,066 PCR-positive visits of 6,125 total visits among 533 children on Pemba Island, Zanzibar, Tanzania, December 2003–January 2005*
| MOI | No. | % |
|---|---|---|
| 1 | 1,237 | 40 |
| 2 | 765 | 25 |
| 3 | 544 | 18 |
| 4 | 368 | 12 |
| 5 | 126 | 4 |
| 6 | 21 | 1 |
| 7 | 4 | |
| 8 | 1 | |
| Total | 3,066 |
Multiplicity of infection (MOI) was calculated as the maximum number of alleles per visit for three microsatellite markers. PCR = polymerase chain reaction.
Figure 3.
Multiplicity of infection (moi) by age in months, in 3,066 polymerase chain reaction–positive visits among 533 children on Pemba Island, Xanzibar, Tanzania. A, Absolute frequency (1–4+); B, Relative frequency of monoclonal infections (including 95% confidence interval [CI]).
Risk factors for severe anemia incidence.
Risk factors for severe anemia (Hb level < 7 mg/dL) are shown in Table 3. Two multivariate models are presented: model 1, without MOI as a risk factor; and model 2, which included MOI, but at the cost of a considerably reduced number of total observations (not parasitemic or MOI not available). Univariate regressions were conducted on an extended list of candidate risk factors for severe anemia (Supplemental Table 2). In the univariate regressions, arm circumference was the strongest predictor among the nutritional variables. None of the nutritional variables was found to be statistically significant in the multivariate models.
Table 3.
Final model for conditional logistic regression on anemia matched for day of presentation on Pemba Island, Zanzibar, Tanzania*
| Predictor | Univariate regressions (n = 120 cases and 556 controls) 95% CI | Multivariate model 1 (n = 114 cases and 527 controls) | Multivariate model 2 (n = 49 cases and 243 controls) | |||||
|---|---|---|---|---|---|---|---|---|
| No. cases (%) | No, controls (%) | Univariate OR (P) | 95% CI | Multivariate OR (P) | 95% CI | Multivariate OR (P) | 95% CI | |
| Age, months | ||||||||
| 1–6 | 15 (12.5) | 80 (14.3) | Reference | Reference | Reference | |||
| 7–12 | 68 (56.6) | 217 (39) | 2.36 (0.003) | 1.28–4.38 | 1.82 (0.060) | 0.98–3.41 | 0.36 (0.284) | 0.54–2.35 |
| ≥ 13 | 37 (30.8) | 259 (45.5) | 0.94 (0.846) | 0.49–1.18 | 0.88 (0.699) | 0.46–1.68 | 0.11 (0.011) | 0.21–0.61 |
| Sex | ||||||||
| M | 65 (54.1) | 274 (49.2) | Reference | Reference | Reference | |||
| F | 55 (45.8) | 282 (50.8) | 0.69 (0.037) | 0.49–0.98 | 0.82 (0.351) | 0.55–1.24 | 0.58 (0.381) | 0.17–1.97 |
| Household income ($/month) | ||||||||
| < 10 | 48 (40) | 201 (36.1) | Reference | Reference | Reference | |||
| ≥ 10 | 72 (60) | 355 (63.9) | 0.55 (0.003) | 0.37–0.82 | 0.53 (0.006) | 0.38–0.84 | 0.61 (0.469) | 0.15–2.31 |
| Malaria at previous visit† | ||||||||
| No | 74 (64.9) | 404 (76.6) | Reference | Reference | ||||
| Yes | 40 (35.1) | 123 (23.4) | 1.89 (0.003) | 1.23–2.87 | 1.78 (0.014) | 1.12–2.81 | ||
| Parasite density at previous visit (log10/μL) | 2.1 (SD 1.7) | 1.6 (SD 1.7) | 1.22 (< 0.001) | 1.09–1.37 | 1.75 (0.031) | 1.05–2.91 | ||
| Parasite density at current visit (log10/μL) | 2.1 (SD 1.9) | 1.5 (SD 1.8) | 1.29 (< 0.001) | 1.15–1.45 | 0.95 (0.731) | 0.70–1.29 | ||
| Pneumonia at visit prior to current visit | ||||||||
| No | 107 (93.8) | 511 (96.4) | Reference | Reference | ||||
| Yes | 7 (6.2) | 19 (3.6) | 14.07 (0.001) | 2.90–68.15 | 11.63 (0.005) | 2.07–65.21 | ||
| Gametocytes at current visit | ||||||||
| No | 114 (95) | 550 (98.9) | Reference | Reference | Reference | |||
| Yes | 6 (5) | 6 (1.1) | 6.13 (0.001) | 2.04–18.43 | 6.24 (0.005) | 1.72–22.58 | 11.07 (0.030) | 1.27–96.54 |
| Information on MOI only available in a limited data set | ||||||||
| MOI at current visit‡ | ||||||||
| 1 | 30 (38.9) | 128 (42.1) | Reference | Reference | ||||
| 2 | 26 (33.7) | 76 (25) | 2.66 (0.005) | 1.35–5.25 | 7.05 (0.036) | 1.14–43.57 | ||
| 3 | 17 (22.0) | 46 (15.1) | 2.00 (0.114) | 0.85–4.68 | 2.00 (0.328) | 0.50–7.90 | ||
| ≥ 4 | 4 (5.1) | 54 (17.7) | 0.82 (0.627) | 0.38–1.79 | 0.06 (0.012) | 0.01–0.55 | ||
| MOI at prior visit§ | ||||||||
| 1 | 29 (39.1) | 175 (40.5) | Reference | Reference | ||||
| 2 | 22 (29.7) | 116 (26.8) | 1.39 (0.279) | 0.77–2.52 | 3.04 (0.134) | 0.71–13.02 | ||
| 3 | 14 (18.9) | 62 (14.3) | 1.84 (0.115) | 0.86–3.93 | 3.58 (0.256) | 0.40–32.32 | ||
| ≥ 4 | 9 (12.1`) | 79 (18.2) | 0.60 (0.250) | 0.24–1.44 | 0.24 (0.129) | 0.04–1.51 | ||
OR = odds ratio; CI = confidence interval; MOI = multiplicity of infection.
As per definition 3: ≥ 10 parasites/μL plus history of fever or documented fever. Information on previous malaria was not available for the time window –5 to –21 days in 10 cases and 84 controls.
Information on MOI at current visit (–5 to –21 days) was not available in 43 cases and 252 controls. These are microscopy-negative cases and microscopy-positive cases in which the polymerase chain reaction did not yield a result.
Information on MOI at previous visit (–5 to –21 days) was not available in 46 cases and 124 controls. These are microscopy-negative cases and microscopy-positive cases in which the polymerase chain reaction did not yield a result.
Within the demographic and socioeconomic status variables age, sex, and family income were associated with severe anemia incidence. The association of male sex with a 30–40% reduction in severe anemia risk was only significant in the univariate regression. The effect of age group on severe anemia incidence corresponds to the pattern shown in Figure 2 in which the intermediate age group of 7–12 months showed a greater than two-fold increased risk for severe anemia compared with children 1–6 months of age in univariate and in the first multivariate model (without MOI). In the children 13–22 months of age, the severe anemia risk is comparable with the risk in children 1–6 months of age. In the second multivariate model, the effect size of age group is higher because of smaller sample size. As expected, household income was associated with a significant protection against severe anemia in the category of ≥ $10 per month in the univariate and in the first multivariate model (without MOI), which reflected the nutritional and sociodemographic causes of severe anemia.
Infectious diseases are the most important risk factor for severe anemia in this study. Two independent risk factors highlight the importance of malaria infections for severe anemia incidence, namely diagnosis of clinical malaria at prior visit (–5 to –21 days), which increases the severe anemia risk by 80%; and gametocytes at current visit, which increases the severe anemia risk by a factor of six. Parasite density at prior visit did not significantly alter the estimates (except displaying partial collinearity with diagnosis of clinical malaria at prior visit) and was not included in the best model. Among the four candidate definitions tested for clinical malaria, the third definition (fever or history of fever and any parasites, corresponding to a rapid diagnostic test) performed best in the univariate regressions and was therefore included in the multivariate models (Supplemental Table 2). Pneumonia at prior visit (–5 to –21 days) was associated with a more than 10-fold increase in severe anemia risk, which highlights the importance of bacterial infections in the causation of anemia. In the multivariate model, this risk increase was independent of malaria status and gametocyte presence.
When MOI at current/prior visit was added to the multivariate model (resulting in a reduced number of observations in the model), malaria diagnosis at prior visit as a risk factor was replaced by parasite density at prior visit, which increased the anemia risk by 70%.
An MOI of 2 or 3 was associated with a 2–7-fold increase in severe anemia risk (reference category MOI = 1), but a protective effect (odds ratio = 0.82/0.60 in univariate and 0.06/0.24 in multivariate analysis) was seen with MOI values > 4. This pattern reached borderline statistical significance and was present for MOI at current and at previous visit, which supports the robustness of this finding in our data. Collinearity between gametocytes, malaria definitions (1–4), and MOI was negligible.
Discussion
Incidence of severe anemia (Hb level < 7 mg/dL) showed a clear peak in the second six months of the first year of life on Pemba Island. This age distribution matches the age range in which young children are confronted with malaria parasites and develop their own immunity after gradually losing the protection by passively transferred maternal antibodies and by fetal Hb. Severe anemia is often reported as prevalence. We report incidence and can therefore better display the dynamic nature of the changes in Hb level with malaria infection and MOI.
Malaria incidence (as by any parasites plus fever/history of fever) is increasing synchronous in time with anemia incidence at approximately seven months of age, reaches a plateau, and then slowly continues to increase at approximately 13 months of age when anemia incidence decreases back to the baseline risk experienced by children < 1–6 months of age. We confirm the important role of infectious diseases (malaria and pneumonia) for anemia and can report about independent contributions of malaria diagnosis, gametocyte presence, or MOI to anemia incidence. Family income is the only non-infectious risk factor still significant in the multivariate model, which suggests the underlying nutritional and sociodemographic reasons of anemia (e.g., micronutrient malnutrition).18 This study was nested into a micronutrient intervention trial, in which iron supplementation was found to lead to an increased hospitalization rate,12 which was attributed to the high malaria endemicity in the study area.
Enrollment for this study was independent of the ongoing micro-nutrient trial, and not all children enrolled in this study were also included in the micro-nutrient trial. Information on micro-nutrient substitution status (zinc or control arm) was not investigated as a risk factor for anemia. The focus of this study was on risk factors of anemia related to malaria and bacterial infections. The association of anemia with zinc substitution is better reflected in the entire dataset of the intervention trial.
Anemia (especially microcytic anemia) is often multifactorial in origin. In malaria transmission areas, malaria-specific factors coincide with factors that are present in any acute or chronic infection, e.g., bacterial infections. The malaria-specific factors are described in detail in a review by Ghosh and Ghosh.19 One of the key features that explain chronic anemia as one complication of severe malaria is a low reticulocyte response and high serum erythropoietin levels and ongoing hemolysis.20 These factors interact with other coexisting infections and nutritional deficiencies.21 Severe malaria anemia is recognized as a serious complication of malaria predominantly in high transmission areas.22 Our data show the magnitude of the problem in a hyperendemic transmission setting.10 The pattern that children 7–12 months of age are at highest risk for severe anemia is similar to that described for holoendemic areas.23 In our study, malaria-specific risk factors outweighed nutritional or demographic risk factors for severe anemia. Prior malaria episodes and current gametocyte carriage were independent risk factors for incident severe anemia.
The role of MOI as a risk factor for severe anemia could only be assessed with a limited data set. The MOI at the current visit outweighed the MOI at the previous visit as a predictor, but the effect of MOI at previous visit (–5 to –21 days) was similar to the effect of MOI at the current visit (when severe anemia was diagnosed). An MOI of 2 or 3 was associated with an increased risk for anemia compared with an MOI of 1. An MOI ≥ 4 was associated with a moderate protection from anemia in our data independent of parasite density at prior visit, parasite density at present visit, age group, and gametocyte status at current visit. This finding can be interpreted as a signal that the ability to harbor or tolerate a higher number of multiple parallel infections represents a correlate of an immune mechanism in malaria. In the past, the role of MOI as a risk factor for malaria and anemia was mainly assessed in a binary (single versus multiple) way. In spite of the fact that inter-laboratory differences exist and that MOI is frequently measured by genotyping polymorphic regions of merozoite surface proteins 1 and 2 (which is different from our approach), we believe that a more quantitative assessment of MOI is needed. A high MOI was associated with anemia in another study, in which MOI was included as a continuous predictor.24 In our analysis, we allowed for a non-continuous relationship with an interesting result as reported above. The MOI and severe anemia were not associated with bed net use (as by enrollment) (Supplemental Table 2).
Bacteremia has been identified as an important, often underestimated, cause for anemia in malaria-endemic areas.25 In our data, pneumonia and leukocyte counts were investigated and showed significant associations with increased severe anemia risk by univariate regressions. However, only pneumonia at prior visit was significant in the multivariate model. The odds ratio of 11.6 in the multivariate model highlights the importance, but also raises caution because of the limited number of cases and the large confidence interval (95% confidence interval = 2.1–65.2). Because pneumonia was identified on the basis of clinical criteria (according to IMCI guidelines), a considerable number of pneumonia cases might have been missed.
Treating bacterial infections could open up another avenue to control severe anemia in malaria-endemic regions, especially because malaria transmission has decreased substantially in some areas of eastern Africa, and the relative importance of pneumonia or other bacterial infections could increase in the future.
Supplementary Material
ACKNOWLEDGMENTS
We thank Lirong Shi (laboratory technician in the laboratory of David J. Sullivan), for help with DNA extraction and PCR tests; Roger Gaczkowski for help with the statistical analysis and with preparation of the figures; Dr. Mashavu K Othman for clinical assistance; and Public Health Laboratory–Ivo de Carneri in Pemba and the Zanzibar Ministry of Health and Social Welfare for assistance.
Footnotes
Financial support: The laboratory work was supported by the Johns Hopkins Malaria Research Institute and the Bloomberg Family Foundation. The field study was within the sponsored study by The Department of Child and Adolescent Health and Development/World Health Organization, Geneva, Switzerland (ISRCTN59549825).
Authors' addresses: Thomas Jaenisch, Department of Infectious Diseases, Parasitology, Heidelberg University Hospital, Heidelberg, Germany, E-mail: thomas.jaenisch@urz.uni-heidelberg.de. Sunil Sazawal, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, E-mail: ssazawal@jhsph.edu. Arup Dutta and Saikat Deb, Public Health Laboratory, Ivo de Carneri, Pemba, Zanzibar, Tanzania, E-mails: adutta@cphealthkinetics.org and saikatdeb@gmail.com. Mahdi Ramsan, Public Health Laboratory, Ivo de Carneri, Zanzibar Malaria Control Programme, Zanzibar, Tanzania, E-mail: mmohamed@rti.org. David J. Sullivan, Department of Microbiology and Immunology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, E-mail: dsulliva@jhsph.edu.
References
- 1.Milman N. Anemia–still a major health problem in many parts of the world! Ann Hematol. 2011;90:369–377. doi: 10.1007/s00277-010-1144-5. [DOI] [PubMed] [Google Scholar]
- 2.Ellman R, Maxwell C, Finch R, Shayo D. Malaria and anaemia at different altitudes in the Muheza district of Tanzania: childhood morbidity in relation to level of exposure to infection. Ann Trop Med Parasitol. 1998;92:741–753. doi: 10.1080/00034989858989. [DOI] [PubMed] [Google Scholar]
- 3.Kitua AY, Smith TA, Alonso PL, Urassa H, Masanja H, Kimario J, Tanner M. The role of low level Plasmodium falciparum parasitaemia in anaemia among infants living in an area of intense and perennial transmission. Trop Med Int Health. 1997;2:325–333. doi: 10.1111/j.1365-3156.1997.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 4.Premji Z, Hamisi Y, Shiff C, Minjas J, Lubega P, Makwaya C. Anaemia and Plasmodium falciparum infections among young children in an holoendemic area, Bagamoyo, Tanzania. Acta Trop. 1995;59:55–64. doi: 10.1016/0001-706x(94)00079-g. [DOI] [PubMed] [Google Scholar]
- 5.Shiff C, Checkley W, Winch P, Premji Z, Minjas J, Lubega P. Changes in weight gain and anaemia attributable to malaria in Tanzanian children living under holoendemic conditions. Trans R Soc Trop Med Hyg. 1996;90:262–265. doi: 10.1016/s0035-9203(96)90240-0. [DOI] [PubMed] [Google Scholar]
- 6.Olney DK, Pollitt E, Kariger PK, Khalfan SS, Ali NS, Tielsch JM, Sazawal S, Black R, Mast D, Allen LH, Stoltzfus RJ. Young Zanzibari children with iron deficiency, iron deficiency anemia, stunting, or malaria have lower motor activity scores and spend less time in locomotion. J Nutr. 2007;137:2756–2762. doi: 10.1093/jn/137.12.2756. [DOI] [PubMed] [Google Scholar]
- 7.Olney DK, Kariger PK, Stoltzfus RJ, Khalfan SS, Ali NS, Tielsch JM, Sazawal S, Black R, Allen LH, Pollitt E. Development of nutritionally at-risk young children is predicted by malaria, anemia, and stunting in Pemba, Zanzibar. J Nutr. 2009;139:763–772. doi: 10.3945/jn.107.086231. [DOI] [PubMed] [Google Scholar]
- 8.Stoltzfus RJ, Chwaya HM, Albonico M, Schulze KJ, Savioli L, Tielsch JM. Serum ferritin, erythrocyte protoporphyrin and hemoglobin are valid indicators of iron status of school children in a malaria-holoendemic population. J Nutr. 1997;127:293–298. doi: 10.1093/jn/127.2.293. [DOI] [PubMed] [Google Scholar]
- 9.Stoltzfus RJ, Chwaya HM, Montresor A, Albonico M, Savioli L, Tielsch JM. Malaria, hookworms and recent fever are related to anemia and iron status indicators in 0- to 5-y old Zanzibari children and these relationships change with age. J Nutr. 2000;130:1724–1733. doi: 10.1093/jn/130.7.1724. [DOI] [PubMed] [Google Scholar]
- 10.Jaenisch T, Sullivan DJ, Dutta A, Deb S, Ramsan M, Othman MK, Gaczkowski R, Tielsch J, Sazawal S. Malaria incidence and prevalence on Pemba island before the onset of the successful control intervention on the Zanzibar archipelago. Malar J. 2010;9:32. doi: 10.1186/1475-2875-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattarai A, Ali AS, Kachur SP, Martensson A, Abbas AK, Khatib R, Al-Mafazy AW, Ramsan M, Rotllant G, Gerstenmaier JF, Molteni F, Abdulla S, Montgomery SM, Kaneko A, Bjorkman A. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007;4:e309. doi: 10.1371/journal.pmed.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, Kabole FM. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 13.Trape JF. Rapid evaluation of malaria parasite density and standardization of thick smear examination for epidemiological investigations. Trans R Soc Trop Med Hyg. 1985;79:181–184. doi: 10.1016/0035-9203(85)90329-3. [DOI] [PubMed] [Google Scholar]
- 14.Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg. 1995;52:565–568. doi: 10.4269/ajtmh.1995.52.565. [DOI] [PubMed] [Google Scholar]
- 15.Anderson TJ, Su XZ, Bockarie M, Lagog M, Day KP. Twelve microsatellite markers for characterization of Plasmodium falciparum from finger-prick blood samples. Parasitology. 1999;119:113–125. doi: 10.1017/s0031182099004552. [DOI] [PubMed] [Google Scholar]
- 16.Smith T, Schellenberg JA, Hayes R. Attributable fraction estimates and case definitions for malaria in endemic areas. Stat Med. 1994;13:2345–2358. doi: 10.1002/sim.4780132206. [DOI] [PubMed] [Google Scholar]
- 17.Fraser-Hurt N, Felger I, Edoh D, Steiger S, Mashaka M, Masanja H, Smith T, Mbena F, Beck HP. Effect of insecticide-treated bed nets on haemoglobin values, prevalence and multiplicity of infection with Plasmodium falciparum in a randomized controlled trial in Tanzania. Trans R Soc Trop Med Hyg. 1999;93((Suppl 1)):47–51. doi: 10.1016/s0035-9203(99)90327-9. [DOI] [PubMed] [Google Scholar]
- 18.Nussenblatt V, Semba RD. Micronutrient malnutrition and the pathogenesis of malarial anemia. Acta Trop. 2002;82:321–337. doi: 10.1016/s0001-706x(02)00049-9. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh K, Ghosh K. Pathogenesis of anemia in malaria: a concise review. Parasitol Res. 2007;101:1463–1469. doi: 10.1007/s00436-007-0742-1. [DOI] [PubMed] [Google Scholar]
- 20.Roberts DJ, Casals-Pascual C, Weatherall DJ. The clinical and pathophysiological features of malarial anaemia. Curr Top Microbiol Immunol. 2005;295:137–167. doi: 10.1007/3-540-29088-5_6. [DOI] [PubMed] [Google Scholar]
- 21.Weatherall DJ, Miller LH, Baruch DI, Marsh K, Doumbo OK, Casals-Pascual C, Roberts DJ. Malaria and the red cell. Hematology Am Soc Hematol Educ Program. 2002:35–57. doi: 10.1182/asheducation-2002.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 23.Snow RW, Omumbo JA, Lowe B, Molyneux CS, Obiero JO, Palmer A, Weber MW, Pinder M, Nahlen B, Obonyo C, Newbold C, Gupta S, Marsh K. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 24.Mockenhaupt FP, Ehrhardt S, Eggelte TA, Markert M, Anemana S, Otchwemah R, Bienzle U. Plasmodium falciparum multiplicity correlates with anaemia in symptomatic malaria. Trop Med Int Health. 2003;8:857–859. doi: 10.1046/j.1365-3156.2003.01117.x. [DOI] [PubMed] [Google Scholar]
- 25.Calis JC, Phiri KS, Faragher EB, Brabin BJ, Bates I, Cuevas LE, de Haan RJ, Phiri AI, Malange P, Khoka M, Hulshof PJ, van Lieshout L, Beld MG, Teo YY, Rockett KA, Richardson A, Kwiatkowski DP, Molyneux ME, van Hensbroek MB. Severe anemia in Malawian children. N Engl J Med. 2008;358:888–899. doi: 10.1056/NEJMoa072727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.